In vitro Evaluation of Acyclovir/Chitosan Floating Systems
Abstract
:1. Introduction
2. Results and Discussion
| Formulations (F) | Composition of suspensions before freeze-drying process (g/100 mL) | |
|---|---|---|
| ACV | CS | |
| L1 | 0.5 | 1 |
| L2 | 0.5 | 2 |
| L3 | 0.5 | 3 |
| L4 | 0.5 | 4 |
| L5 | 0.5 | 5 |
| L6 | 2 | 1 |
| L7 | 2 | 2 |
| L8 | 2 | 3 |
| L9 | 2 | 4 |
| L10 | 2 | 5 |
| B1 | - | 1 |
| B2 | - | 2 |
| B3 | - | 3 |
| B4 | - | 4 |
| B5 | - | 5 |
2.1. X-ray Diffraction Analysis
 L10). Furthermore, slight displacements on ACV characteristic peaks were detected on some diffraction patterns (from 26.2° to 26.3° 2θ; from 29.2° to 29.3° 2θ and from 23.9° to 24.0° 2θ). It can be related to some modifications in the crystallite size during the lyophilization process. However, results suggest that there are no interactions between ACV and CS within systems in the studied ratios.
L10). Furthermore, slight displacements on ACV characteristic peaks were detected on some diffraction patterns (from 26.2° to 26.3° 2θ; from 29.2° to 29.3° 2θ and from 23.9° to 24.0° 2θ). It can be related to some modifications in the crystallite size during the lyophilization process. However, results suggest that there are no interactions between ACV and CS within systems in the studied ratios.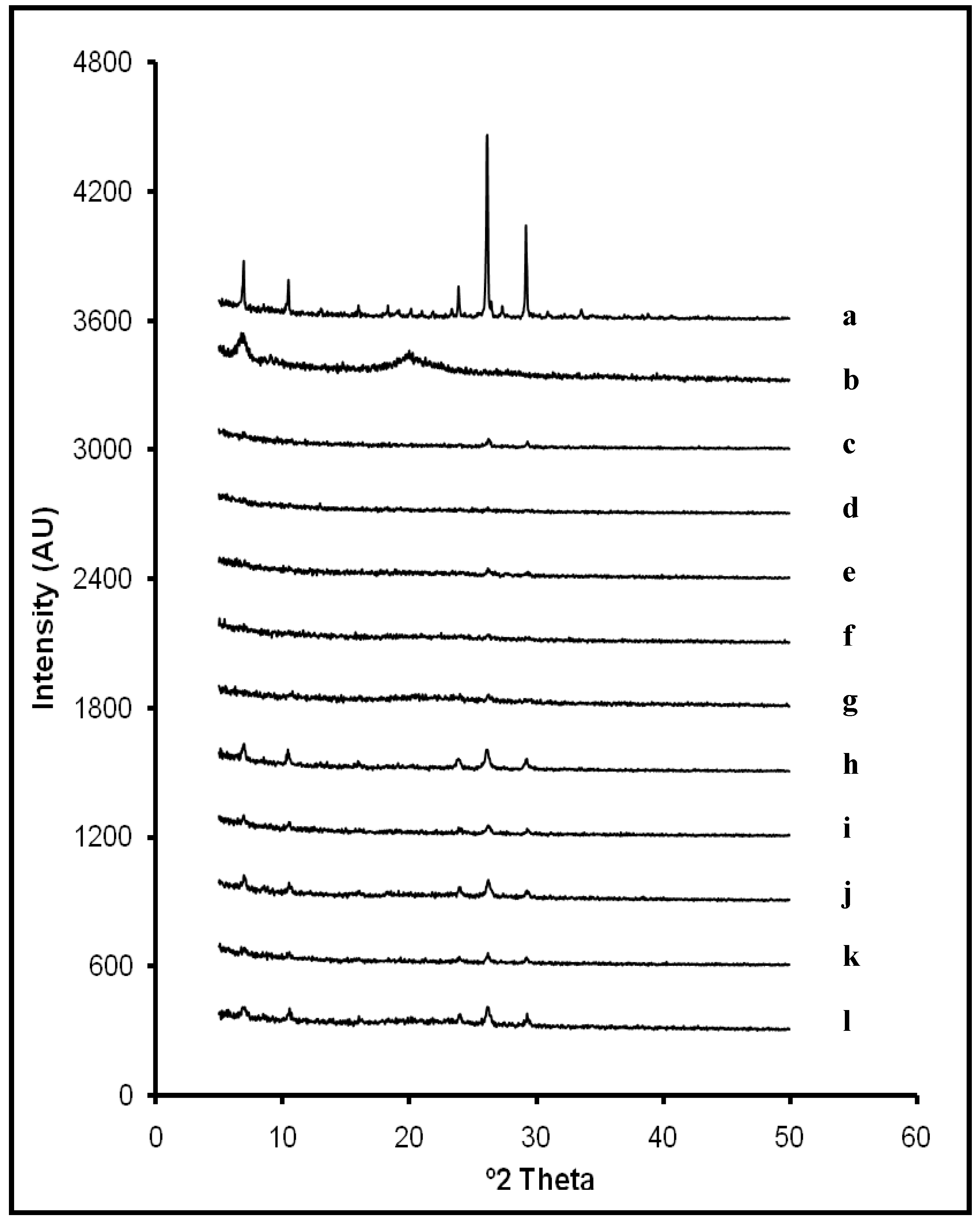
2.2. Swelling Test
 pH 4) (B).
pH 4) (B).
 pH 4) (B).
pH 4) (B).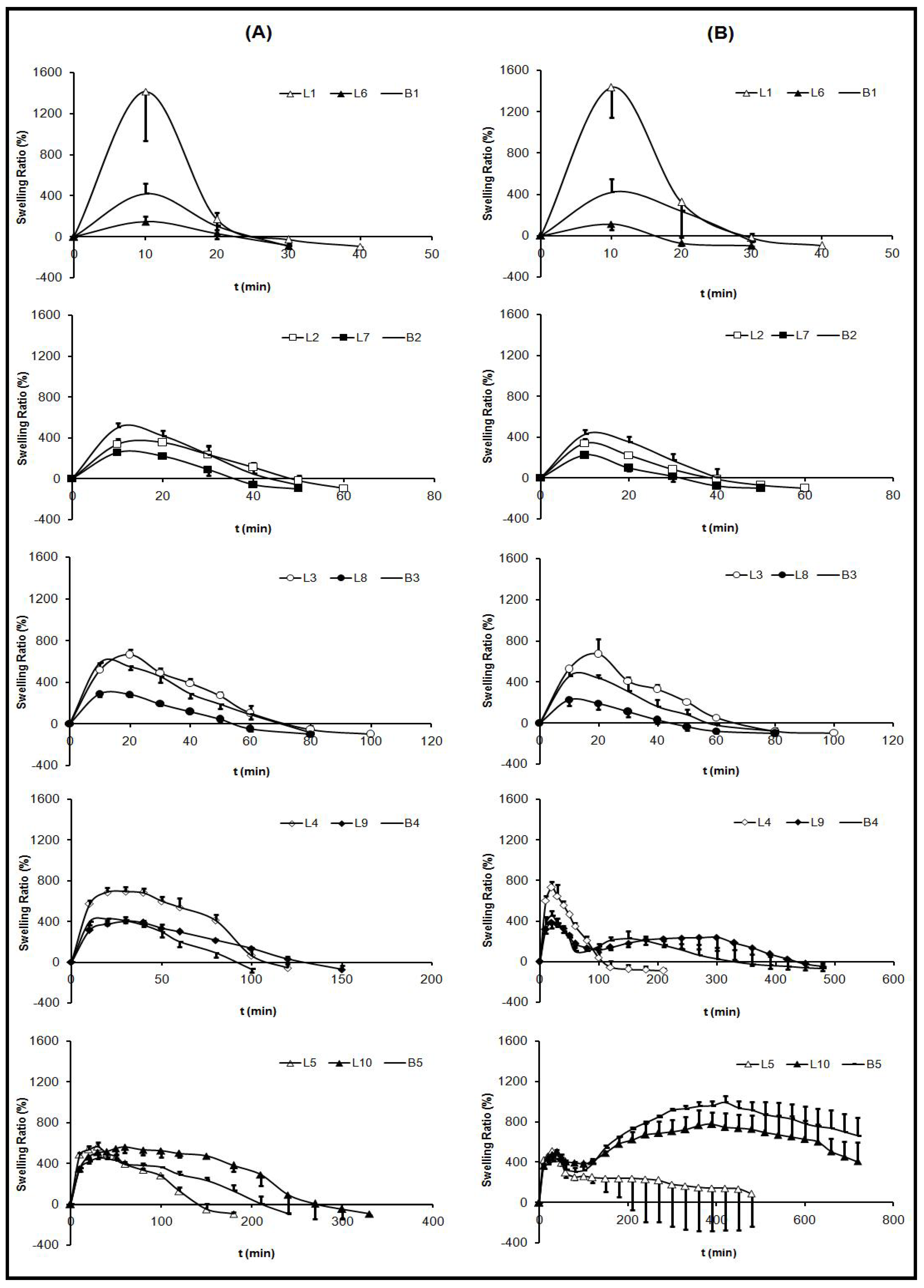
 pH 4).
pH 4).
| Formulations | 0.1 M HCl | medium | progressive pH | medium |
|---|---|---|---|---|
| Maximum SR (%) | T max (min) | Maximum SR (%) | T max (min) | |
| B1 | 419.16 ± 99.16 | 10 | 420.41 ± 125.25 | 10 |
| B2 | 501.50 ± 47.11 | 10 | 428.44 ± 45.24 | 10 |
| B3 | 587.41 ± 52.87 | 10 | 457.51 ± 75.75 | 10 |
| B4 | 425.75 ± 61.66 | 20 | 453.11 ± 43.88 | 20 |
| B5 | 454.09 ± 43.44 | 30 | 992.05 ± 63.13 | 420 |
| L1 | 1413.92 ± 476.74 | 10 | 1433.65 ± 289.10 | 10 |
| L2 | 337.03 ± 52.79 | 10 | 338.01 ± 48.10 | 10 |
| L3 | 666.92 ± 50.33 | 20 | 673.52 ± 146.14 | 20 |
| L4 | 694.25 ± 41.99 | 30 | 730.73 ± 58.05 | 20 |
| L5 | 565.66 ± 41.08 | 30 | 511.96 ± 63.49 | 30 |
| L6 | 146.33 ± 54.28 | 10 | 111.92 ± 58.52 | 10 |
| L7 | 258.19 ± 11.41 | 10 | 225.02 ± 24.29 | 10 |
| L8 | 287.95 ± 24.75 | 10 | 227.24 ± 59.78 | 10 |
| L9 | 404.99 ± 37.59 | 30 | 387.88 ± 59.51 | 10 |
| L10 | 560.52 ± 58.53 | 60 | 777.00 ± 114.73 | 390 |

 pH 4).
pH 4).
 pH 4).
pH 4).
2.3. Buoyancy Test
2.4. Dissolution Test
 4), the influence of ACV proportion over its selfsame dissolution was a little more remarkable. At 120 minutes the percentages of released ACV from the systems with an equal amount of CS and different amounts of ACV were: from L3, 96.7% and from L8, 76.8%; from L4, 68.1 % and from L9, 63.1%; from L5, 64.6% and from L10, 40.0%. These different behaviors were due to the pH dependent ACV dissolution [52].
4), the influence of ACV proportion over its selfsame dissolution was a little more remarkable. At 120 minutes the percentages of released ACV from the systems with an equal amount of CS and different amounts of ACV were: from L3, 96.7% and from L8, 76.8%; from L4, 68.1 % and from L9, 63.1%; from L5, 64.6% and from L10, 40.0%. These different behaviors were due to the pH dependent ACV dissolution [52].  pH 4) (B).
pH 4) (B).
 pH 4) (B).
pH 4) (B).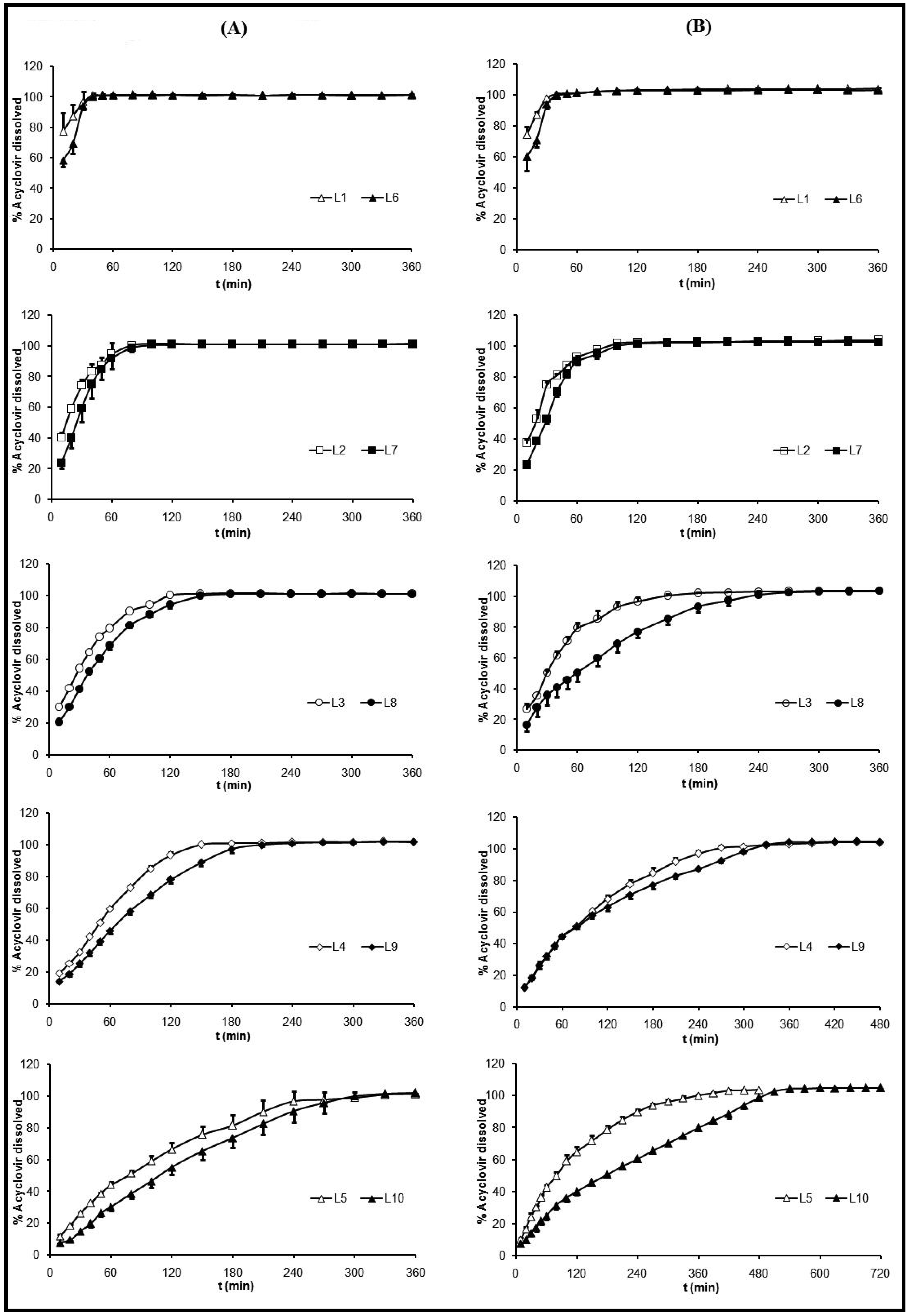
3. Experimental Section
3.1. Materials
3.2. Preparation of ACV/CS Freeze-Dried Formulations (L)
 B5) were also prepared, as described above, in order to compare them to the ACV/CS formulations in the characterization studies. The dimensions of systems were measured, and the results were 22.5 ± 0.7 mm (length), 8.9 ± 0.3 mm (width) and 6.1 ± 0.2 mm (thickness) (n = 15).
B5) were also prepared, as described above, in order to compare them to the ACV/CS formulations in the characterization studies. The dimensions of systems were measured, and the results were 22.5 ± 0.7 mm (length), 8.9 ± 0.3 mm (width) and 6.1 ± 0.2 mm (thickness) (n = 15). 3.3. Characterization of ACV/CS Lyophilized Formulations
3.3.1. X-ray diffraction analysis
3.3.2. Swelling test
 4). This progressive medium was composed of an aqueous mixture of 0.05 M hydrochloric acid 37%, 0.05 M ortho-phosphoric acid 85% and 0.05 M acetic acid glacial with a final pH value of 1.5, which was maintained during the first hour. After this hour, a sufficient quantity of 10 M NaOH was added until pH reached a value of 4.0, which was maintained until the test was finished.
4). This progressive medium was composed of an aqueous mixture of 0.05 M hydrochloric acid 37%, 0.05 M ortho-phosphoric acid 85% and 0.05 M acetic acid glacial with a final pH value of 1.5, which was maintained during the first hour. After this hour, a sufficient quantity of 10 M NaOH was added until pH reached a value of 4.0, which was maintained until the test was finished. 3.3.3. Buoyancy test
3.3.4. Dissolution test
4. Conclusions
Acknowledgements
References and Notes
- Illum, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Skaugrud, O. Chitosan––New biopolymer for cosmetics and drugs. Drug Cosmetic Ind. 1991, 148, 24–29. [Google Scholar]
- Ilango, R.; Jayakar, B.; Kavimani, S. Chitosan as a new pharmaceutical excipient. The East. Pharm. 1998, 41, 47–49. [Google Scholar]
- Singla, A.K.; Chawla, M. Chitosan: some pharmaceutical and biological aspects-an update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Burkatovskaya, M.; Castano, A.P.; Demidova-Rice, T.N.; Tegos, G.P.; Hamblin, M.R. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 2008, 16, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Wang, Y.M.; Liu, C.F.; Wang, J.Y. The effect of water-soluble chitosan on macrophage activation and the attenuation of mite allergen-induced airway inflammation. Biomaterials 2008, 29, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Seyfarth, F.; Schliemann, S.; Elsner, P.; Hipler, U.C. Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-D-glucosamine against Candida albicans, Candida krusei and Candida glabrata. Int. J. Pharm. 2008, 353, 139–148. [Google Scholar] [PubMed]
- Gallaher, D.D.; Gallaher, C.M.; Mahrt, G.J.; Carr, T.P.; Hollingshead, C.H.; Hesslink, R., Jr.; Wise, J. A glucomannan and chitosan fiber supplement decreases plasma cholesterol and increases cholesterol excretion in overweight normocholesterolemic humans. J. Am. Coll. Nutr. 2002, 21, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.T.; Yao, H.T.; Chen, H.C. Effect of dietary chitosans with different viscosity on plasma lipids and lipid peroxidation in rats fed on a diet enriched with cholesterol. Biosci. Biotechnol. Biochem. 2000, 64, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Ubaidulla, U.; Khar, R.K.; Ahmad, F.J.; Sultana, Y.; Panda, A.K. Development and characterization of chitosan succinate microspheres for the improved oral bioavailability of insulin. J. Pharm. Sci. 2007, 96, 3010–3023. [Google Scholar] [CrossRef] [PubMed]
- Lueßen, H.L.; Lehr, C.M.; Rentel, C.O.; Noach, A.B.J.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Bioadhesive polymers for the peroral delivery of peptide drugs. J. Control. Release 1994, 29, 329–338. [Google Scholar] [CrossRef]
- Lueßen, H.L.; de Leeuw, B.J.; Langemeÿer, M.W.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Mucoadhesive polymers in peroral peptide drug delivery. VI. Carbomer and chitosan improve the intestinal absorption of the peptide drug buserelin in vivo. Pharm. Res. 1996, 13, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Lueßen, H.L.; Rentel, C.O.; Kotzé, A.F.; Lehr, C.M.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Mucoadhesive polymers in peroral peptide drug delivery. IV. Polycarbophil and chitosan are potent enhancers of peptide transport across intestinal mucosae in vitro. J. Control. Release 1997, 45, 15–23. [Google Scholar] [CrossRef]
- Hassan, E.E.; Gallo, J.M. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm. Res. 1990, 7, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, K.; Shibata, K.; Murata, Y.; Miyamoto, E.; Kawashima, S. Preparation and drug retention of biodegradable chitosan gel beads. Chem. Pharm. Bull. 1999, 47, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Ganza-González, A.; Anguiano-Igea, S.; Otero-Espinar, F.J.; Blanco Méndez, J. Chitosan and chondroitin microspheres for oral-administration controlled release of metoclopramide. Eur. J. Pharm. Biopharm. 1999, 48, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Shantha, K.L.; Harding, D.R.K. Preparation and in-vitro evaluation of poly[N-vinyl-2-pyrrolidone-polyethylene glycol diacrylate]-chitosan interpolymeric pH-responsive hydrogels for oral drug delivery. Int. J. Pharm. 2000, 207, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Chun, M.K.; Choi, H.K. Preparation of an extended-release matrix tablet using chitosan/Carbopol interpolymer complex. Int. J. Pharm. 2008, 347, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Nunthanid, J.; Laungtana-Anan, M.; Sriamornsak, P.; Limmatvapirat, S.; Puttipipatkhachorn, S.; Lim, L.Y.; Khor, E. Characterization of chitosan acetate as a binder for sustained release tablets. J. Control. Release 2004, 99, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Zerrouk, N.; Mennini, N.; Maestrelli, F.; Chemtob, C.; Mura, P. Comparison of the effect of chitosan and polyvinylpyrrolidone on dissolution properties and analgesic effect of naproxen. Eur. J. Pharm. Biopharm. 2004, 57, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Bae, J.W.; Joung, Y.K.; Shin, J.W.; Park, K.D. Nanoaggregate of thermosensitive chitosan-Pluronic for sustained release of hydrophobic drug. Colloids Surf. B. 2008, 63, 1–6. [Google Scholar] [CrossRef]
- Lim Soo, P.; Cho, J.; Grant, J.; Ho, E.; Piquette-Miller, M.; Allen, C. Drug release mechanism of paclitaxel from a chitosan-lipid implant system: Effect of swelling, degradation and morphology. Eur. J. Pharm. Biopharm. 2008, 69, 149–157. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Murdan, S.; Basit, A.W. An Investigation into the Digestion of Chitosan (Noncrosslinked and Crosslinked) by Human Colonic Bacteria. J. Pharm. Sci. 2008, 97, 3820–3829. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Sandri, G.; Rossi, S.; Ferrari, F.; Gibin, S.; Caramella, C. Chitosan citrate as multifunctional polymer for vaginal delivery. Evaluation of penetration enhancement and peptidase inhibition properties. Eur. J. Pharm. Sci. 2008, 33, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.G.; Strauss, J.H. Viruses and Human Disease, 1st ed.; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Whitley, R.J. Herpes simplex virus. In Fields virology, 4th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2001; pp. 2461–2511. [Google Scholar]
- Whitley, R.J.; Roizman, B. Herpes simplex virus infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Spruance, S.L.; Overall, J.C., Jr.; Kern, E.R.; Krueger, G.G.; Pliam, V.; Miller, W. The natural history of recurrent herpes simplex labialis: implications for antiviral therapy. N. Engl. J. Med. 1977, 297, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; el-Ghorr, A.A. UV radiation and mouse models of herpes simplex virus infection. Photochem. Photobiol. 1996, 64, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Erlich, K.S.; Mills, J.; Chatis, P.; Mertz, G.J.; Busch, D.F.; Follansbee, S.E.; Grant, R.M.; Crumpacker, C.S. Acyclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 1989, 320, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Spear, P.G. Infections with herpes simplex viruses (1). N. Engl. J. Med. 1986, 314, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Spear, P.G. Infections with herpes simplex viruses (2). N. Engl. J. Med. 1986, 314, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Erlich, K.S. Management of herpes simplex and varicella-zoster virus infections. West. J. Med. 1997, 166, 211–215. [Google Scholar] [PubMed]
- Fujioka, Y.; Mizuno, N.; Morita, E.; Motozono, H.; Takahashi, K.; Yamanaka, Y.; Shinkuma, D. Effect of age on the gastrointestinal absorption of acyclovir in rats. J. Pharm. Pharmacol. 1991, 43, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Meadows, K.C.; Dressman, J.B. Mechanism of acyclovir uptake in rat jejunum. Pharm. Res. 1990, 7, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Vergin, H.; Kikuta, C.; Mascher, H.; Metz, R. Pharmacokinetics and bioavailability of different formulations of acyclovir. Arzneimittelforschung 1995, 45, 508–515. [Google Scholar] [PubMed]
- de Miranda, P.; Krasny, H.C.; Page, D.A.; Elion, C.B. Species differences in the disposition of acyclovir. Am. J. Med. 1982, 73, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.; Bauer, D.J. Relative potencies of anti-herpes compounds. Ann. N. Y. Acad. Sci. 1977, 284, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Krenitsky, T.A.; Hall, W.W.; de Miranda, P.; Beauchamp, L.M.; Schaeffer, H.J.; Whiteman, P. 6-Deoxy acyclovir: a xanthine oxidase-activated prodrug of acyclovir. Proc. Natl. Acad. Sci. USA 1984, 81, 3209–3213. [Google Scholar] [CrossRef] [PubMed]
- Lycke, J.; Malmeström, C.; Ståhle, L. Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob. Agents Chemother. 2003, 47, 2438–2441. [Google Scholar] [CrossRef] [PubMed]
- Luengo, J.; Aránguiz, T.; Sepulveda, J. Preliminary pharmacokinetic study to different preparations of acyclovir with β-cyclodextrin. J. Pharm. Sci. 2002, 91, 2593–2598. [Google Scholar] [CrossRef] [PubMed]
- Attia, I.A.; El-Gizawy, S.A; Fouda, M.A.; Donia, A.M. Influence of a niosomal formulation on the oral bioavailability of acyclovir in rabbits. AAPS PharmSciTech. 2007, 8, E106. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.D.; Fowle, A.S.; Bittiner, S.B.; Bye, A.; Isaacs, P.E. Human gastrointestinal absorption of acyclovir from tablet duodenal infusion and sipped solution. Br. J. Clin. Pharmacol. 1986, 21, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, N.; Ordu, S.; Özkan, Y. Studies of floating dosage forms of furosemide: in vitro and in vivo evaluations of bilayer tablet formulations. Drug Dev. Ind. Pharm. 2000, 26, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Stepensky, D.; Lavy, E.; Eyal, S.; Klausner, E.; Friedman, M. Pharmacokinetic and pharmacodynamic aspects of gastroretentive dosage forms. Int. J. Pharm. 2004, 277, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Gröning, R.; Cloer, C.; Georgarakis, M.; Müller, R.S. Compressed collagen sponges as gastroretentive dosage forms: in vitro and in vivo studies. Eur. J. Pharm. Sci. 2007, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sheth, P.R.; Tossounian, J.L. Novel sustained release tablet formulations. U.S. Patent 4,167,558, 1979. [Google Scholar]
- Junyaprasert, V.B.; Pornsuwannapha, S. Floating properties and release characteristics of hollow microspheres of acyclovir. Drug Deliv. 2008, 15, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Gröning, R.; Berntgen, M.; Georgarakis, M. Acyclovir serum concentrations following peroral administration of magnetic depot tablets and the influence of extracorporal magnets to control gastrointestinal transit. Eur. J. Pharm. Biopharm. 1998, 46, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, L.; Fell, J.T.; Collett, J.H.; Sharma, H.L.; Smith, A.M. Floating dosage forms: an in vivo study demonstrating prolonged gastric retention. J. Control. Release 1998, 55, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Talukder, R.; Fassihi, R. Gastroretentive delivery systems: hollow beads. Drug Dev. Ind. Pharm. 2004, 30, 405–412. [Google Scholar] [CrossRef] [PubMed]
- US FDA. Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid dosage forms based on a biopharmaceutics classification system. 2000. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070246.pdf (accessed on 1 September 2010). [Google Scholar]
- Dressman, J.B.; Berardi, R.R.; Dermentzoglou, L.C.; Russell, T.L.; Schmaltz, S.P.; Barnett, J.L.; Jarvenpaa, K.M. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm. Res. 1990, 7, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.L.; Berardi, R.R.; Barnett, J.L.; Dermentzoglou, L.C.; Jarvenpaa, K.M.; Schmaltz, S.P.; Dressman, J.B. Upper gastrointestinal pH in seventy-nine healthy, elderly, North American men and women. Pharm. Res. 1993, 10, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Lui, C.Y.; Amidon, G.L.; Berardi, R.R.; Fleisher, D.; Youngberg, C.; Dressman, J.B. Comparison of gastrointestinal pH in dogs and humans: implications on the use of the beagle dog as a model for oral absorption in humans. J. Pharm. Sci. 1986, 75, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Ali, J.; Ahuja, A.; Khar, R.K.; Baboota, S. Floating drug delivery systems: a review. AAPS PharmSciTech 2005, 6, E372–E390. [Google Scholar] [CrossRef] [PubMed]
- Haupt, S.; Zioni, T.; Gati, I.; Kleinstern, J.; Rubinstein, A. Luminal delivery and dosing considerations of local celecoxib administration to colorectal cancer. Eur. J. Pharm. Sci. 2006, 28, 204–211. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ruiz-Caro, R.; Veiga, M.D. In vitro Evaluation of Acyclovir/Chitosan Floating Systems. Materials 2010, 3, 5195-5211. https://doi.org/10.3390/ma3125195
Ruiz-Caro R, Veiga MD. In vitro Evaluation of Acyclovir/Chitosan Floating Systems. Materials. 2010; 3(12):5195-5211. https://doi.org/10.3390/ma3125195
Chicago/Turabian StyleRuiz-Caro, Roberto, and María D. Veiga. 2010. "In vitro Evaluation of Acyclovir/Chitosan Floating Systems" Materials 3, no. 12: 5195-5211. https://doi.org/10.3390/ma3125195




