Toxicity of Transition Metal Oxide Nanoparticles: Recent Insights from in vitro Studies
Abstract
:1. Overview
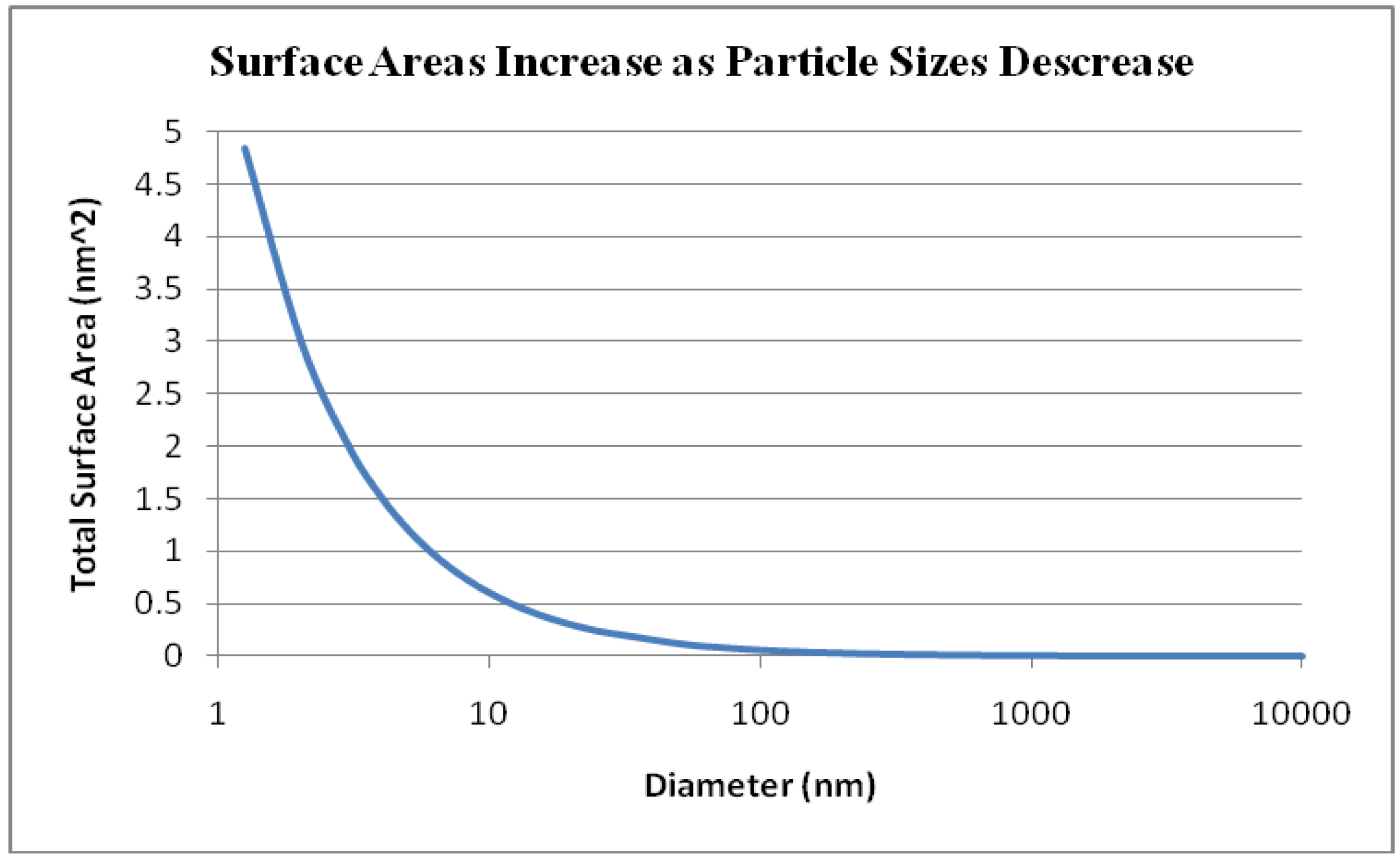
2. Transition Metal Oxide Nanoparticles and their Applications
3. Methodologies to Study Toxicity of Transition Metal Oxides
4. Mechanisms of Cellular Uptake and Intracellular Interactions
5. Toxicity of Transition Metal Oxide Particles
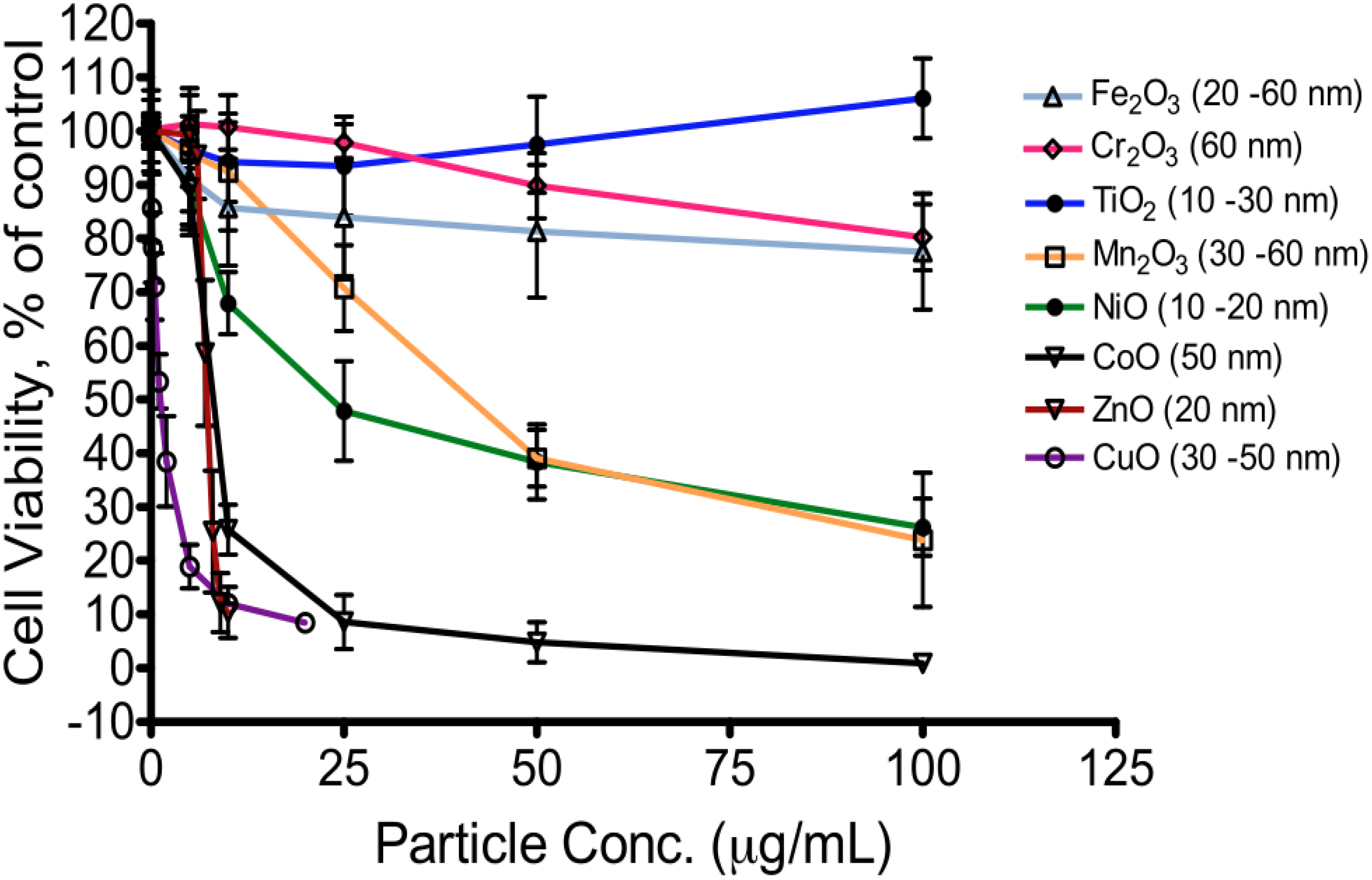
6. Mechanisms of Action of Toxicity
6.1. Exposure to Metal Oxides Tips Off Cellular Redox State—Elevated Oxidative Stress
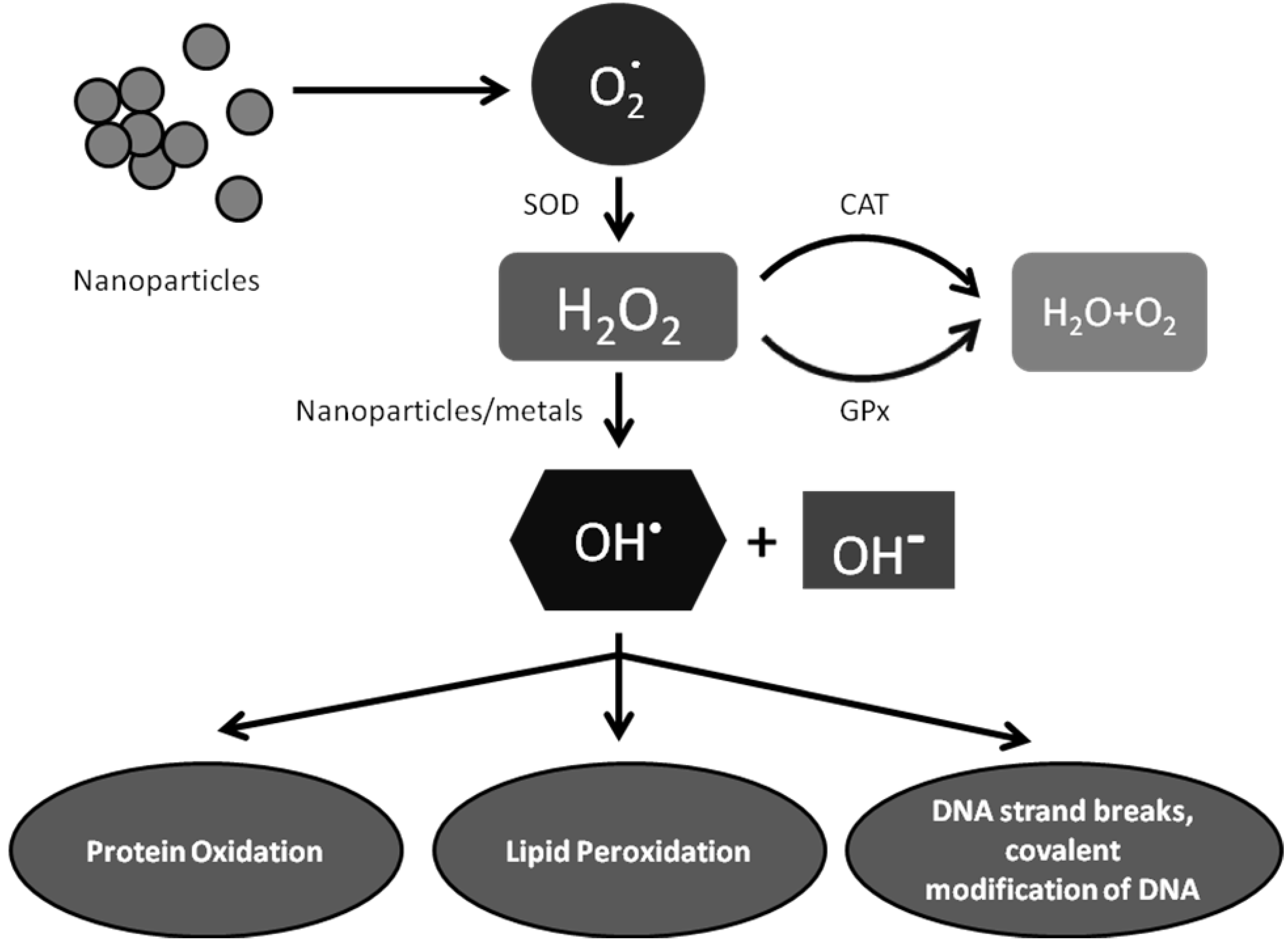
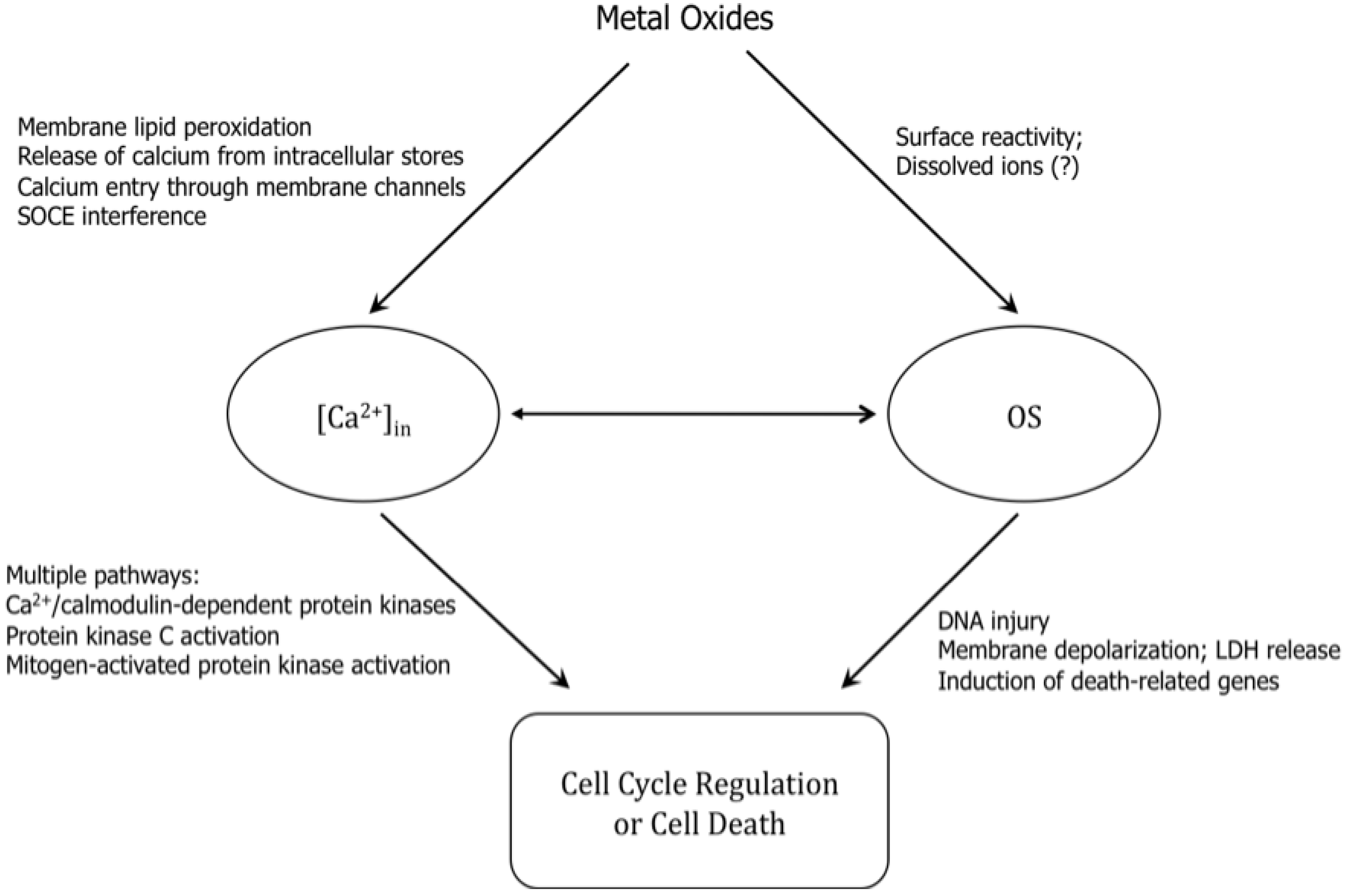
6.2. Oxidative Stress and Perturbation of Intracellular Calcium Homeostasis

6.3. Pro-Inflammatory Response

| Nanoparticle | Size (nm, diameter) | Cell type/animal | Effect | Ref. |
|---|---|---|---|---|
| TiO2 | N/A | A549 cells | mRNA and protein of IL-8 ↑ | [81] |
| TiO2 | 20–80 | A549 cells | IL-8 mRNA ↑ | [80] |
| TiO2 | N/A | Human neutrophils | IL-6, IL-8, MIP-1α, MIP-1β ↑ | [86] |
| ZnO | 24–70 | Lung lavage; BEAS-2B cells | IL-8 mRNA ↑ in both cell types | [82] |
| Al2O3, Al | Al2O3 (33); Al (48) | U937 & A549 (co-cultured) | Phagocytosis activity ↓ (Al); suppress immune response (Al & Al2O3) | [45] |
| Au-NPs | 50 | Bovine retinal pigment epithelial cells | Inhibit VEGF and IL-1β induced proliferation and migration | [83] |
| Silica | 20 | HUVEC (human umbilical vein endothelial cells) | IL-6, IL-8, monocyte chemotactic protein-1 α (MIP-1α) ↑ | [85] |
| Fe | 20–50 | HL1-NB cells (mouse cardiac cells) | IL-8 & MCP-1 not changed | [84] |
| Fe3O4 | 5.3 | ICR mouse (♂) | IL-1, IL-2, IL-4, IL-5, IL-6, IL-12, TNF-α, TGF-β, IgE, & B cell distribution ↑. T cell (CD4+/CD8+) diminished. | [87] |
| Fe3O4 (superpara magnetic) | 36 | BALB/c mouse (♂) | PMN & lymphocyte ↑; IL-1β, IL-6, TNF-α, MIP-1α mRNA ↑ | [88] |
| SWCNT; MWCNT | SWCNT: 4(W) x 1E5(L) MWCNT:30(W) x 2E5(L) | BALB/c mouse (♀) | TNF-α & MIP-1 ↑ | [89] |
7. Conclusions
References and Notes
- National Nanotechnology Coordination Center. National Nanotechnology Initiative. Available online: http://www.nano.gov/html/facts/home_facts.html. (accessed on 22 October 2006).
- Jiang, J.; Oberdorster, G.; Elder, A.; Gelein, R.; Mercer, P.; Biswas, P. Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology 2008, 2, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Mossman, B.T.; Churg, A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Respir. Crit. Care Med. 1998, 157, 1666–1680. [Google Scholar] [CrossRef] [PubMed]
- Schins, R.P. Mechanisms of genotoxicity of particles and fibers. Inhal. Toxicol. 2002, 14, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Knaapen, A.M.; Borm, P.J.; Albrecht, C.; Schins, R.P. Inhaled particles and lung cancer. Part A: Mechanisms. Int. J. Cancer. 2004, 109, 799–809. [Google Scholar] [CrossRef]
- van Maanen, J.M.; Borm, P.J.; Knaapen, A.; van Herwijnen, M.; Schilderman, P.A.; Smith, K.R.; Aust, A.E.; Tomatis, M.; Fubini, B. In vitro effects of coal fly ashes: hydroxyl radical generation, iron release, and DNA damage and toxicity in rat lung epithelial cells. Inhalation Toxicol. 1999, 11, 1123–1141. [Google Scholar]
- Driscoll, K.E.; Deyo, L.C.; Carter, J.M.; Howard, B.W.; Hassenbein, D.G.; Bertram, T.A. Effects of particle exposure and particle-elicited inflammatory cells on mutation in rat alveolar epithelial cells. Carcinogenesis 1997, 18, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.Z.; Whong, W.Z.; Ong, T.M. Detection of mineral-dust-induced DNA damage in two mammalian cell lines using the alkaline single cell gel/comet assay. Mutat. Res. 1997, 393, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Leanderson, P.; Tagesson, C. Hydrogen peroxide release and hydroxyl radical formation in mixtures containing mineral fibres and human neutrophils. Br. J. Ind. Med. 1992, 49, 745–759. [Google Scholar] [PubMed]
- Borm, P.J.; Schins, R.P.; Albrecht, C. Inhaled particles and lung cancer, part B: Paradigms and risk assessment. Int. J. Cancer 2004, 110, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Noronha, F.B.; Schmal, M.; Nicot, C.; Moraweck, B.; Frety, R. Characterization of graphite-supported palladium-cobalt catalysts by temperature-programmed reduction and magnetic measurements. J. Catal. 1997, 168, 42–50. [Google Scholar] [CrossRef]
- Roy, S.; Das, D.; Chakravorty, D.; Agrawal, D.C. Magnetic-properties of glass-metal nanocomposites prepared by the sol-gel route and hot-pressing. J. Appl. Phys. 1993, 74, 4746–4749. [Google Scholar] [CrossRef]
- Prinz, G.A. Magnetoelectronics. Science 1999, 283, 330. [Google Scholar]
- Vassiliou, J.K.; Mehrotra, V.; Russell, M.W.; Giannelis, E.P.; Mcmichael, R.D.; Shull, R.D.; Ziolo, R.F. Magnetic and optical-properties of gamma-Fe2O3 nanocrystals. J. Appl. Phys. 1993, 73, 5109–5116. [Google Scholar] [CrossRef]
- Airapetyan, S.S.; Balayan, G.G.; Khachatryan, A.G. Synthesis and some characteristics of magnetic matrices for fixation of biologically active substances. Russ. J. Appl. Chem. 2001, 74, 519–521. [Google Scholar] [CrossRef]
- Kobe, S.; Drazic, G.; McGuiness, P.J.; Strazisar, J. The influence of the magnetic field on the crystallisation form of calcium carbonate and the testing of a magnetic water-treatment device. J. Magn. Mag. Mater. 2001, 236, 71–76. [Google Scholar] [CrossRef]
- Gruttner, C.; Teller, J. Preparation and characterization of magnetic nanoparticles for in-vivo applications. In Scientific and Clinical Applications of Magnetic Carriers; Hafeli, U., Schutt, W., Teller, J., Zborowski, M., Eds.; Plenum Publishing Corporation: New York, NY, USA, 1997; p. 53. [Google Scholar]
- Howard, M.A., III; Dacey, R.G.; Grady, M.S.; Ritter, R.C.; Gillies, G.T. Magnetically guided stereotaxis. In Advanced Neurosurgical Navigation; Alexander, E., III, Maciunas, R.J., Eds.; Thieme Medical Publishers: New York, NY, USA, 1999; pp. 549–563. [Google Scholar]
- Howard, M.A., III; Grady, M.S.; Ritter, R.C.; Gillies, G.T.; Broaddus, W.C.; Dacey, R.G. Magnetic neurosurgery. In Stereotactic and Functional Neurosurgery; Lozano, Gildenberg & Tasker, Ed.; Karger Pulbisher: Basel, Switzerland, 1996; pp. 102–107. [Google Scholar]
- Grady, M.S.; Howard, M.A.; Dacey, R.G.; Blume, W.; Lawson, M.; Werp, P.; Ritter, R.C. Experimental study of the magnetic stereotaxis system for catheter manipulation within the brain. J. Neurosurg. 2000, 93, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Wellman, B.J.; Howard, M.A., III; Dacey, R.G.; Grady, M.S.; Ritter, R.C.; Gillies, G.T. Magnetically guided interventional medicine. In Proceedings of the Biomedical Optics. Surgical-Assist Systems, San Jose, CA, USA, January 25-28, 1998; pp. 15–25.
- Zhu, B.L.; Xie, C.S.; Zeng, D.W.; Song, W.L.; Wang, A.H. Investigation of gas sensitivity of Sb-doped ZnO nanoparticles. Mater. Chem. Phys. 2005, 89, 148–153. [Google Scholar] [CrossRef]
- Ramakrishna, G.G. Effect of particle size on the reactivityof quantum size ZnO nanoparticles and charge-transfer dynamics with adsorbed catechols. Langmuir 2003, 19, 3006–3012. [Google Scholar] [CrossRef]
- Huang, G.G.; Wang, C.T.; Tang, H.T.; Huang, Y.S.; Yang, J. ZnO nanoparticle-modified infrared internal relfection elements for selective detection of volatile organic compounds. Anal. Chem. 2006, 78, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Z.L. Structure analysis of nanowires and nanobelts by transmission electron microscopy. J. Phys. Chem. 2004, 108, 12280–12291. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglieri, G.; Pan, Z.; Wang, Z.L. Stable and highly sensitive gas sensors based on semiconducting oxide nanobelts. Appl. Phys. Lett. 2002, 81, 1869–1871. [Google Scholar] [CrossRef]
- Bai, X.D.; Gao, P.X.; Wang, Z.L.; Wang, E.G. Dual-mode mechanical resonance of individual ZnO nanobelts. Appl. Phys. Lett. 2003, 28, 4806–4808. [Google Scholar] [CrossRef]
- Bae, S.Y.; Seo, H.W. Vertically aligned sulfur-doped ZnO nanowires synthesized via chemical vapor deposition. J. Phys. Chem. 2004, 108, 5206–5210. [Google Scholar] [CrossRef]
- Kumar, S.A.; Chen, S.M. Nanostructured Zinc Oxide Particles in Chemically Modified Electrodes for Biosensor Applications. Anal. Lett. 2008, 41, 141–158. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Madler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Lai, M.B.; Jandhyam, S.; Dukhande, V.V.; Bhushan, A.; Daniels, C.K.; Leung, S.W. Exposure to titanium dioxide and other metallic oxide nanoparticles induces cytotoxicity on human neural cells and fibroblasts. Int. J. Nanomed. 2008, 3, 533–545. [Google Scholar]
- Lin, W.; Stayton, I.; Huang, Y.W.; Zhou, X.D.; Ma, Y. Cytotoxicity and cell membrane depolarization induced by aluminum oxide nanoparticles in human lung epithelial cells A549. Toxico. Environ. Chem. 2008, 90, 983–996. [Google Scholar] [CrossRef]
- Lin, W.; Xu, Y.; Huang, C.C.; Ma, Y.; Shannon, K.B.; Chen, D.R.; Huang, Y.W. Toxicity of nano- and micro-sized ZnO particles in human lung epithelial cells. J. Nanopart. Res. 2009, 11, 25–39. [Google Scholar] [CrossRef]
- Kang, S.G.; Brown, A.L.; Chung, J.H. Oxygen tension regulates the stability of insulin receptor substrate-1 (IRS-1) through caspase-mediated cleavage. J. Biol. Chem. 2007, 282, 6090–6097. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, Y.W.; Zhou, X.D.; Ma, Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxico. Appl. Pharmacol. 2006, 217, 252–259. [Google Scholar] [CrossRef]
- Lin, W.; Huang, Y.W.; Zhou, X.D.; Ma, Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int. J. Toxicol. 2006, 25, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, B.; Cormier, S.A. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol. in vitro 2009, 23, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, C.I.; Cimpan, M.R.; Hol, P.J.; Sornes, S.; Lie, S.A.; Gjerdet, N.R. Induction of cell death by TiO2 nanoparticles: Studies on a human monoblastoid cell line. Toxicol. in vitro 2008, 22, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Tabata, M.; Kubo-Irie, M.; Shimizu, T.; Suzuki, K.; Nihei, Y.; Takeda, K. The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicol. in vitro 2008, 22, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Ivankovic, S.; Music, S.; Gotic, M.; Ljubesic, N. Cytotoxicity of nanosize V2O5 particles to selected fibroblast and tumor cells. Toxicol. in vitro 2006, 20, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Aronstam, R.S.; Chen, D.R.; Huang, Y.W. Oxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticles. Toxicol. in vitro 2010, 24, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Sharma, C.; Yog, R.; Periakaruppan, A.; Jejelowo, O.; Thomas, R.; Barrera, E.V.; Rice-Ficht, A.C.; Wilson, B.L.; Ramesh, G.T. Analysis of stress responsive genes induced by single-walled carbon nanotubes in BJ Foreskin cells. J. Nanosci. Nanotechnol. 2007, 7, 584–592. [Google Scholar] [PubMed]
- Braydich-Stolle, L.K.; Speshock, J.L.; Castle, A.; Smith, M.; Murdock, R.C.; Hussain, S.M. Nanosized aluminum altered immune function. ACS Nano 2010, 4, 3661–3670. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Borm, P.J.; Castranova, V.; Gulumian, M. The limits of testing particle-mediated oxidative stress in vitro in predicting diverse pathologies; relevance for testing of nanoparticles. Part. Fibre. Toxicol. 2009, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Limbach, L.K.; Li, Y.; Grass, R.N.; Brunner, T.J.; Hintermann, M.A.; Muller, M.; Gunther, D.; Stark, W.J. Oxide nanoparticle uptake in human lung fibroblasts: Effects of particle size, agglomeration, and diffusion at low concentrations. Environ. Sci. Technol. 2005, 39, 9370–9376. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Niwa, M.; Takeuchi, T.; Sonomura, K.; Kawabata, N.; Koike, Y.; Takehashi, M.; Tanaka, S.; Ueda, K.; Simpson, J.C.; Jones, A.T.; Sugiura, Y.; Futaki, S. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol. Ther. 2004, 10, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Wadia, J.S.; Dowdy, S.F. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv. Drug Deliv. Rev. 2005, 57, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, M.; Rusnati, M.; Presta, M.; Giacca, M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 2001, 276, 3254–3261. [Google Scholar] [CrossRef] [PubMed]
- Bahattacharjee, S.; Haan, L.H.J.; Evers, N.M.; Jiang, X.; Marcelis, A.T.M.; Zuihof, H.; Rietjens, I.M.C.M.; Alink, G.M. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part. Fibre. Toxicol. 2010, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Kam, N.W.; Dai, H. Carbon nanotubes as intracellular protein transporters: Generality and biological functionality. J. Am. Chem. Soc. 2005, 127, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, B.R.; Lee, H.J.; Shannon, K.B.; Winiarz, J.G.; Wang, T.C.; Chiang, H.J.; Huang, Y.W. Nona-arginine facilitates delivery of quantum dots into cells via multiple pathways. J. Biomed. Biotechnol. 2010, in press. [Google Scholar]
- Liu, B.R.; Li, J.F.; Lu, S.W.; Lee, H.J.; Shannon, K.B.; Huang, Y.W.; Aronstam, R.S. Cellular internalization of quantum dots noncovalently conjugated with arginine-rich intracellular delivery peptides. J. Nanosci. Nanotechnol. 2010, 10, 6534–6543. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.; Agrawal, A.; Marcus, A.I.; Nie, S. Imaging and tracking of Tat peptide-conjugated quantum dots in living Cells: New Insights into nanoparticle uptake, intracellular transport, and vesicle shedding. J. Am. Chem. Soc. 2007, 129, 14759–14766. [Google Scholar] [CrossRef] [PubMed]
- Krpetic, Z.; Porta, F.; Caneva, E.; Dal Santo, V.; Scari, G. Phagocytosis of biocompatible gold nanoparticles. Langmuir 2010, 26, 14799–14805. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.Y.; Zhu, B.S.; Wang, X.F.; Lu, Q.H. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem. Res. Toxicol. 2008, 21, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Cook, S.; Wang, P.; Hwang, H.M. In vitro evaluation of cytotoxicity of engineered metal oxide nanoparticles. Sci. Total. Environ. 2009, 407, 3070–3072. [Google Scholar] [CrossRef] [PubMed]
- Kasemets, K.; Ivask, A.; Dubourguier, H.C.; Kahru, A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. in vitro 2009, 23, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Limbach, L.K.; Wick, P.; Manser, P.; Grass, R.N.; Stark, W.J. Exposure of engineered nanoparticles to human lung epithelial cells: Influence of chemical composition and catalytic activity on oxidative stress. Eviron. Sci. Technol. 2007, 41, 4158–4163. [Google Scholar] [CrossRef]
- Horie, M.; Nishio, K.; Fujita, K.; Endoh, S.; Miyauchi, A.; Saito, Y.; Iwahashi, H.; Yamamoto, K.; Murayama, H.; Nakano, H.; Nanashima, N.; Niki, E.; Yoshida, Y. Protein adsorption of ultrafine metal oxide and its influence on cytotoxicity toward cultured cells. Chem. Res. Toxicol. 2009, 22, 543–553. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Pokhrel, S.; Xia, T.; Gilbert, B.; Ji, Z.; Schowalter, M.; Rosenauer, A.; Damoiseaux, R.; Bradley, K.A.; Madler, L.; Nel, A.E. Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping. ACS Nano 2010, 4, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Kan, Y.W. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 12731–12736. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Chanas, S.A.; Henderson, C.J.; McMahon, M.; Sun, C.; Moffat, G.J.; Wolf, C.R.; Yamamoto, M. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem. Soc. Trans. 2000, 28, 33–41. [Google Scholar] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; Yamamoto, M.; Nabeshima, Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.J.; Choi, J. Oxidative stress of CeO2 nanoparticles via p38-Nrf-2 signaling pathway in human bronchial epithelial cell, Beas-2B. Toxicol. Lett. 2009, 187, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Sato, H.; Nishimura, N.; Takahashi, S.; Itoh, K.; Yamamoto, M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol. Appl. Pharmacol. 2001, 173, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.W.; Jamall, I.S. Relative importance of intracellular glutathione peroxidase and catalase in vivo for prevention of peroxidation to the heart. Cardiovasc. Res. 1989, 23, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kotamraju, S.; Konorev, E.; Kalivendi, S.; Joseph, J.; Kalyanaraman, B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: The role of hydrogen peroxide. Biochem. J. 2002, 367, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Davoren, M.; Boertz, J.; Schins, R.P.; Hoffmann, E.; Dopp, E. Titanium dioxide nanoparticles induce oxidative stress and DNA-adduct formation but not DNA-breakage in human lung cells. Part. Fibre. Toxicol. 2009, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Falck, G.C.; Lindberg, H.K.; Suhonen, S.; Vippola, M.; Vanhala, E.; Catalan, J.; Savolainen, K.; Norppa, H. Genotoxic effects of nanosized and fine TiO2. Hum. Exp. Toxicol. 2009, 28, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Q.; Lohani, M.; Dopp, E.; Pemsel, H.; Jonas, L.; Weiss, D.G.; Schiffmann, D. Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ. Health Perspect 2002, 110, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Jeng, H.A.; Swanson, J. Toxicity of metal oxide nanoparticles in mammalian cells. J. Environ. Sci. Health, Part A: Toxic/ Hazardous Subst. Environ. Eng. 2006, 41, 2699–2711. [Google Scholar] [CrossRef]
- Stone, V.; Tuinman, M.; Vamvakopoulos, J.E.; Shaw, J.; Brown, D.; Petterson, S.; Faux, S.P.; Borm, P.; MacNee, W.; Michaelangeli, F.; Donaldson, K. Increased calcium influx in a monocytic cell line on exposure to ultrafine carbon black. Eur. Respir. J. 2000, 15, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Moller, W.; Brown, D.M.; Kreyling, W.G.; Stone, V. Ultrafine particles cause cytoskeletal dysfunctions in macrophages: Role of intracellular calcium. Part. Fibre. Toxicol. 2005, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Aronstam, R.S.; Patil, P. Receptors on autonomic neurons and neuroeffector cells: Muscarinic receptors. In Encyclopedia of Neuroscience; Adelman, G., Smith, B., Eds.; Elsevier Press: Amstersam, The Netherlands, 2008. [Google Scholar]
- Smyth, J.T.; Dehaven, W.I.; Bird, G.S.; Putney, J.W., Jr. Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J. Cell Sci. 2008, 121, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Putney, J.W., Jr. Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here). Cell Calcium 2007, 42, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Shi, T.; Duffin, R.; Albrecht, C.; van Berlo, D.; Hohr, D.; Fubini, B.; Martra, G.; Fenoglio, I.; Borm, P.J.; Schins, R.P. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: Role of the specific surface area and of surface methylation of the particles. Toxicol. Appl. Pharmacol. 2007, 222, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Monteiller, C.; Tran, L.; MacNee, W.; Faux, S.; Jones, A.; Miller, B.; Donaldson, K. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: The role of surface area. Occup. Environ. Med. 2007, 64, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Samet, J.M.; Peden, D.B.; Bromberg, P.A. Phosphorylation of p65 is required for zinc oxide nanoparticle-induced interleukin 8 expression in human bronchial epithelial cells. Environ. Health Perspect 2010, 118, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, B.; Kalishwaralal, K.; Sheikpranbabu, S.; Deepak, V.; Haribalaganesh, R.; Gurunathan, S. Gold nanoparticles downregulate VEGF-and IL-1beta-induced cell proliferation through Src kinase in retinal pigment epithelial cells. Exp. Eye Res. 2010, 91, 769–778. [Google Scholar] [CrossRef]
- Kahn, E.; Baarine, M.; Pelloux, S.; Riedinger, J.M.; Frouin, F.; Tourneur, Y.; Lizard, G. Iron nanoparticles increase 7-ketocholesterol-induced cell death, inflammation, and oxidation on murine cardiac HL1-NB cells. Int. J. Nanomed. 2010, 5, 185–195. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J. Endothelial cells dysfunction induced by silica nanoparticles through oxidative stress via JNK/P53 and NF-kappaB pathways. Biomaterials. 2010, 31, 8198–8209. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, D.M.; Chiasson, S.; Girard, D. Activation of human neutrophils by titanium dioxide (TiO2) nanoparticles. Toxicol. in vitro 2010, 24, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, H.; Kim, Y.; Yi, J.; Choi, K.; Park, K. Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice. Toxicology 2010, 275, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Cho, M.; Kim, S.R.; Choi, M.; Lee, J.Y.; Han, B.S.; Park, S.N.; Yu, M.K.; Jon, S.; Jeong, J. Pulmonary toxicity and kinetic study of Cy5.5-conjugated superparamagnetic iron oxide nanoparticles by optical imaging. Toxicol. Appl. Pharmacol. 2009, 239, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.C.; Hansen, J.S.; Samuelsen, M.; Alberg, T.; Marioara, C.D.; Lovik, M. Single-walled and multi-walled carbon nanotubes promote allergic immune responses in mice. Toxicol. Sci. 2009, 109, 113–123. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, Y.-W.; Wu, C.-h.; Aronstam, R.S. Toxicity of Transition Metal Oxide Nanoparticles: Recent Insights from in vitro Studies. Materials 2010, 3, 4842-4859. https://doi.org/10.3390/ma3104842
Huang Y-W, Wu C-h, Aronstam RS. Toxicity of Transition Metal Oxide Nanoparticles: Recent Insights from in vitro Studies. Materials. 2010; 3(10):4842-4859. https://doi.org/10.3390/ma3104842
Chicago/Turabian StyleHuang, Yue-Wern, Chi-heng Wu, and Robert S. Aronstam. 2010. "Toxicity of Transition Metal Oxide Nanoparticles: Recent Insights from in vitro Studies" Materials 3, no. 10: 4842-4859. https://doi.org/10.3390/ma3104842





