Synthesis, X-ray Diffraction Study and Antimicrobial Activity of Calcium Sulphate Nanocomposites from Plant Charcoal
Abstract
:1. Introduction
2. Results and Discussion
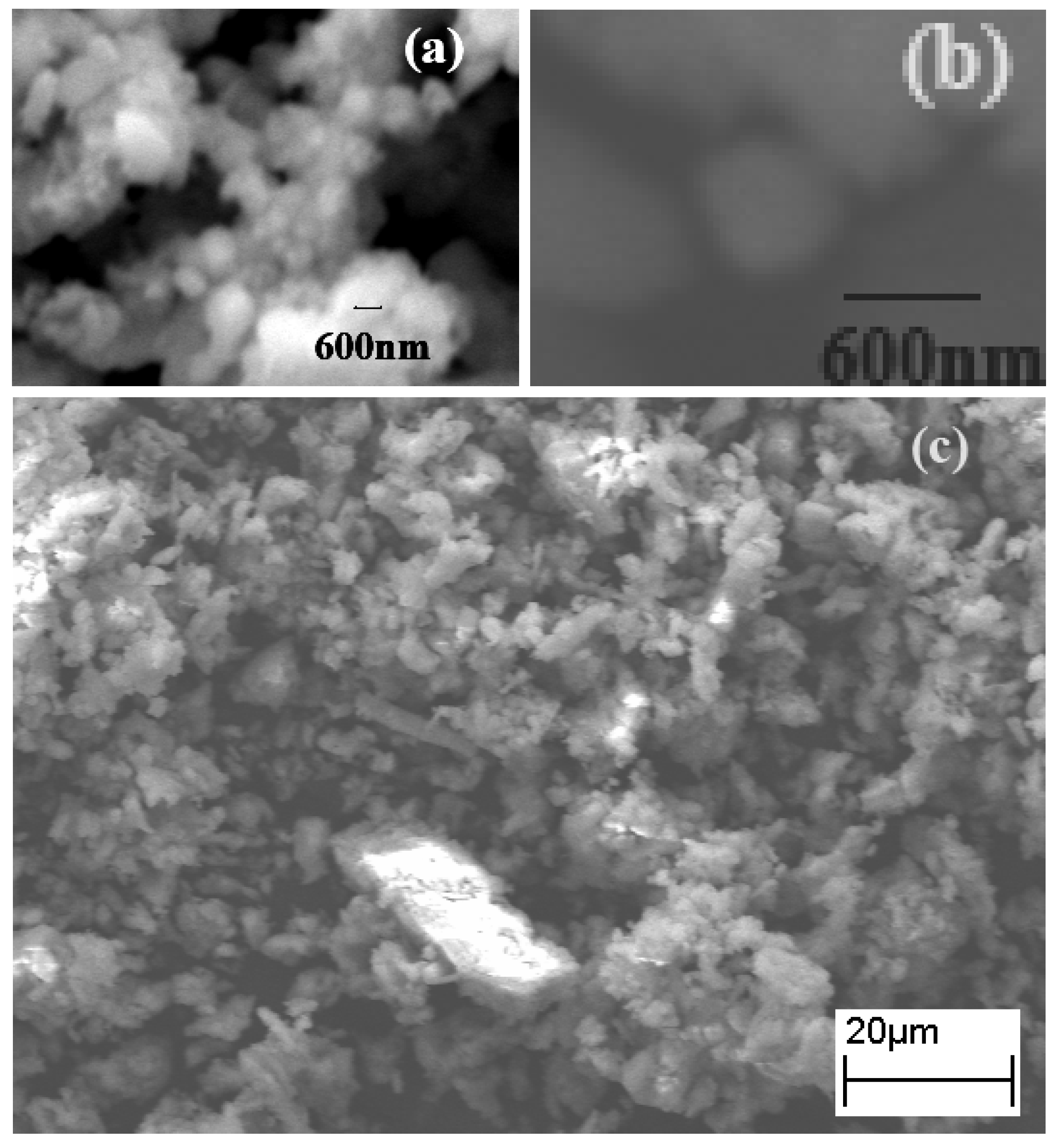
2.1. SEM Studies
2.2. Elemental Analysis

| Element | App Conc. | Intensity | Weight% | Weight% Sigma | Atomic% |
|---|---|---|---|---|---|
| O | 37.55 | 0.4370 | 49.94 | 0.78 | 68.23 |
| Na | 1.55 | 0.6542 | 1.38 | 0.18 | 1.31 |
| Mg | 2.30 | 0.6318 | 2.12 | 0.15 | 1.90 |
| Al | 1.13 | 0.7350 | 0.89 | 0.11 | 0.72 |
| Si | 4.65 | 0.8396 | 3.22 | 0.15 | 2.50 |
| S | 25.86 | 0.9334 | 16.10 | 0.34 | 10.97 |
| Cl | 1.49 | 0.7180 | 1.21 | 0.12 | 0.75 |
| K | 1.27 | 1.0228 | 0.72 | 0.10 | 0.40 |
| Ca | 39.14 | 0.9582 | 23.74 | 0.43 | 12.94 |
| Fe | 0.95 | 0.8093 | 0.68 | 0.15 | 0.27 |
| Total | 100.00 |
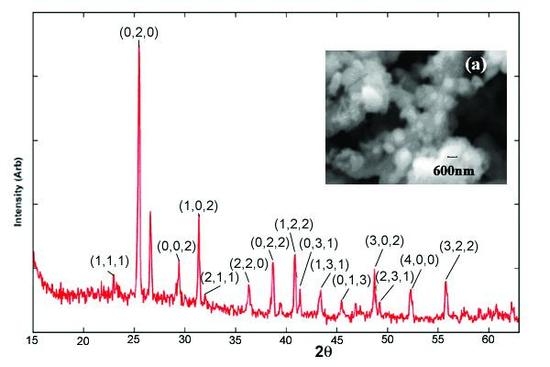
2.3. XRD Studies
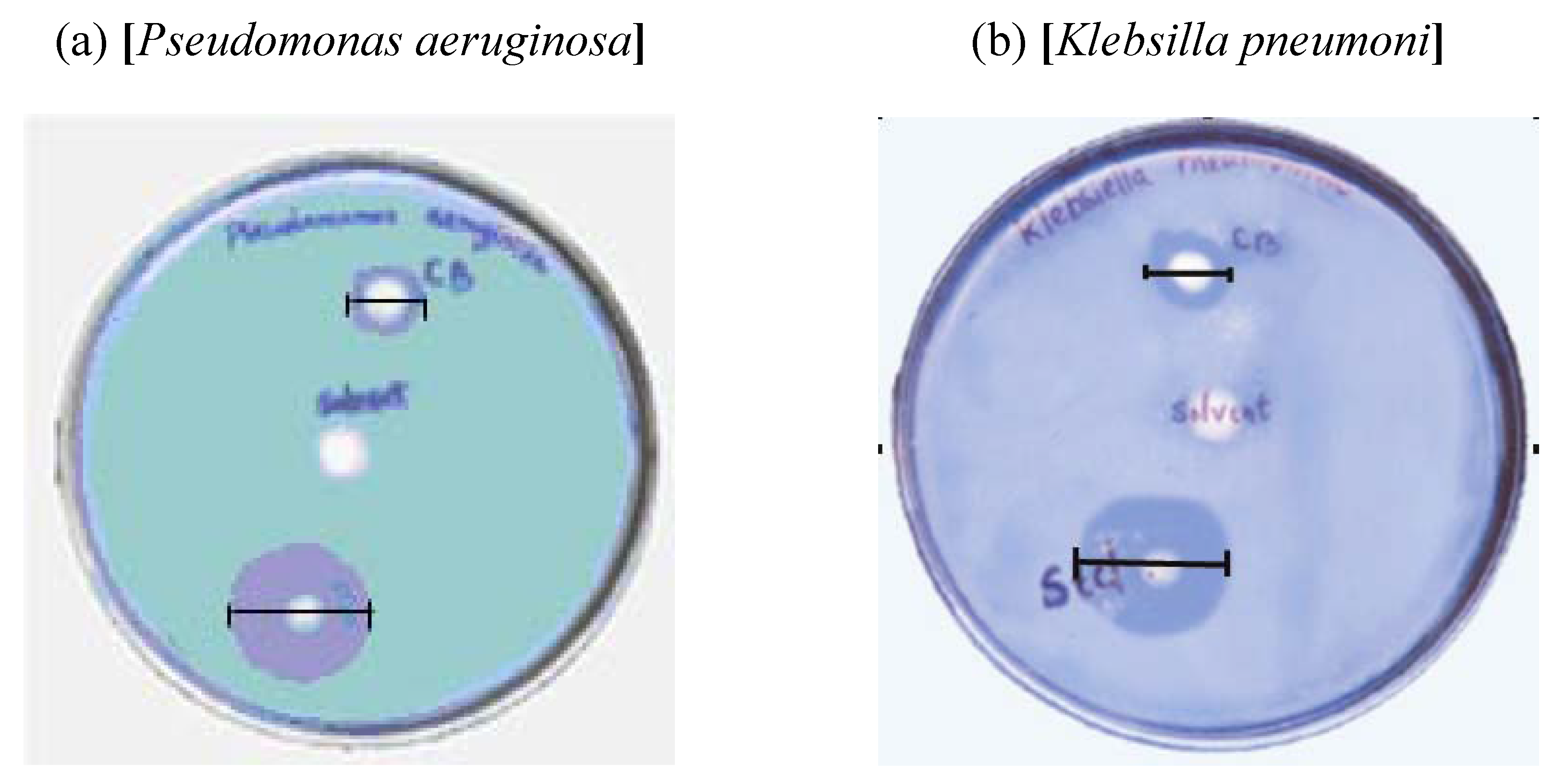
2.4. Study of Antimicrobial Activity

| Sl No. | Microbe | Standard (Zone of inhibition) | Solvent (CHCl3) | CB (Zone of inhibition) |
|---|---|---|---|---|
| 1 | Streptococcus faecaelis | 40 mm | Nil | 18 mm |
| 2 | Bacillus subtilis | 32 mm | Nil | 20 mm |
| 3 | Klebsilla pneumoni | 30 mm | Nil | 12 mm |
| 4 | E. coli | 35 mm | Nil | 13 mm |
| 5 | Proteus vulgaris | 40 mm | Nil | 12 mm |
| 6 | Pseudomonas aeruginosa | 30 mm | Nil | 12 mm |
3. Experimental Section
3.1. Preparation of the materials
3.2. Study of antimicrobial activity
3.3. X-Ray Diffraction (XRD) Study
4. Conclusions
Acknowledgements
References and Notes
- Robinson, K.L.; Weaver, J.V.M.; Armes, S.P.; Marti, E.D.; Meldrum, F.C. Synthesis of controlled-structure sulfate-based copolymers via atom transfer radical polymerisation and their use as crystal habit modifiers for BaSO4. J. Mater. Chem. 2002, 12, 890–896. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, J.; Tang, H.; Lin, J. Facile synthesis of monodispersed barium sulphate particles via an in situ templated process. J. Coll. Int. Sci. 2007, 311, 89–93. [Google Scholar] [CrossRef]
- Available online: http://www.musketeer.ch/blackpowder/charcoal.html accessed 20 February, 2009.
- “Wood Ash in the Garden”. Available online: http://www.hort.purdue.edu/ext/woodash.html accessed 20 February, 2009.
- Misra, M.K.; Ragland, K.W.; Baker, A.J. Wood ash composition as a function of furnace temperature. Biom. Bioen. 1993, 4, 103–116. [Google Scholar] [CrossRef]
- Ferraz, M.P.; Mateus, A.Y.; Sousa, J.C.; Monteiro, F.J. Nanohydroxyapatite microspheres as delivery system for antibiotics: Release kinetics, antimicrobial activity, and interaction with osteoblasts. J. Biomed. Mater. Res. Part A 2007, 81, 994–1004. [Google Scholar] [CrossRef]
- Sabbour, M.M. The role of chemical additives in enhancing the efficacy of Beauveria bassiana and Metarhizium anisopliae against the potato tuber moth Phthorimaea operculella (Zeller) (Lepidoptera:Gelechiidae). Pak. J. Biol. Sci. 2002, 5, 1155–1159. [Google Scholar] [CrossRef]
- Davis, C.J.; Eschenazi, E.; Papadopoulos, K.D. Combined effects of Ca2+ and humic acid on colloid transport through porous media. Coll. Polym. Sci. 2002, 280, 52–58. [Google Scholar] [CrossRef]
- Pelley, J.; Tufenkji, N. Effect of particle size and natural organic matter on the migration of nano- and microscale latex particles in saturated porous media. J. Coll. Int. Sci. 2008, 321, 74–83. [Google Scholar] [CrossRef]
- Southam, D.C.; Lewis, T.W.; McFarlane, A.J.; Johnston, J.H. Amorphous calcium silicate as a chemisorbent for phosphate. Curr. Appl. Phys. 2004, 4, 355–358. [Google Scholar] [CrossRef]
- Southam, D.C.; Lewis, T.W.; McFarlane, A.J.; Borrmann, T.; Johnston, J.H. Calcium–phosphorus interactions at a nano-structured silicate surface. J. Coll. Int. Sci. 2008, 319, 489–497. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- CIF files. File number: 5000040. Available online: http://cod.ibt.lt/ accessed 11 December, 2008.
- Cheng, G.C.H.; Zussman, J. The crystal structure of anhydrite (CaSO4). Acta Crystallogr. 1963, 16, 767–769. [Google Scholar] [CrossRef]
- Mercury 1.4.1 ®. Available online: http://www.ccdc.cam.ac.uk/ accessed 15 November, 2007.
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Sample availability: Available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bhattacharjee, C.R.; Paul, S.B.; Nath, A.; Choudhury, P.P.N.; Choudhury, S. Synthesis, X-ray Diffraction Study and Antimicrobial Activity of Calcium Sulphate Nanocomposites from Plant Charcoal. Materials 2009, 2, 345-352. https://doi.org/10.3390/ma2020345
Bhattacharjee CR, Paul SB, Nath A, Choudhury PPN, Choudhury S. Synthesis, X-ray Diffraction Study and Antimicrobial Activity of Calcium Sulphate Nanocomposites from Plant Charcoal. Materials. 2009; 2(2):345-352. https://doi.org/10.3390/ma2020345
Chicago/Turabian StyleBhattacharjee, Chira R., Satya B. Paul, Abhijit Nath, Pinak P. N. Choudhury, and Sudip Choudhury. 2009. "Synthesis, X-ray Diffraction Study and Antimicrobial Activity of Calcium Sulphate Nanocomposites from Plant Charcoal" Materials 2, no. 2: 345-352. https://doi.org/10.3390/ma2020345
APA StyleBhattacharjee, C. R., Paul, S. B., Nath, A., Choudhury, P. P. N., & Choudhury, S. (2009). Synthesis, X-ray Diffraction Study and Antimicrobial Activity of Calcium Sulphate Nanocomposites from Plant Charcoal. Materials, 2(2), 345-352. https://doi.org/10.3390/ma2020345