Inorganic Hybrid Materials with Encapsulated Polyoxometalates
Abstract
:1. Introduction
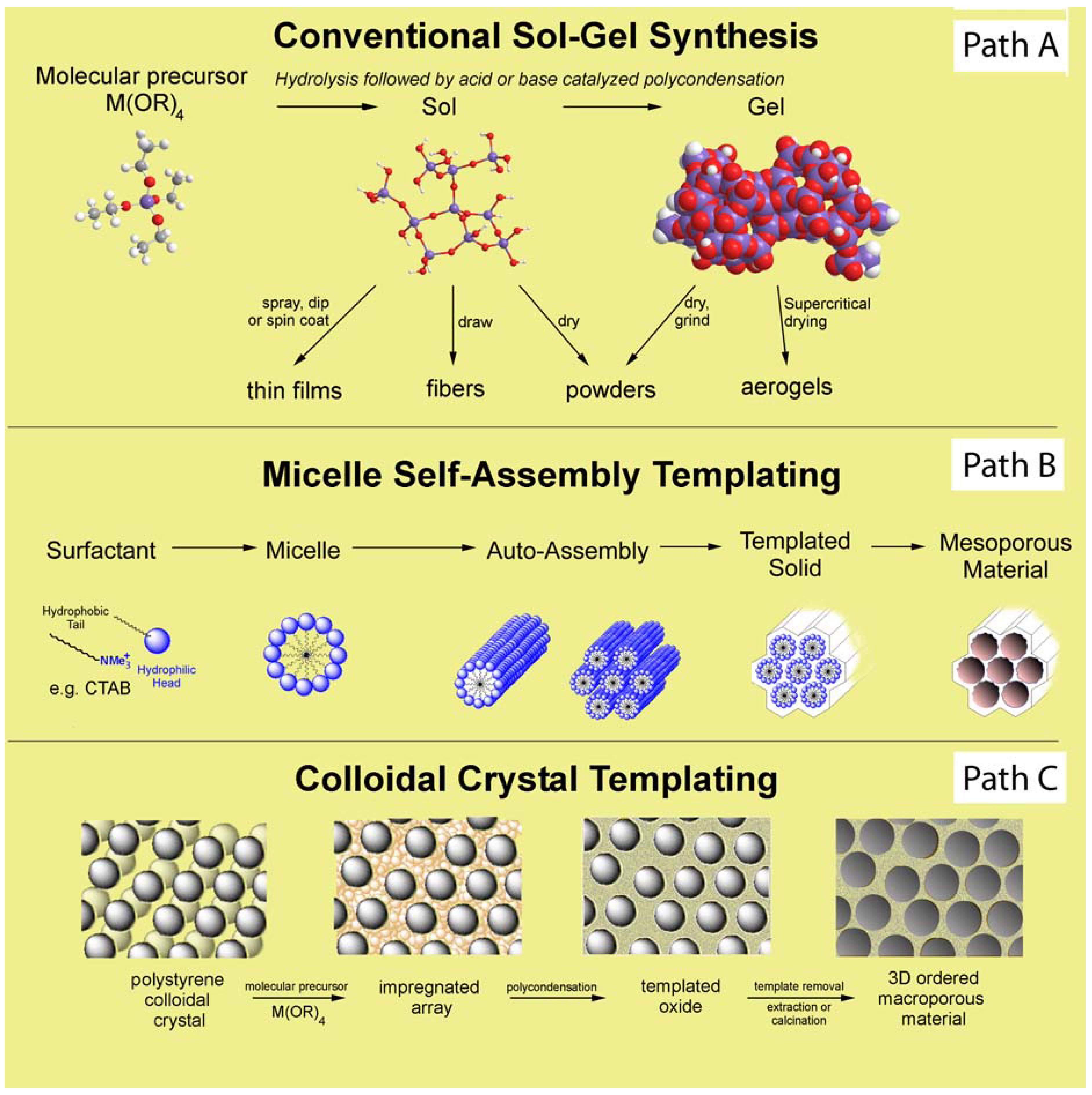
2. Non-Structured Silica Based Materials
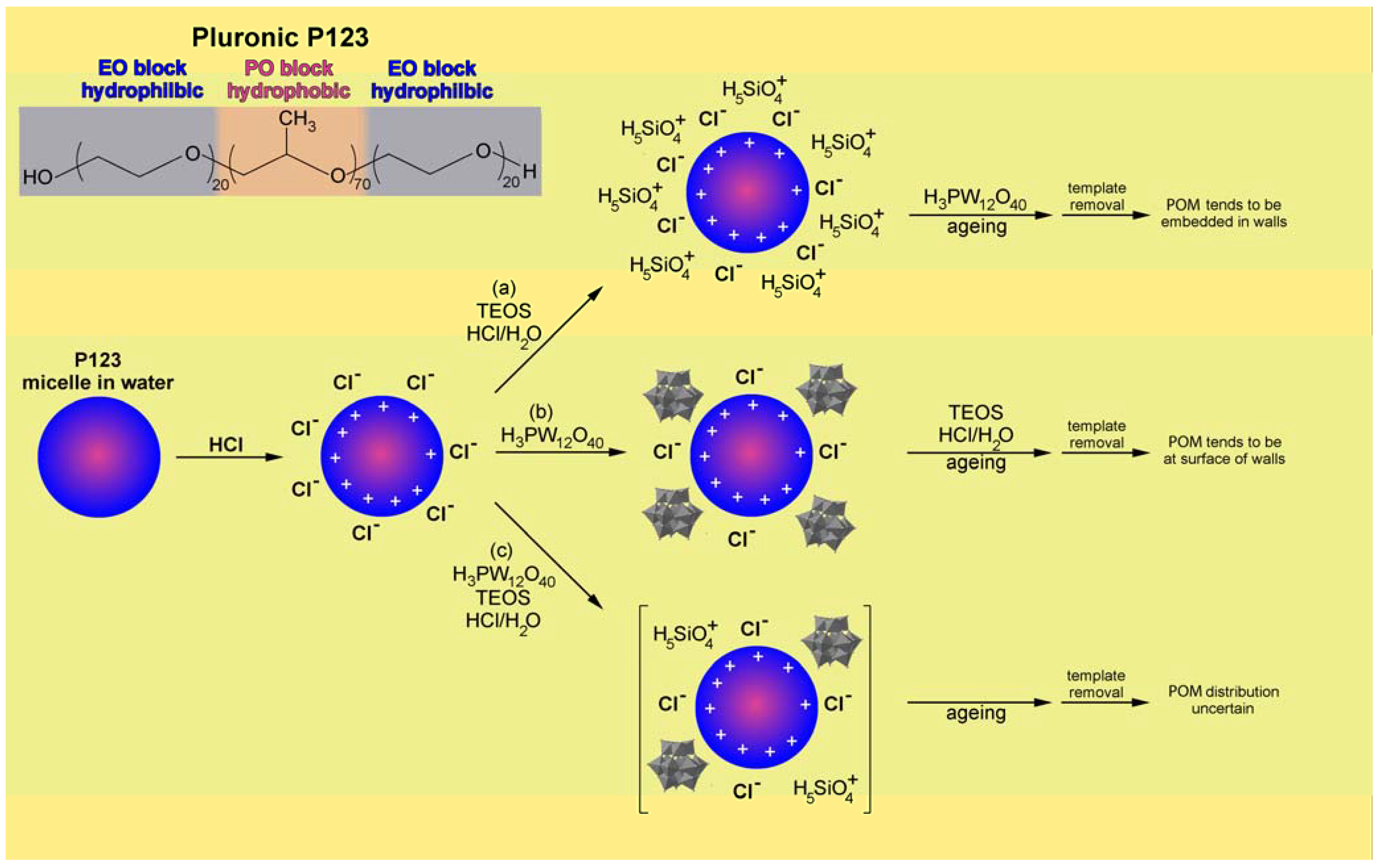
3. Mesostructured Silica Based Materials
| Polyoxo-metalate | Other function | Structure directing agent | Type of mesostructured silica | Type of bonding | Template removal technique | Refer-ence |
|---|---|---|---|---|---|---|
| H3PW12O40 | CTAB + Triton (TX-100) | MSU | Electrostatic bond | Calcination | [24,25,26] | |
| H3PW12O40 | H2PtCl6.6H2O(a) | CTAB + Triton (TX-100) | MSU | Electrostatic bond | Calcination | [27] |
| H3PW12O40 | MTMABr | SBA-3 | Electrostatic bond | Calcination | [28] | |
| H3PW12O40 | Pluronic 123 | SBA-15 | Electrostatic bond | Calcination | [29,30,32] | |
| H3PW12O40 H3PMo12O40 | CTAB + Pluronic 123 | SBA-15 | Electrostatic bond | Subsequent calcination & extraction | [31] | |
| H3PW12O40 | [Pt(NH3)4]Cl2(b) | Pluronic 123 | SBA-15 | Electrostatic bond | Calcination | [34] |
| Na2HPO4 + Na2WO4 | Pluronic 123 | SBA-15 | Electrostatic bond | Extraction | [35] | |
| K8SiW11O39 (R4N)8SiW11O39 | Pluronic 123 | SBA-15 | Covalent bond | Extraction | [37] |
3.1. MSU-type Mesoporous Material
3.2. SBA-3-Type Mesoporous Materials
3.3. SBA-15-Type Mesoporous Materials

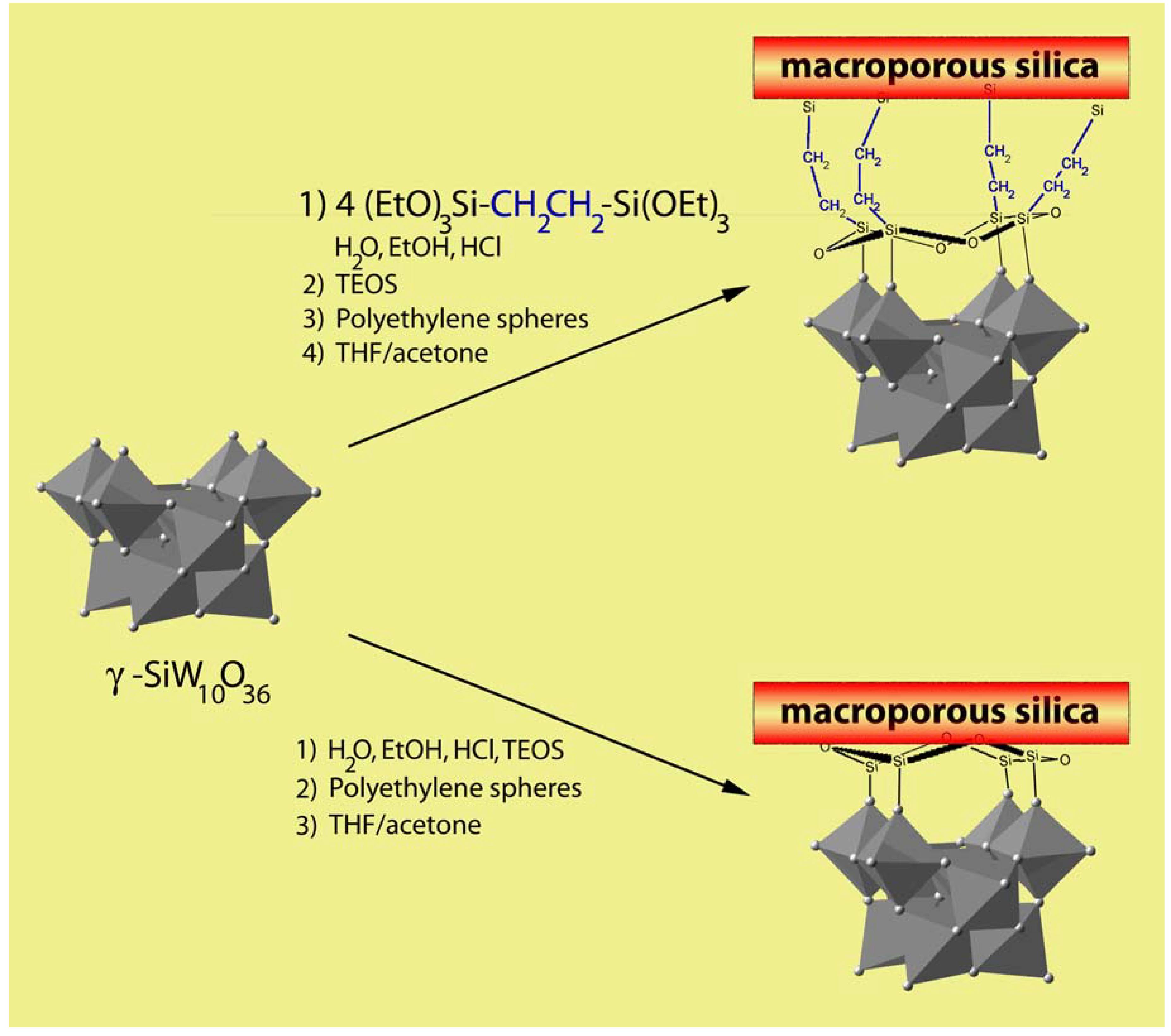
4. Macrostructured Silica Based Materials
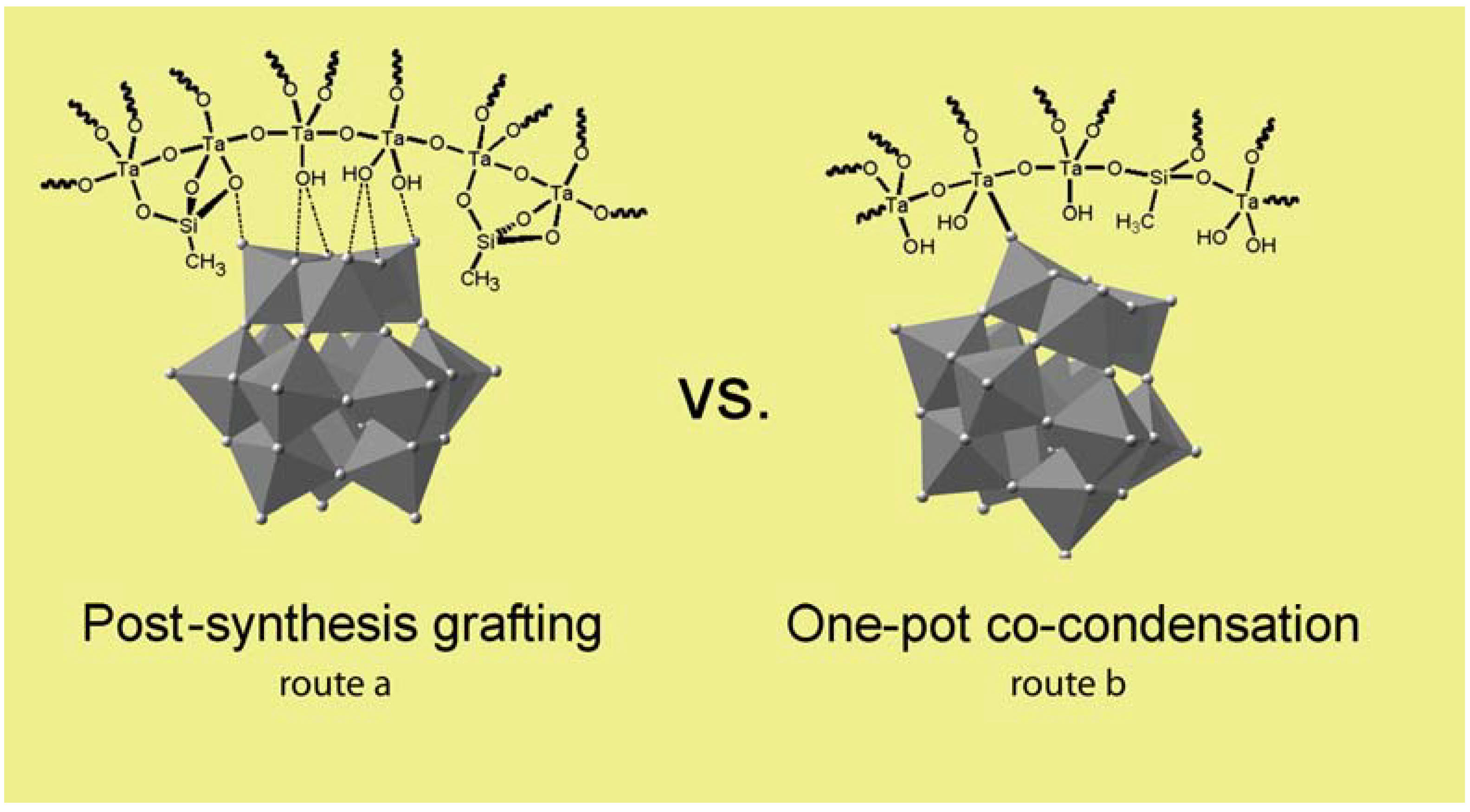
5. Other Oxides
6.Conclusions
Acknowledgements
References and Notes
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer-Verlag Berlin: New York, NY. USA, 1983. [Google Scholar]
- Mizuno, N.; Misono, M. Heterogeneous catalysis. Chem. Rev. 1998, 98, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Katsoulis, D.E. A survey of applications of polyoxometalates. Chem. Rev. 1998, 98, 359–387. [Google Scholar] [CrossRef] [PubMed]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in medicine. Chem. Rev. 1998, 98, 327–357. [Google Scholar] [CrossRef] [PubMed]
- Kozhevnikov, I.V. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Ono, M.; Kitagawa, M.; Yoshida, M.; Urabe, K. Silica-included heteropoly compounds as solid acid catalysts. Microporous Mater. 1995, 5, 255–262. [Google Scholar] [CrossRef]
- Izumi, Y. Recent advances in immobilization of heteropolyacids. Res. Chem. Intermediates 1998, 24, 461–471. [Google Scholar] [CrossRef]
- Izumi, Y.; Hisano, K.; Hida, T. Acid catalysis of silica-included heteropolyacid in polar reaction media. Appl. Catal. A-Gen. 1999, 181, 277–282. [Google Scholar] [CrossRef]
- Kukovecz, A.; Balogi, Z.; Konya, Z.; Toba, M.; Lentz, P.; Niwa, S.-I.; Mizukami, F.; Molnar, A.; Nagy, J.B.; Kiricsi, I. Synthesis, characterisation and catalytic applications of sol-gel derived silica-phosphotungstic acid composites. Appl. Catal. A-Gen. 2002, 228, 83–94. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Hu, C.; Wang, Y.; Wang, E.; Zhou, Y.; Feng, S. Microporous polyoxometalates POMs/SiO2: Synthesis and photocatalytic degradation of aqueous organocholorine pesticides. Chem. Mater. 2000, 12, 3501–3508. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, C.; Wang, X.; Wang, Y.; Wang, E.; Zou, Y.; Ding, H.; Feng, S. Microporous decatungstates: Synthesis and photochemical behavior. Chem. Mater. 2001, 13, 4058–4064. [Google Scholar] [CrossRef]
- Thouvenot, R.; Fournier, M.; Rocchiccioli-Deltcheff, C. Catalysis by polyoxometalates. 2. Silicon-29 nuclear magnetic resonance evidence for 12-molybdosilicate in silica-supported molybdenum catalysts. J. Chem. Soc. Faraday Trans. 1991, 87, 2829–2835. [Google Scholar] [CrossRef]
- Lefebvre, F. 31P MAS NMR study of H3PW12O40 supported on silica: Formation of (SiOH2+)(H2PW12O40)-. J. Chem. Soc. Chem. Commn. 1992, 756–757. [Google Scholar] [CrossRef]
- Popa, A.; Sasca, V.; Kiss, E.E.; Marinkovic-Neducin, R.; Bokorov, M.T.; Holclajtner-Antunović, I. Studies in structural characterization of silica–heteropolyacids composites prepared by sol–gel method. Mater. Chem. Phys. 2010, 119, 465–470. [Google Scholar] [CrossRef]
- Pito, D.S.; Matos, I.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Methoxylation of alpha-pinene over heteropolyacids immobilized in silica. Appl. Catal. A-Gen. 2010, 373, 140–146. [Google Scholar] [CrossRef]
- Xu, B.; Xu, L.; Gao, G.; Li, Z.; Liu, Y.; Guo, W.; Jia, L. Polyoxometalate-based gasochromic silica. New J. Chem. 2008, 32, 1008–1013. [Google Scholar] [CrossRef]
- Klonkowski, A.M.; Grobelna, B.; But, S.; Lis, S. Luminescent materials consisting of Eu(III) ions complexed in heteropolyoxometalates incorporated into silica xerogels. J. Non-Cryst. Solids 2006, 352, 2213–2219. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.-W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; Higgins, J.B.; Schlenker, J.L. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 Angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star copolymer and oligomeric surfactant syntheses of highly ordered hydrothermally stable mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Kaleta, W.; Nowinska, K. Immobilisation of heteropoly anions in Si-MCM-41 channels by means of chemical bonding to aminosilane groups. Chem. Commun. 2001, 535–536. [Google Scholar] [CrossRef]
- Johnson, B.J.S.; Stein, A. Surface modification of mesoporous, macroporous, and amorphous silica with catalytically active polyoxometalate clusters. Inorg. Chem. 2001, 40, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Toufaily, J.; Guth, J.-L.; Soulard, M.; Patarin, J.; Hamad, H.; Hamieh, T. RMN du 31P et mesure de temps de relaxation de l'acide 12-tungstophosphorique incorporé par synthèse directe dans une charpente silicique mésoporeuse. J. Phys. IV Fr. 2005, 124, 69–73. [Google Scholar] [CrossRef]
- Toufaily, J.; Soulard, M.; Guth, J.-L. Etude physicochimique de nouveaux composés formés par incorporation directe d'hétéropolyacide dans une silice mésoporeuse organisée. C. R. Acad. Sci. Chem. 2001, 4, 675–681. [Google Scholar]
- Toufaily, J.; Soulard, M.; Guth, J.-L.; Patarin, J.; Delmote, L.; Hamieha, T.; Kodeih, M.; Naoufal, D.; Hamad, H. Synthesis and characterization of new catalysts formed by direct incorporation of heteropolyacids into organized mesoporous silica. Colloid. Surface. A 2008, 316, 285–291. [Google Scholar] [CrossRef]
- Hamad, H.; Soulard, M.; Lebeau, B.; Patarin, J.; Hamieh, T.; Toufaily, J.; Mahzoul, H. A new way for deNOx catalyst preparation: Direct incorporation of 12-tungstophosphoric acid H3PW12O40 and platinum into mesoporous molecular sieves material. J. Mol. Catal. A-Chem. 2007, 278, 53–63. [Google Scholar] [CrossRef]
- Nowinska, K.; Formaniak, R.; Kaleta, W.; Waclaw, A. Heteropoly compounds incorporated into mesoporous material structure. Appl. Catal. A-Gen. 2003, 256, 115–123. [Google Scholar] [CrossRef]
- Yun, H.-S.; Kuwabara, M.; Zhou, H.S.; Honma, I. One-pot synthesis of mesoporous PWA/SiO2 composite materials using triblock copolymer templates. J. Mater. Sci. 2004, 39, 2341–2347. [Google Scholar] [CrossRef]
- Yang, L.; Qi, Y.; Yuan, X.; Shen, J.; Kim, J. Direct synthesis, characterization and catalytic application of SBA-15 containing heteropolyacid H3PW12O40. J. Mol. Catal. A-Chem. 2005, 229, 199–205. [Google Scholar] [CrossRef]
- Dufaud, V.; Lefebvre, F.; Niccolai, G.P.; Aouine, M. New insights into the encapsulation and stabilization of heteropolyacids inside the pore walls of mesostructured silica materials. J. Mater. Chem. 2009, 19, 1142–1150. [Google Scholar] [CrossRef]
- Guo, Y.; Li, K.; Yu, X.; Clark, J.H. Mesoporous H3PW12O40-silica composite: Efficient and reusable solid acid catalyst for the synthesis of diphenolic acid from levulinic acid. Appl. Catal. B-Environ. 2008, 81, 182–191. [Google Scholar] [CrossRef]
- Galarneau, A.; Cambon, H.; Renzo, F.D.; Fajula, F. True microporosity and surface area of mesoporous SBA-15 silicas as a function of synthesis temperature. Langmuir 2001, 17, 8328–8335. [Google Scholar] [CrossRef]
- Gagea, B.C.; Lorgouilloux, Y.; Altintas, Y.; Jacobs, P.A.; Martens, J.A. Bifunctional conversion of n-decane over HPW heteropoly acid incorporated into SBA-15 during synthesis. J. Catal. 2009, 265, 99–108. [Google Scholar] [CrossRef]
- Shi, C.; Wang, R.; Zhu, G.; Qiu, S.; Long, J. Synthesis, characterization, and catalytic properties of SiPW-X mesoporous silica with heteropolyacid encapsulated into their framework. Eur. J. Inorg. Chem. 2005, 4801–4807. [Google Scholar] [CrossRef]
- Wang, J.; Yan, L.; Qian, G.; Yang, K.; Liu, H.; Wang, X. Heteropolyacid encapsulated into mesoporous silica framework for an efficient preparation of 1,1-diacetates from aldehydes under a solvent-free condition. Tetrahedron Lett. 2006, 47, 8309–8312. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, C. A novel polyoxometalate-functionalized mesoporous hybrid silica: Synthesis and characterization. J. Mater. Chem. 2008, 18, 2691–2703. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, Y.; Hu, C.; Guo, C.; Wang, E.; Zou, Y.; Feng, S. Preparation, characterization and photochemical properties of ordered macroporous hybrid silica materials based on monovacant Keggin-type polyoxometalates. J. Mater. Chem. 2002, 12, 3046–3052. [Google Scholar] [CrossRef]
- Judeinstein, P.; Deprun, C.; Nadjo, L. Synthesis and multispectroscopic characterization of organically modified polyoxometalates. J. Chem. Soc., Dalton Trans. 1991, 1991. [Google Scholar]
- Stein, A.; Schroden, R.C. Colloidal crystal templating of three-dimensionally ordered macroporous solids: Materials for photonics and beyond. Curr. Opin. Solid State Mater. Sci. 2001, 5, 553–564. [Google Scholar] [CrossRef]
- Blanford, C.F.; Yan, H.; Schroden, R.C.; Al-Daous, M.; Stein, A. Gems of chemistry and physics: Macroporous metal oxides with 3D order. Adv. Mater. 2001, 13, 401–407. [Google Scholar] [CrossRef]
- Schroden, R.C.; Blanford, C.F.; Brian, J.; Melde; Johnson, B.J.S.; Stein, A. Direct synthesis of ordered macroporous silica materials functionalized with polyoxometalate clusters. Chem. Mater. 2001, 13, 1074–1081. [Google Scholar] [CrossRef]
- Mayer, C.R.; Fournier, I.; Thouvenot, R. Bis- and tetrakis(organosilyl) decatungstosilicate, [γ-SiW10O36(RSi)2O]4- and [γ-SiW10O36(RSiO)4]4-: Synthesis and structural determination by multinuclear NMR spectroscopy and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Chem. Eur. J. 2000, 6, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.R.; Thouvenot, R.; Lalot, T. New hybrid covalent networks based on polyoxometalates: Part 1. hybrid networks based on poly(ethyl methacrylate) chains covalently cross-linked by heteropolyanions: Synthesis and swelling properties. Chem. Mater. 2000, 12, 257–260. [Google Scholar] [CrossRef]
- Farhadi, S.; Zaidi, M. Polyoxometalate–zirconia (POM/ZrO2) nanocomposite prepared by sol–gel process: A green and recyclable photocatalyst for efficient and selective aerobic oxidation of alcohols into aldehydes and ketones. Appl. Catal. A-Gen. 2009, 354, 119–126. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Yang, X.; Yu, X.; Guo, Y.; Clark, J.H. Preparation of mesoporous polyoxometalate-tantalum pentoxide composite catalyst and its application for biodiesel production by esterification and transesterification. Green Chem. 2008, 10, 746–755. [Google Scholar] [CrossRef]
- Xu, L.; Li, W.; Hu, J.; Yang, X.; Guo, Y. Biodiesel production from soybean oil catalyzed by multifunctionalized Ta2O5/SiO2-[H3PW12O40/R] (R = Me or Ph) hybrid catalyst. Appl. Catal. B-Environ. 2009, 90, 587–594. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Wu, L. Organic−inorganic hybrid supramolecular gels of surfactant-encapsulated polyoxometalates. Langmuir 2009. [Google Scholar]
- Clemente-León, M.; Agricole, B.; Mingotaud, C.; Gómez-García, C.J.; Coronado, E.; Delhaes, P. Toward new organic/inorganic superlattices: Keggin polyoxometalates in Langmuir and Langmuir−Blodgett films. Langmuir 1997, 13, 2340–2347. [Google Scholar] [CrossRef]
- Clemente-León, M.; Mingotaud, C.; Agricole, B.; Gómez-Garcia, C.J.; Coronado, E.; Delhaès, P. Application of the Langmuir-Blodgett technique to polyoxometalates: Towards new magnetic films. Angew. Chem., Int. Ed. Engl. 1997, 36, 1114–1116. [Google Scholar]
- Li, H.; Sun, H.; Qi, W.; Xu, M.; Wu, L. Onionlike hybrid assemblies based on surfactant-encapsulated polyoxometalates. Angew. Chem., Int. Ed. 2007, 46, 1300–1303. [Google Scholar]
- Pradeep, C.P.; Misdrahi, M.F.; Li, F.-Y.; Zhang, J.; Xu, L.; Long, D.-L.; Liu, T.; Cronin, L. Synthesis of modular inorganic-organic-inorganic polyoxometalates and their assembly into vesicles. Angew. Chem., Int. Ed. 2009, 48, 8309–8313. [Google Scholar] [CrossRef]
- Tagami, H.; Uchida, S.; Mizuno, N. Size-selective sorption of small organic molecules in one-dimensional channels of an ionic crystalline organic-inorganic hybrid compound stabilized by π-π interactions. Angew. Chem., Int. Ed. 2009, 48, 6160–6164. [Google Scholar] [CrossRef]
- Rodriguez-Albelo, L.M.; Ruiz-Salvador, A.R.; Sampieri, A.; Lewis, D.W.; Gomez, A.; Nohra, B.; Mialane, P.; Marrot, J.; Sécheresse, F.; Mellot-Draznieks, C.; Biboum, R.N.; Keita, B.; Nadjo, L.; Dolbecq, A. Zeolitic polyoxometalate-based metal−organic frameworks (Z-POMOFs): Computational evaluation of hypothetical polymorphs and the successful targeted synthesis of the redox-active Z-POMOF1. J. Am. Chem. Soc. 2009, 131, 16078–16087. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qi, W.; Li, W.; Wu, L. Covalent dispersion of surfactant-encapsulated polyoxometalates and in situ incorporation of metal nanoparticles in silica spheres. Langmuir 2009. [Google Scholar] [CrossRef]
- He, T.; Yao, J. Photochromism in composite and hybrid materials based on transition-metal oxides and polyoxometalates. Prog. Mater. Sci. 2006, 51, 810–879. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dufaud, V.; Lefebvre, F. Inorganic Hybrid Materials with Encapsulated Polyoxometalates. Materials 2010, 3, 682-703. https://doi.org/10.3390/ma3010682
Dufaud V, Lefebvre F. Inorganic Hybrid Materials with Encapsulated Polyoxometalates. Materials. 2010; 3(1):682-703. https://doi.org/10.3390/ma3010682
Chicago/Turabian StyleDufaud, Véronique, and Frédéric Lefebvre. 2010. "Inorganic Hybrid Materials with Encapsulated Polyoxometalates" Materials 3, no. 1: 682-703. https://doi.org/10.3390/ma3010682





