Performance of Zirconia for Dental Healthcare
Abstract
:1. Introduction
2. Zirconia as a Dental Material
2.1. Pre- and Fully-Sintered 3Y-TZP for Dental Application
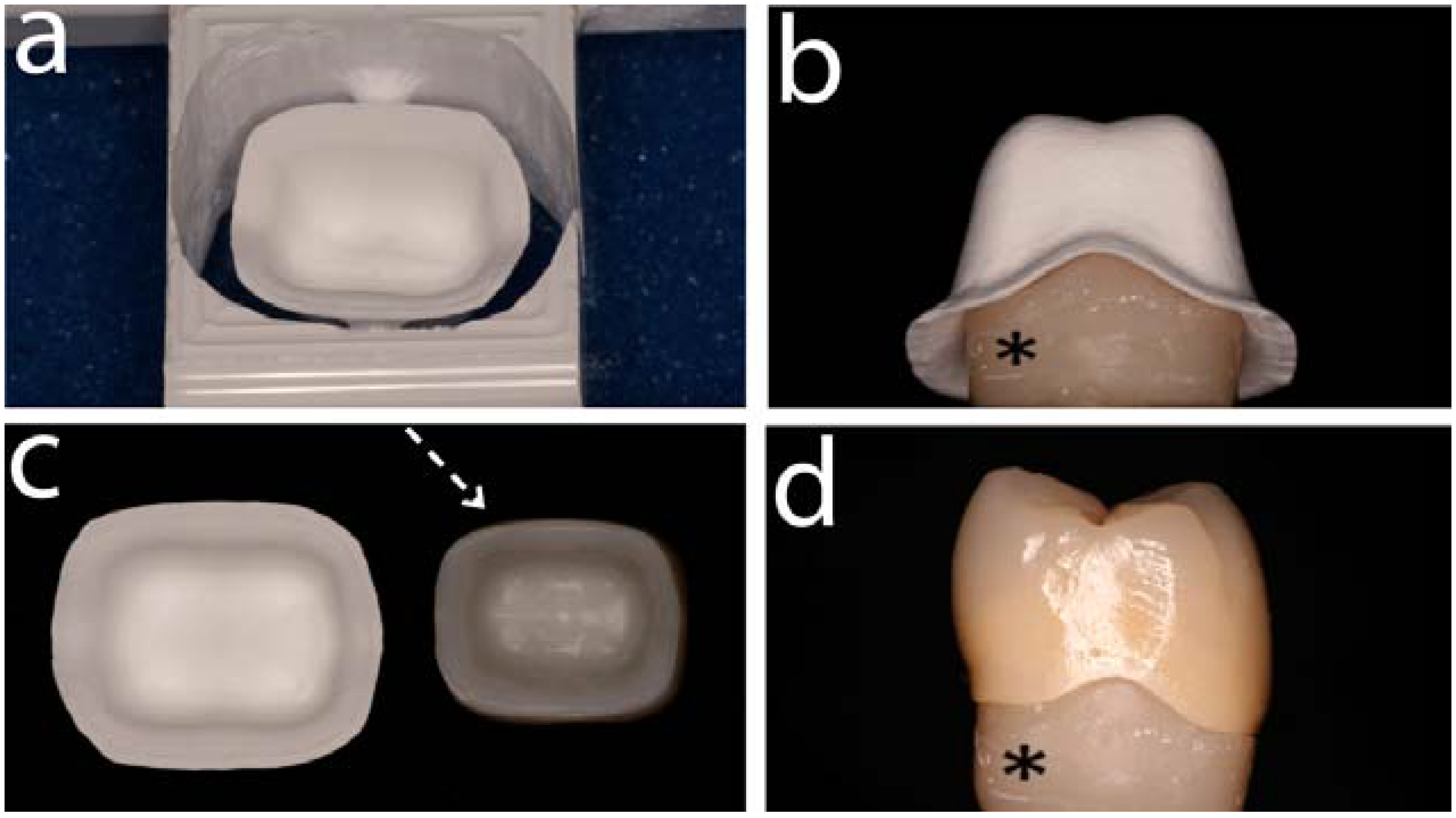
3. Clinical Aspects of Zirconia Restorations
3.1. Clinical Survival and Complication Rates
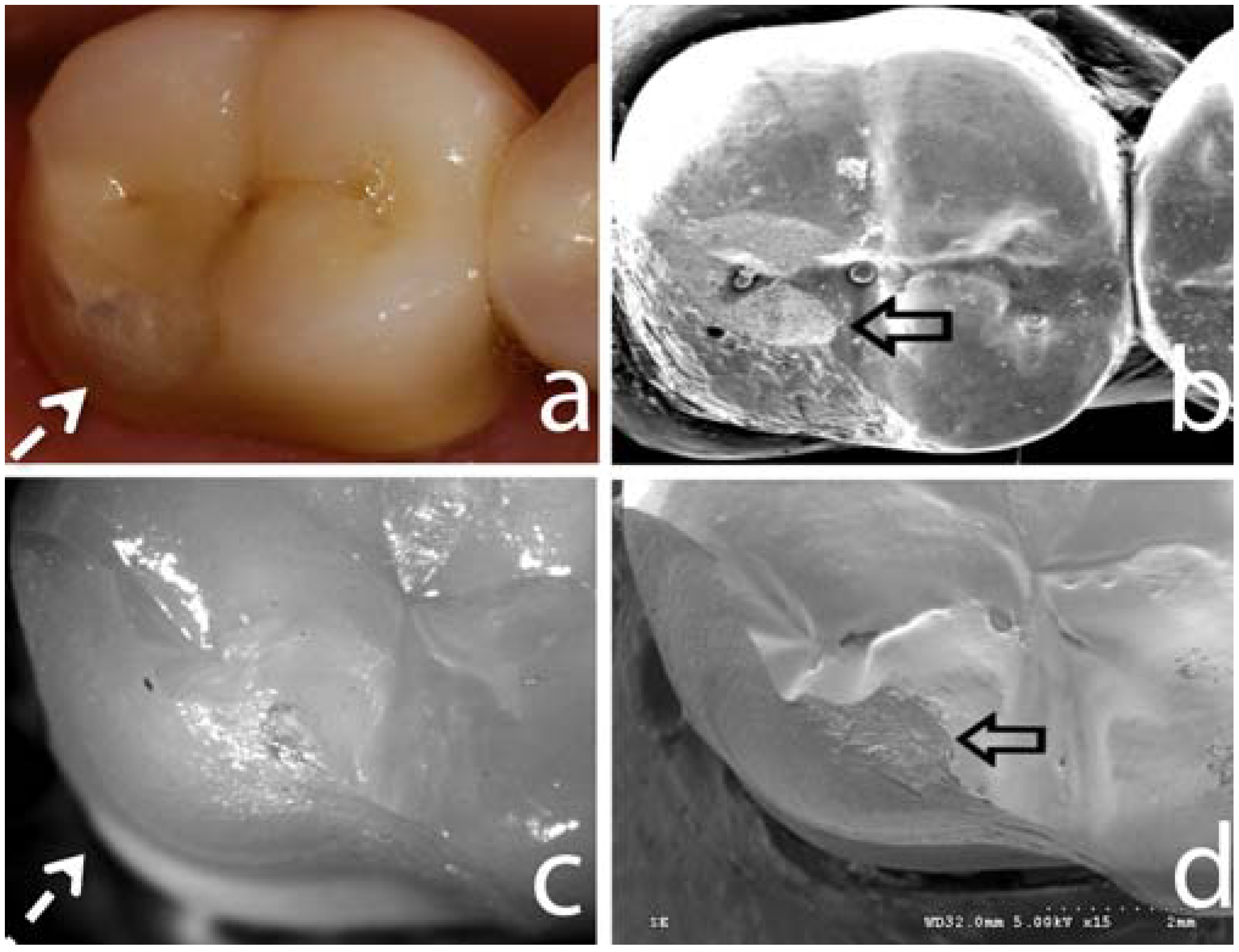
4. Mechanical Response of Zirconia-Based Restorations
4.1. Flat Model Veneered Zirconia System
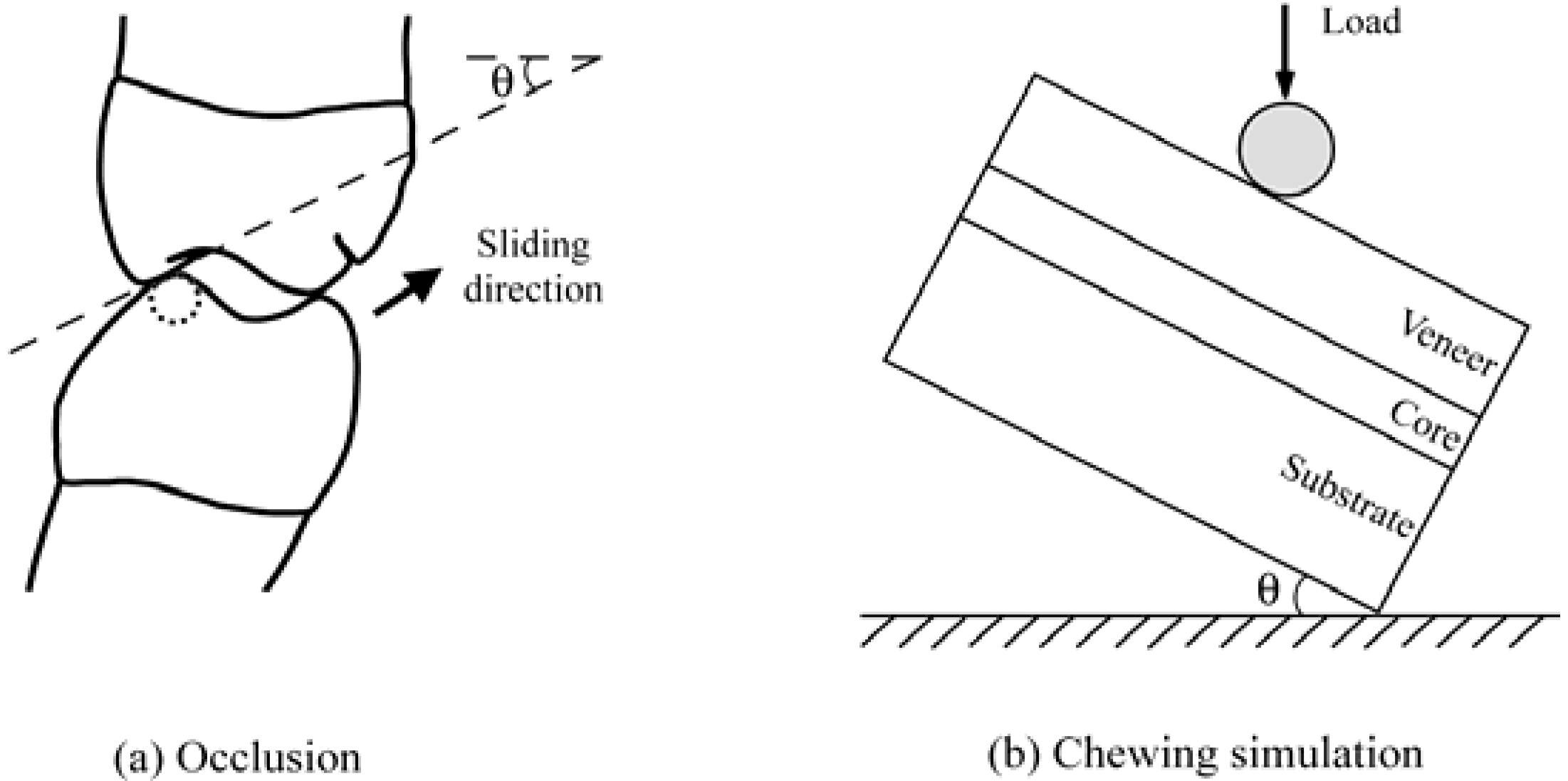
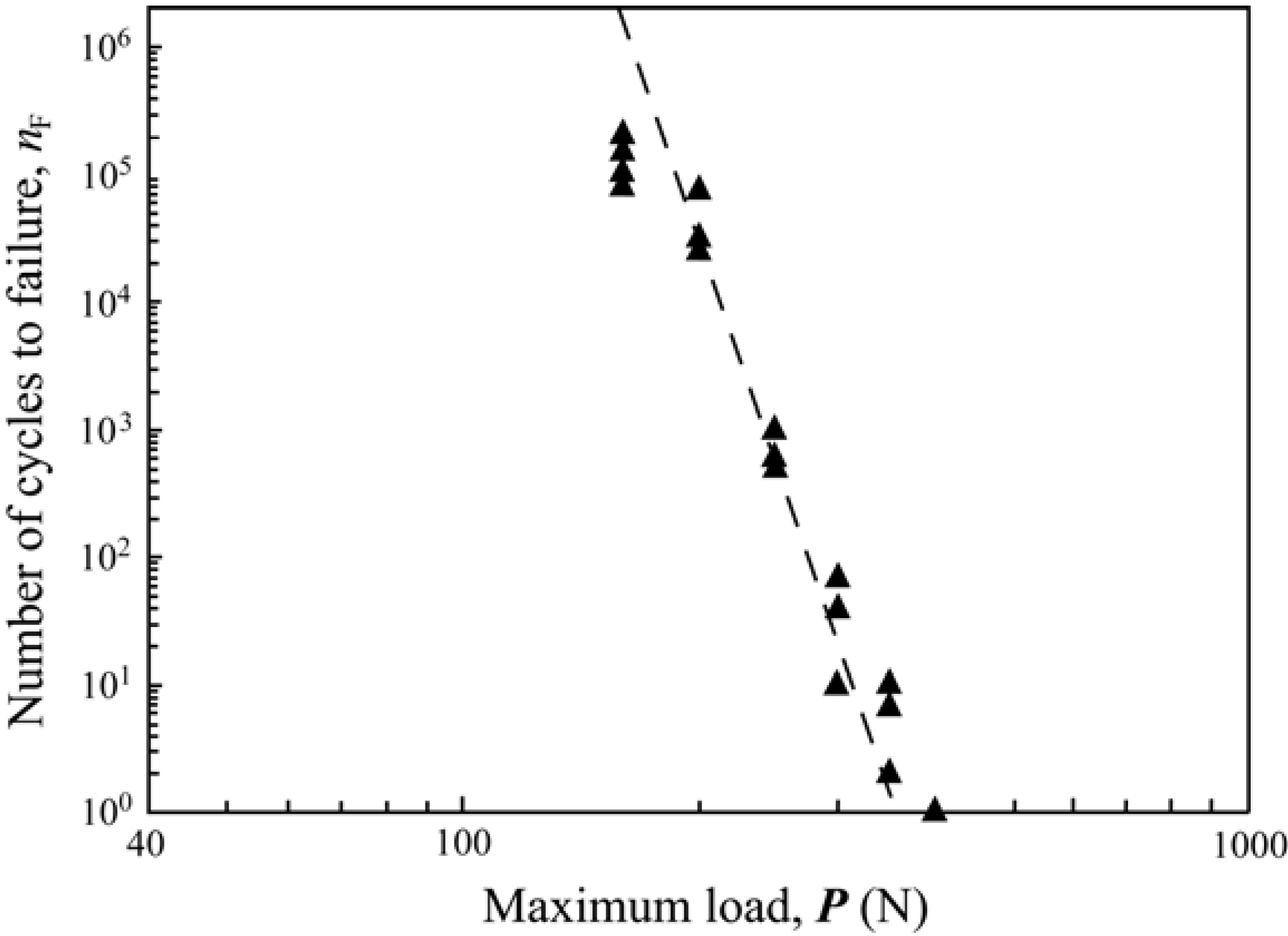
4.2. Anatomically correct Zirconia-Supported Restorations
4.2.1. Test Method
4.2.2. Failure Modes and Veneering Technique
4.2.3. Framework Design
4.2.4. Innovative Veneering Techniques
5. Zirconia Abutments for Oral Implants
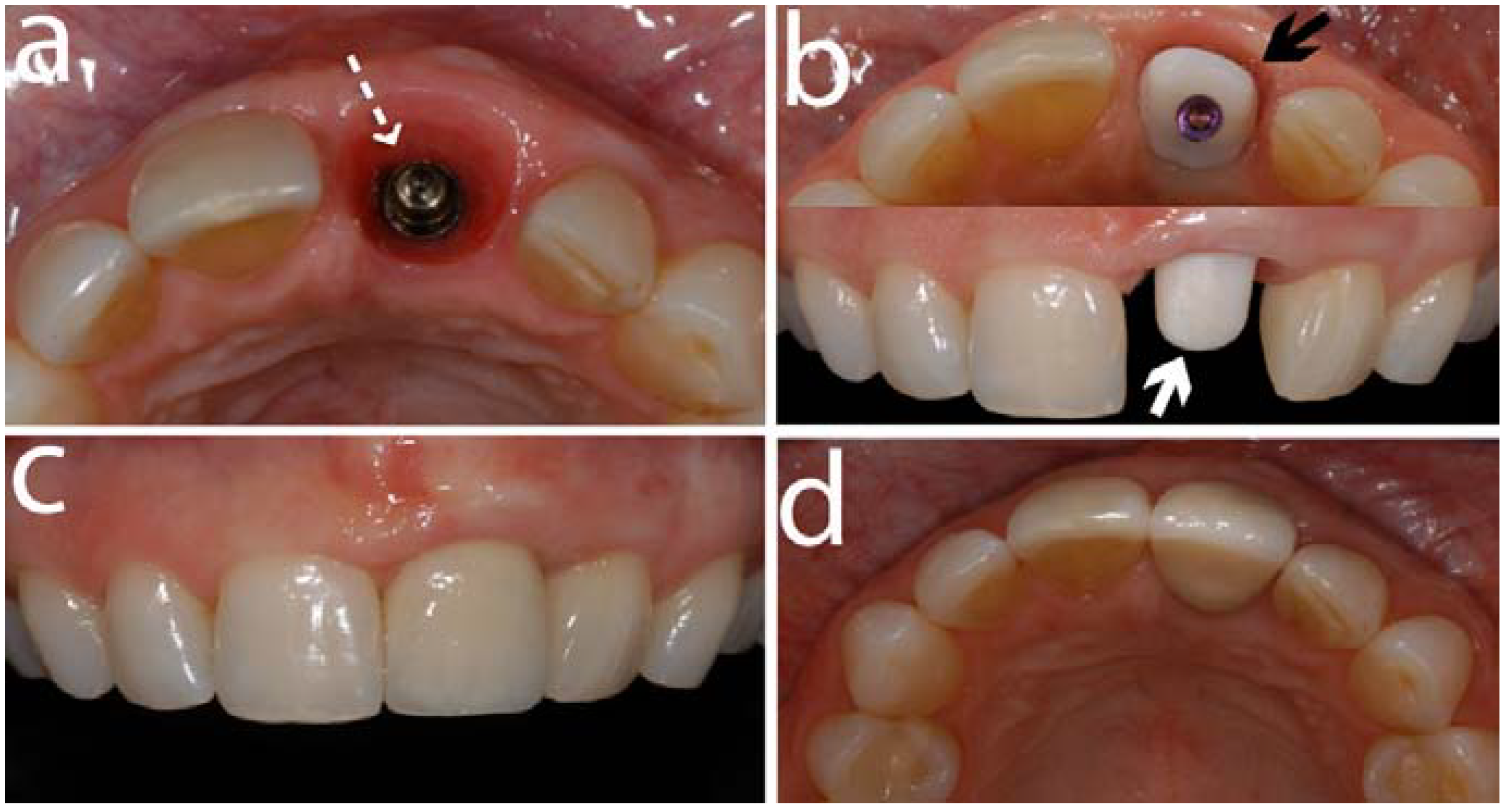
5.1. Abutment Screw Loosening
- Furthermore, an in vivo study on customized zirconia abutments fabricated by CAD/CAM procedures, exhibited an excellent fit with a rotational freedom of less than 3° [92].
5.2. Fractures of the Veneering Ceramic
5.3. Biological Response on Mucosa and Bone
6. Zirconia Oral implants
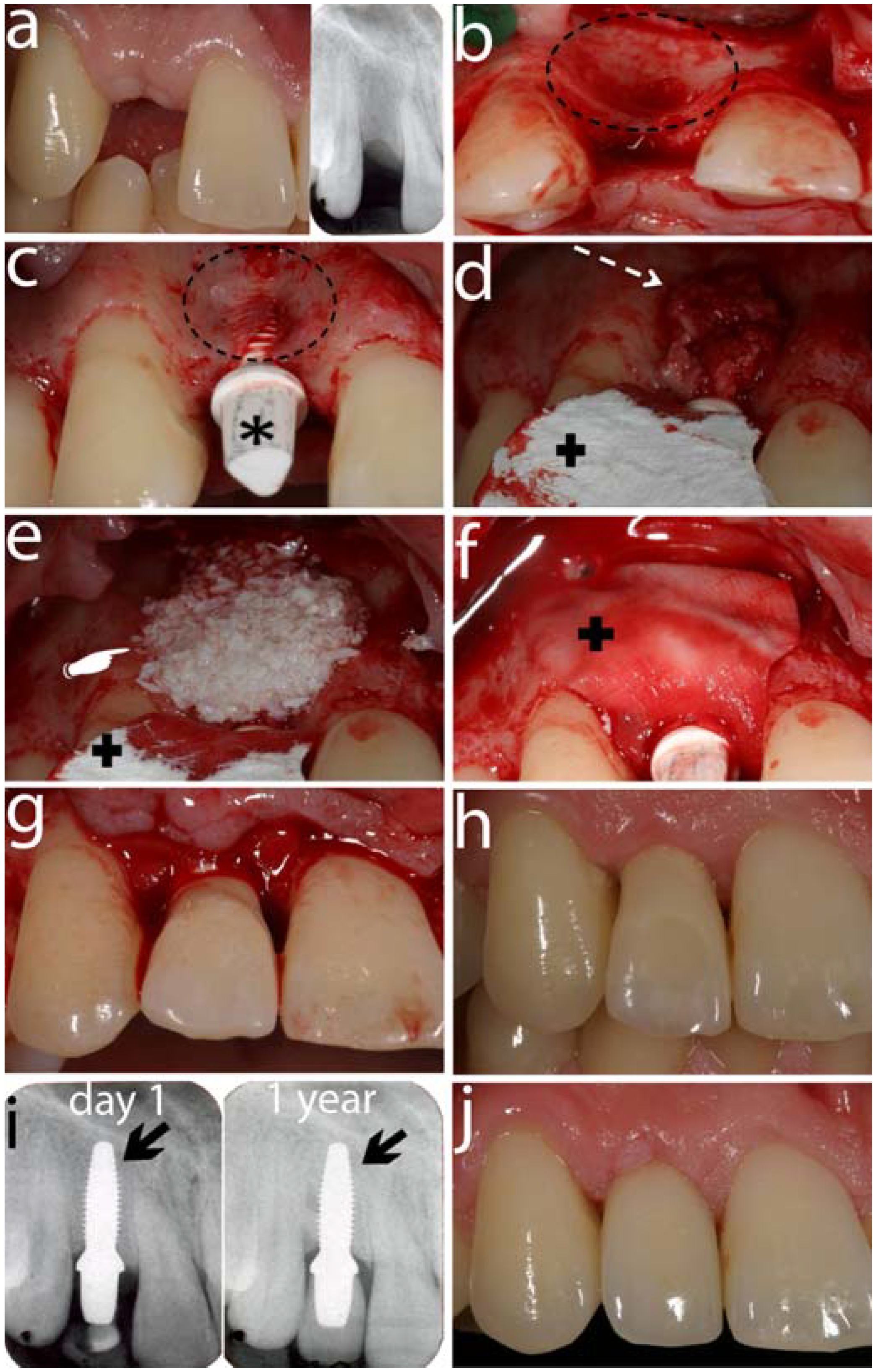
6.1. Laboratory Studies
| Author (year) | Animal model | Bone-implant Contact |
|---|---|---|
| Akagawa et al. (1993) [137] | dogs | Unloaded implants: 82% |
| Loaded implants: 70% | ||
| Akagawa et al. (1998) [138] | monkey | Loading period: 12 months |
| Single freestanding implants: 54%−71% | ||
| Connected freestanding implants: 58%−77% | ||
| Implant-tooth supported: 70%−75% | ||
| Loading period: 24 months | ||
| Single freestanding implants: 66%−81% | ||
| Connected freestanding implants: 66%−77% | ||
| Implant-tooth supported: 66%−82% | ||
| Scarano et al. (2003) [139] | rabbit | 4 weeks: 68% |
| Kohal et al. (2004) [151] | monkey | 9 months; Y-TZP implants: 68%; Ti implants: 73% |
| Sennerby et al. (2005) [140] | rabbit | 6 weeks |
| Zr-Ctr: femur: 46%; tibia: 19% | ||
| Zr-A: femur: 60%; tibia: 31% | ||
| Zr-B: femur: 70%; tibia: 22% | ||
| Ti-ox: femur: 68%; tibia: 24% | ||
| Hoffman et al. (2008) [141] | rabbit | 2 weeks: |
| Y-TZP: 55% | ||
| Ti: 47.6% | ||
| 4 weeks: | ||
| Y-TZP: 71.5% | ||
| Ti: 80% | ||
| Depprich et al. (2008) [142] | minipig | 1 week; Y-TZP: 35%; Ti: 48% |
| 4 weeks;Y-TZP: 45%;Ti: 99% | ||
| 12 weeks; Y-TZP: 71%; Ti: 83% | ||
| Lee et al. (2009) [143] | rabbit | 3 weeks: |
| ZiUnite: 70.5% | ||
| Nano-modified surface A: 64.6% | ||
| Nano-modified surface B: 62.2% | ||
| TiUnite: 77.6% | ||
| 6 weeks: | ||
| ZiUnite: 69.7% | ||
| Nano-modified surface A: 68.6% | ||
| Nano-modified surface B: 64.5% | ||
| TiUnite: 67.1% | ||
| Kohal et al. (2009) [144] | rat | 14 days: |
| ZrO2modified: 45.3% | ||
| TiUnite: 36.4% | ||
| 28 days: | ||
| ZrO2modified: 59.4% | ||
| TiUnite: 55.2% | ||
| Rocchietta et al. (2009) [145] | rabbit | 3 weeks: |
| ZiUnite: 27.5% | ||
| Promimic: 42.5% | ||
| CoAT sputtered: 36.1% | ||
| TiUnite: 58.3% | ||
| Gahlert et al. (2009) [148] | minipig | 4 weeks: |
| Zirconia: 51.1% | ||
| Ti-SLA: 55.1% | ||
| 8 weeks: | ||
| Zirconia: 53,7% | ||
| Ti-SLA: 70.4% | ||
| 12 weeks: | ||
| Zirconia: 64.2% | ||
| Ti-SLA: 54.4% |
6.2. Animal Studies
6.3. Clinical Studies
6.4. Discussion and Critical Appraisal
6.4.1. Zirconia Oral Implants and Stability
6.4.2. Zirconia Oral Implants and Osseointegration
6.4.3. Clinical Investigations
7. Future of Zirconia for Dental Healthcare
Acknowledgements
References
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Garvie, R.C.; Hannink, R.H.J.; Pascoe, R.T. Ceramic Steel? Nature 1975, 258, 703. [Google Scholar] [CrossRef]
- Deville, S.; Chevalier, J.; Gremillard, L. Influence of surface finish and residual stresses on the ageing sensitivity of biomedical grade zirconia. Biomaterials 2006, 27, 2186–2192. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Gremillard, L. Zirconia ceramics. In Bioceramics and their clinical applications; Kokubo, T., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 243–265. [Google Scholar]
- Green, D.J.; Hannink, R.H.J.; Swain, M.V. Transformation Toughening of Ceramics; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Leach, C.A. Sintering of magnesium partially-stabilized zirconia-behavior of an impurity silicate phase. Mater. Sci. Technol. 1987, 3, 321–324. [Google Scholar] [CrossRef]
- Sundh, A.; Sjogren, G. Fracture resistance of all-ceramic zirconia bridges with differing phase stabilizers and quality of sintering. Dent. Mater. 2006, 22, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Lange, F.F. Transformation toughening. Part 4. Fabrication, fracture-toughness and strength of Al2O3-ZrO2 composites. J. Mater. Sci. 1982, 17, 247–254. [Google Scholar] [CrossRef]
- Lange, F.F. Transformation toughening. Part 5. Effect of temperature and alloy on fracture-toughness. J. Mater. Sci. 1982, 17, 255–262. [Google Scholar] [CrossRef]
- Guazzato, M.; Albakry, M.; Quach, L.; Swain, M.V. Influence of grinding, sandblasting, polishing and heat treatment on the flexural strength of a glass-infiltrated alumina-reiforced dental ceramic. Bioceramics 2004, 25, 2153–2160. [Google Scholar]
- Tsukuma, K. Mechanical properties and thermal stability of CeO2 containing tetragonal zirconia zirocnia polycrystals. Am. Ceram. Soc. Bull. 1986, 65, 1386–1389. [Google Scholar]
- Guazzato, M.; Albakry, M.; Swain, M.V.; Ringer, S.P. Microstructure of alumina- and alumina/zirconia-glass infiltrated dental ceramics. Bioceramics 2003, 15, 879–882. [Google Scholar]
- Glauser, R.; Sailer, I.; Wohlwend, A.; Studer, S.; Schibli, M.; Schärer, P. Experimental zirconia abutments for implant-supported single-tooth restorations in esthetically demanding regions: 4-year results of a prospective clinical study. Int. J. Prosthodont. 2004, 17, 285–290. [Google Scholar] [PubMed]
- Heuer, A.H.; Claussen, N.; Kriven, W.M.; Ruhle, M. Stability of tetragonal ZrO2 particles in ceramic matrices. J. Am. Ceram. Soc. 1982, 65, 642–650. [Google Scholar] [CrossRef]
- Cottom, B.A.; Mayo, M.J. Fracture toughness of nanocrystalline ZrO2–3 mol % Y2O3 determined by Vickers indentation. Scripta Mater. 1996, 34, 809–814. [Google Scholar] [CrossRef]
- Subbarao, E.C. Zirconia—an overview. In Science and Technology of Zirconia; Heuer, A.H., Hobbs, L.W., Eds.; The American Ceramic Society: Columbus, OH, USA, 1981; pp. 1–24. [Google Scholar]
- Filser, F.; Kocher, P.; Gauckler, L.J. Net-shaping of ceramic components by dirct machining. Assembly Autom. 2003, 23, 382–390. [Google Scholar] [CrossRef]
- Sttor, D.; Hauptmann, H.; Schnagl, R.; Frank, S. Coloring ceramics by way of ionic or complex-containing solutions. 3M Espe AG. US Pat. 6,709,694, 23 March 2004. [Google Scholar]
- Cales, B. Colored zirconia ceramics for dental applications. In Bioceramics; LeGeros, R.Z., LeGeros, J.P., Eds.; World Scientific Publishing Co.Pte.Ltd: New York, NY, USA, 1998. [Google Scholar]
- Kosmac, T.; Oblak, C.; Jevnikar, P.; Funduk, N.; Marion, L. Strength and reliability of surface treated Y-TZP dental ceramics. J. Biomed. Mater. Res. 2000, 53, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Kosmac, T.; Oblak, C.; Jevnikar, P.; Funduk, N.; Marion, L. The effect of surface grinding and sandblasting on flexural strength and reliability of Y-TZP zirconia ceramic. Dent. Mater. 1999, 15, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Rekow, D.; Zhang, Y.; Thompson, V. Can material properties predict survival of all-ceramic posterior crowns? Compend. Contin. Educ. Dent. 2007, 28, 362–368. [Google Scholar] [PubMed]
- Guazzato, M.; Proos, K.; Sara, G.; Swain, M.V. Strength, reliability, and mode of fracture of bilayered porcelain/core ceramics. Int. J. Prosthodont. 2004, 17, 142–149. [Google Scholar] [PubMed]
- Huang, H. Machining characteristics and surface integrity of yttria stabilized tetragonal zirconia in high speed deep griding. Mater. Sci. Eng. A: Struct. 2003, 345, 155–163. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Sailer, I.; Zwahlen, M.; Hämmerle, C.H. A systematic review of the survival and complication rates of all-ceramic and metal-ceramic reconstructions after an observation period of at least 3 years. Part I: Single crowns. Clin. Oral Implants Res. 2007, 18 (Suppl. 3), 73–85. [Google Scholar]
- Sailer, I.; Feher, A.; Filser, F.; Gauckler, L.J.; Lüthy, H.; Hämmerle, C.H. Five-year clinical results of zirconia frameworks for posterior fixed partial dentures. Int. J. Prosthodont. 2007, 20, 383–388. [Google Scholar] [PubMed]
- Sadan, A.; Blatz, M.B.; Lang, B. Clinical considerations for densely sintered alumina and zirconia restorations: Part 2. Int. J. Periodontics Restor. Dent. 2005, 25, 343–349. [Google Scholar]
- Sadan, A.; Blatz, M.B.; Lang, B. Clinical considerations for densely sintered alumina and zirconia restorations: Part 1. Int. J. Periodontics Restor. Dent. 2005, 25, 213–219. [Google Scholar]
- Cehreli, M.C.; Kokat, A.M.; Akca, K. CAD/CAM Zirconia vs. slip-cast glass-infiltrated Alumina/Zirconia all-ceramic crowns: 2-year results of a randomized controlled clinical trial. J. Appl. Oral Sci. 2009, 17, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ortorp, A.; Kihl, M.L.; Carlsson, G.E. A 3-year retrospective and clinical follow-up study of zirconia single crowns performed in a private practice. J. Dent. 2009, 37, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Edelhoff, D.; Florian, B.; Florian, W.; Johnen, C. HIP zirconia fixed partial dentures--clinical results after 3 years of clinical service. Quintessence Int. 2008, 39, 459–471. [Google Scholar] [PubMed]
- Molin, M.K.; Karlsson, S.L. Five-year clinical prospective evaluation of zirconia-based Denzir 3-unit FPDs. Int. J. Prosthodont. 2008, 21, 223–227. [Google Scholar] [PubMed]
- Raigrodski, A.J.; Chiche, G.J.; Potiket, N.; Hochstedler, J.L.; Mohamed, S.E.; Billiot, S.; Mercante, D.E. The efficacy of posterior three-unit zirconium-oxide-based ceramic fixed partial dental prostheses: A prospective clinical pilot study. J. Prosthet. Dent. 2006, 96, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Tinschert, J.; Schulze, K.A.; Natt, G.; Latzke, P.; Heussen, N.; Spiekermann, H. Clinical behavior of zirconia-based fixed partial dentures made of DC-Zirkon: 3-year results. Int. J. Prosthodont. 2008, 21, 217–222. [Google Scholar] [PubMed]
- Beuer, F.; Edelhoff, D.; Gernet, W.; Sörensen, J.A. Three-year clinical prospective evaluation of zirconia-based posterior fixed dental prostheses (FDPs). Clin. Oral Investig. 2009, 13, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Vult von Steyern, P.; Jonsson, O.; Nilner, K. Five-year evaluation of posterior all-ceramic three-unit (In-Ceram) FPDs. Int. J. Prosthodont. 2001, 14, 379–384. [Google Scholar] [PubMed]
- Olsson, K.G.; Fürst, B.; Andersson, B.; Carlsson, G.E. A long-term retrospective and clinical follow-up study of In-Ceram Alumina FPDs. Int. J. Prosthodont. 2003, 16, 150–156. [Google Scholar] [PubMed]
- Sailer, I.; Zembic, A.; Jung, R.E.; Siegenthaler, D.; Holderegger, C.; Hämmerle, C.H. Randomized controlled clinical trial of customized zirconia and titanium implant abutments for canine and posterior single-tooth implant reconstructions: Preliminary results at 1 year of function. Clin. Oral Implants Res. 2009, 20, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Pjetursson, B.E.; Lang, N.P.; Chan, E.S. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. Clin. Oral Implants Res. 2004, 15, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M.N.; de Jager, N.; Kleverlaan, C.J.; Feilzer, A.J. Microtensile bond strength of different components of core veneered all-ceramic restorations. Dent Mater. 2005, 21, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Stawarczyk, B.; Tomic, M.; Strub, J.R.; Hämmerle, C.H. Effect of thermal misfit between different veneering ceramics and zirconia frameworks on in vivo fracture load of single crowns. Dent. Mater. J. 2007, 26, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Grohmann, P.; Stawarczyk, B. Effect of zirconia surface treatments on the shear strength of zirconia/veneering ceramic composites. Dent. Mater. J. 2008, 27, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M.N.; Feilzer, A.J.; Kleverlaan, C.J. Bridging the gap between clinical failure and laboratory fracture strength tests using a fractographic approach. Dent. Mater. 2008, 25, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Al-Dohan, H.M.; Yaman, P.; Dennison, J.B.; Razzoog, M.E.; Lang, B.R. Shear strength of core-veneer interface in bi-layered ceramics. J. Prosthet. Dent. 2004, 91, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Luthardt, R.G.; Sandkuhl, O.; Reitz, B. Zirconia-TZP and alumina—advanced technologies for the manufacturing of single crowns. Eur. J. Prosthodont. Restor. Dent. 1999, 7, 113–119. [Google Scholar] [PubMed]
- Sailer, I.; Philipp, A.; Zembic, A.; Pjetursson, B.E.; Hämmerle, C.H.; Zwahlen, M. A systematic review of the performance of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin. Oral Implants Res. 2009, 20 (Suppl. 4), 4–31. [Google Scholar] [CrossRef]
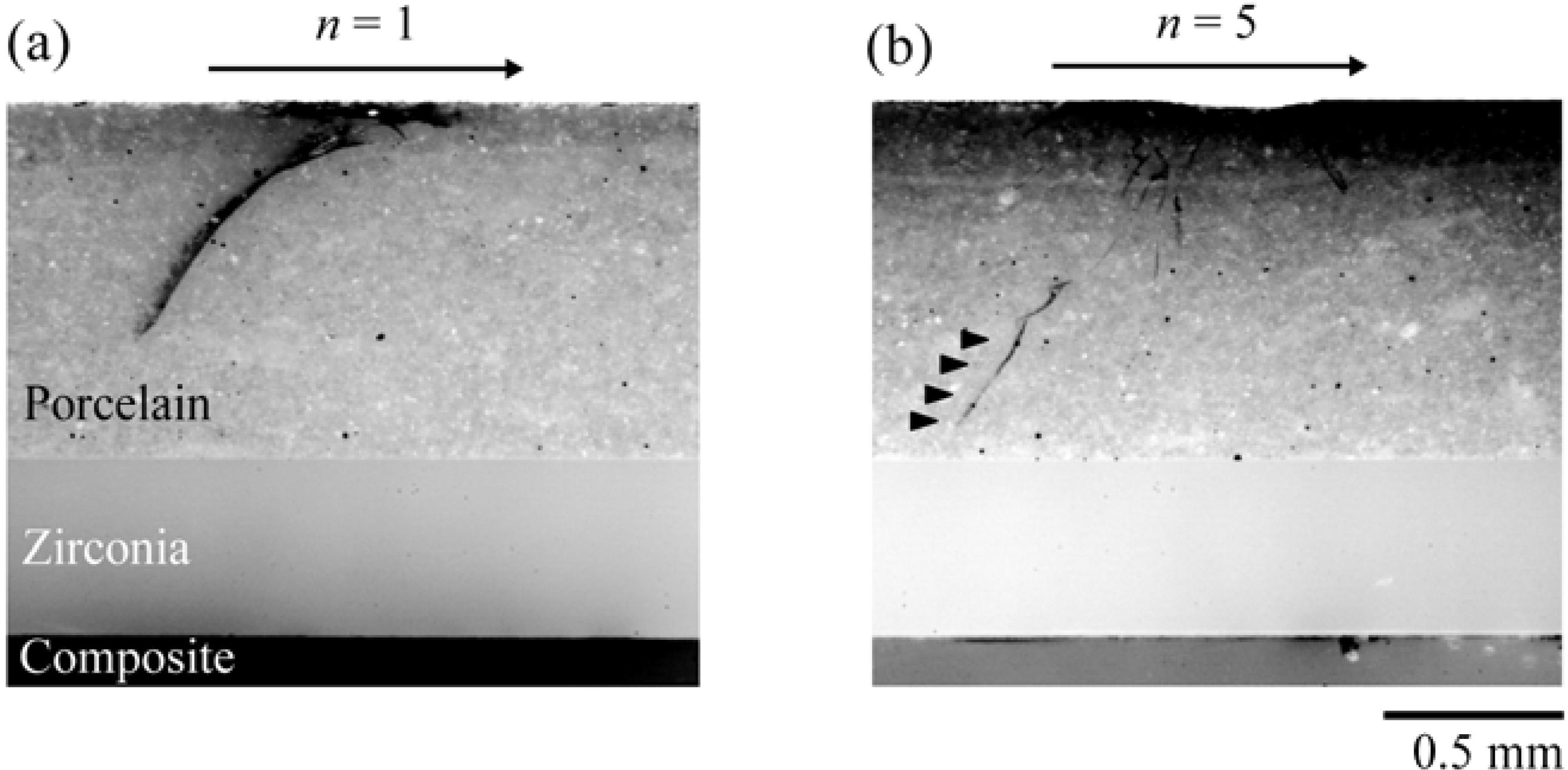
- Kim, J.W.; Kim, J.H.; Thompson, V.P.; Zhang, Y. Sliding contact fatigue damage in layered ceramic structures. J. Dent. Res. 2007, 86, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Delong, R.; Douglas, W.H. Development of an artifical oral environment for the testing of dental restoratives: bi-axial force and movement control. J. Dent. Res. 1983, 62, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Krejci, D.; Albert, P.; Lutz, F. The influence of antagonist standarization on wear. Dent. Res. 1999, 78, 713–719. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, J.H.; Janal, M.N.; Zhang, Y. Damage maps of veneered zirconia under simulated mastication. J. Dent. Res. 2008, 87, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kim, J.W.; Bhowmick, S.; Thompson, V.P.; Rekow, E.D. Competition of fracture mechanisms in monolithic dental ceramics: Flat model systems. J. Biomed. Mater. Res. B: Appl. Biomater. 2009, 88, 402–411. [Google Scholar] [CrossRef]
- Kelly, J.R. Clinically relevant approach to failure testing of all-ceramic restorations. J Prosthet Dent. 1999, 81, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Zhang, Y.; Pines, M.; Thompson, V.P. Fracture of Porcelain-veneered Structures in Fatigue. J. Dent. Res. 2007, 86, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lawn, B.R.; Malament, K.A.; Van Thompson, P.; Rekow, E.D. Damage accumulation and fatigue life of particle-abraded ceramics. Int. J. Prosthodont. 2006, 19, 442–448. [Google Scholar] [PubMed]
- Kelly, J.R. Perspectives on strength. Dent. Mater. 1995, 11, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ritter, J.E. Predicting lifetimes of materials and material structures. Dent Mater. 1995, 11, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Bonfante, E.A.; Silva, N.R.; Rekow, E.D.; Thompson, V.P. Laboratory simulation of Y-TZP all-ceramic crown clinical failures. J. Dent. Res. 2009, 88, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Silva, N.R.; Bonfante, E.A.; Guess, P.C.; Rekow, E.D.; Thompson, V.P. Fatigue testing of two porcelain-zirconia all-ceramic crown systems. Dent. Mater. 2009, 25, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Peterson, I.M.; Wuttiphan, S.; Lawn, B.R.; Chyung, K. Role of microstructure on contact damage and strength degradation of micaceous glass-ceramics. Dent. Mater. 1998, 14, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.G.; Peterson, I.M.; Kim, D.K.; Lawn, B.R. Lifetime-limiting strength degradation from contact fatigue in dental ceramics. J. Dent. Res. 2000, 79, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Guess, P.C.; Zavanelli, R.A.; Silva, N.R.; Bonfante, E.A.; Coehlo, P.; Thompson, V.P. Monolithic CD/CAM lithium disilicate versus veneered Y-TZP crowns: Comparison of failure modes and relibility after fatigue. Int. J. Prosthodont. 2009, in press. [Google Scholar]
- Tsalouchou, E.; Cattell, M.J.; Knowles, J.C.; Pittayachawan, P.; McDonald, A. Fatigue and fracture properties of yttria partially stabilized zirconia crown systems. Dent. Mater. 2007, 24, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Birkby, I.; Stevens, R. Applications of zirconia ceramics. Key Eng. Mater. 1996, 122–124, 527–552. [Google Scholar]
- Swain, M.V. Unstable cracking (chipping) of veneering porcelain on all-ceramic dental crowns and fixed partial dentures. Acta Biomater. 2009, 5, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Hojjatie, B.; Anusavice, K.J. Effects of initial temperature and tempering medium on thermal tempering of dental porcelains. J. Dent. Res. 1993, 72, 566–571. [Google Scholar] [CrossRef] [PubMed]
- De Kler, M.; De Jager, N.; Meegdes, M.; Van Der Zel, J.M. Influence of thermal expansion mismatch and fatigue loading on phase changes in porcelain veneered Y-TZP zirconia discs. J. Oral Rehabil. 2007, 34, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Kollar, A.; Huber, S.; Mericske, E.; Mericske-Stern, R. Zirconia for teeth and implants: A case series. Int. J. Periodontics Restor Dent. 2008, 28, 479–487. [Google Scholar]
- Sturzenegger, B.; Feher, A.; Lüthy, H.; Schumacher, M.; Loeffel, O.; Filser, F.; Kocher, P.; Gauckler, L.; Schärer, P. Clinical evaluation of zirconium oxide bridges in the posterior segments fabricated with the DCM system. Acta. Med. Dent. Helv. 2000, 5, 131–139. [Google Scholar]
- Donovan, T.E. Factors essential for successful all-ceramic restorations. J. Am. Dent. Assoc. 2008, 139 (Suppl.), 14S–18S. [Google Scholar] [CrossRef]
- Marchack, B.; Futatsuki, Y.; Marchack, C.; White, S. Customization of milled zirconia copings for all-ceramic crowns: A clinical report. J. Prosthet. Dent. 2008, 99, 163–173. [Google Scholar]
- Lawn, B.R.; Pajares, A.; Zhang, Y.; Deng, Y.; Polack, M.A.; Lloyd, I.K.; Rekow, E.D.; Thompson, V.P. Materials design in the performance of all-ceramic crown. Biomaterials 2004, 25, 2885–2892. [Google Scholar] [CrossRef] [PubMed]
- Sundh, A.; Sjögren, G. A comparison of fracture strength of yttrium-oxide- partially-stabilized zirconia ceramic crowns with varying core thickness, shapes and veneer ceramics. J. Oral Rehabil. 2004, 31, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Vult von Steyern, P.; Carlson, P.; Nilner, K. All-ceramic fixed partial dentures designed according to the DC-Zirkon technique. A 2-year clinical study. J. Oral Rehabil. 2005, 32, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Schmitter, M.; Mussotter, K.; Rammelsberg, P.; Stober, T.; Ohlmann, B.; Gabbert, O. Clinical performance of extended zirconia frameworks for fixed dental prostheses: Two-year results. J. Oral Rehabil. 2009, 36, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Wohlwend, A.; Studer, S.; Schärer., P. Das Zirkonoxidabutment- ein neues vollkeramisches Konzept zur ästhetischen Verbesserung der Suprastruktur in der Implantologie. Quintessenz Zahntech. 1996, 22, 364–381. [Google Scholar]
- Lüthy, H. Strength and toughness of dental ceramics. In Cad/cam in Aesthetic Dentistry; Mörmann, W.H., Ed.; Quintessence: Chicago, IL, USA, 1996; pp. 229–239. [Google Scholar]
- Canullo, L. Clinical outcome study of customized zirconia abutments for single-implant restorations. Int. J. Prosthodont. 2007, 20, 489–493. [Google Scholar] [PubMed]
- Glauser, R.; Wohlwend, A.; Studer, S. Application of zirconia abutments on single-tooth implants in the maxillary esthetic zone. A 6-year clinical and radiographic follow-up report. Appl. Osseointegration Res. 2004, 4, 41–45. [Google Scholar]
- Zembic, A.; Sailer, I.; Jung, R.E.; Hämmerle, C.H. Randomized-controlled clinical trial of customized zirconia and titanium implant abutments for single-tooth implants in canine and posterior regions: 3-year results. Clin. Oral Implants Res. 2009, 20, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Tan, K.; Lang, N.P.; Brägger, U.; Egger, M.; Zwahlen, M. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. Clin. Oral Implants Res. 2004, 15, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Studart, A.R.; Filser, F.; Kocher, P.; Gauckler, L.J. Fatigue of zirconia under cyclic loading in water and its implications for the design of dental bridges. Dent. Mater. 2007, 23, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, M.; Fischer, H.; Marx, R.; Edelhoff, D. In vivo fracture resistance of implant-supported all-ceramic restorations. J. Prosthet. Dent. 2003, 90, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Haraldson, T.; Carlsson, G.E.; Ingervall, B. Functional state, bite force and postural muscle activity in patients with osseointegrated oral implant bridges. Acta. Odontol. Scand. 1979, 37, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Kurun, S.; Gerds, T.; Strub, J.R. Fracture resistance of single-tooth implant-supported all-ceramic restorations after exposure to the artificial mouth. J. Oral Rehabil. 2006, 33, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.; Urgell, J.P.; Kultje, C.; Klineberg, I.; Goldberg, P.V.; Stevenson-Moore, P.; Alonso, J.M.; Schaller, M.; Corria, R.M.; Engquist, B.; et al. A 5-year multicenter study on implant-supported single crown restorations. Int. J. Oral Maxillofac. Implants 1998, 13, 212–218. [Google Scholar] [PubMed]
- Binon, P.P. The effect of implant/abutment hexagonal misfit on screw joint stability. Int. J. Prosthodont. 1996, 9, 149–160. [Google Scholar] [PubMed]
- Binon, P.P.; McHugh, M.J. The effect of eliminating implant/abutment rotational misfit on screw joint stability. Int. J. Prosthodont. 1996, 9, 511–519. [Google Scholar] [PubMed]
- Jorneus, L.; Jemt, T.; Carlsson, L. Loads and designs of screw joints for single crowns supported by osseointegrated implants. Int. J. Oral Maxillofac. Implants 1992, 7, 353–359. [Google Scholar] [PubMed]
- McGlumphy, E.A.; Mendel, D.A.; Holloway, J.A. Implant screw mechanics. Dent. Clin. North. Am. 1998, 42, 71–89. [Google Scholar] [PubMed]
- Vigolo, P.; Fonzi, F.; Majzoub, Z.; Cordioli, G. An in vivo evaluation of titanium, zirconia, and alumina procera abutments with hexagonal connection. Int. J. Oral Maxillofac. Implants 2006, 21, 575–580. [Google Scholar] [PubMed]
- Sailer, I.; Sailer, T.; Stawarczyk, B.; Jung, R.E.; Hämmerle, C.H. In vivo study of the influence of the type of connection on the fracture load of zirconia abutments with internal and external implant-abutment connections. Int. J. Oral Maxillofac. Implants 2009, 24, 850–858. [Google Scholar] [PubMed]
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin. Oral Implants Res. 2008, 19, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tete, S.; Mastrangelo, F.; Bianchi, A.; Zizzari, V.; Scarano, A. Collagen fiber orientation around machined titanium and zirconia dental implant necks: An animal study. Int. J. Oral Maxillofac. Implants 2009, 24, 52–58. [Google Scholar] [PubMed]
- Berglundh, T.; Lindhe, J.; Marinello, C.; Ericsson, I.; Liljenberg, B. Soft tissue reaction to de novo plaque formation on implants and teeth. An experimental study in the dog. Clin. Oral Implants Res. 1992, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pontoriero, R.; Tonelli, M.P.; Carnevale, G.; Mombelli, A.; Nyman, S.R.; Lang, N.P. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin. Oral Implants Res. 1994, 5, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Grössner-Schreiber, B.; Griepentrog, M.; Haustein, I.; Müller, W.D.; Lange, K.P.; Briedigkeit, H.; Göbel, U.B. Plaque formation on surface modified dental implants. An in vivo study. Clin. Oral Implants Res. 2001, 12, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Rimondini, L.; Cerroni, L.; Carrassi, A.; Torricelli, P. Bacterial colonization of zirconia ceramic surfaces: An in vivo and in vivo study. Int. J. Oral Maxillofac. Implants 2002, 17, 793–798. [Google Scholar] [PubMed]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implants 1986, 1, 11–25. [Google Scholar] [PubMed]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. 1977, Supplementum 16, 1–132. [Google Scholar]
- Buser, D.; Mericske-Stern, R.; Bernard, J.P.; Behneke, A.; Behneke, N.; Hirt, H.P.; Belser, U.C.; Lang, N.P. Long-term evaluation of non-submerged ITI implants. Part I: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin. Oral Implants Res. 1997, 8, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Jemt, T. Fixed implant-supported prostheses in the edentulous maxilla. A five- year follow-up report. Clin. Oral Implants Res. 1994, 5, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Adell, R.; Hansson, B.O.; Brånemark, P.I.; Breine, U. Intra-osseous anchorage of dental prostheses. II. Review of clinical approaches. Scand. J. Plast. Reconstr. Surg. 1970, 4, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T. Direct bone anchorage of dental implants. J. Prosthet. Dent.. 1983, 50, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.I.; Adell, R.; Albrektsson, T.; Lekholm, U.; Lindström, J.; Rockler, B. An experimental and clinical study of osseointegrated implants penetrating the nasal cavity and maxillary sinus. J. Oral Maxillofac. Sur. 1984, 42, 497–505. [Google Scholar] [CrossRef]
- Schroeder, A.; Pohler, O.; Sutter, F. Gewebsreaktion auf ein titan-hohlzylinderimplantat mit titan-spritzschichtoberfläche. Schweiz. Monatsschr. Zahnmed. 1976, 86, 713–727. [Google Scholar]
- Schroeder, A.; Stich, H.; Straumann, F.; Sutter, F. Über die anlagerung von osteozement an einen belasteten Implantatkörper. Schweiz. Monatsschr. Zahnmed. 1978, 88, 1051–1058. [Google Scholar]
- Schulte, W.; Heimke, A. Das tübinger sofort-implantat. Quintessenz. 1976, 27, 17–23. [Google Scholar] [PubMed]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Sur. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- Albrektsson, T.; Dahl, E.; Enbom, L.; Engevall, S.; Engquist, B.; Eriksson, A.R.; Feldmann, G.; Freiberg, N.; Glantz, P.-O.; Kjellman, O.; Kristersson, L.; Kvint, S.; Köndell, P.-Å.; Palmquist, J.; Werndahl, L.; Åstrand, P. Osseointegrated oral implants. A swedish multicenter study of 8139 consecutively inserted Nobelpharma implants. J. Periodontol. 1988, 59, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Tschernitschek, H.; Borchers, L.; Geurtsen, W. Nonalloyed titanium as a bioinert metal--a review. Quintessence Int. 2005, 36, 523–530. [Google Scholar] [PubMed]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin Oral Implants Res. 2008, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Keith, O.; Kusy, R.P.; Whitley, J.Q. Zirconia brackets: An evaluation of morphology and coefficients of friction. Am. J. Orthod. Dentofacial Orthop. 1994, 106, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Meyenberg, K.H.; Lüthy, H.; Schärer, P. Zirconia posts: A new all-ceramic concept for nonvital abutment teeth. J. Esthet. Dent. 1995, 7, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Oblak, C.; Jevnikar, P.; Kosmac, T.; Funduk, N.; Marion, L. Fracture resistance and reliability of new zirconia posts. J. Prosthet. Dent. 2004, 91, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Klaus, G. A zirconia implant-crown system: A case report. Int. J. Periodontics Restor. Dent. 2004, 24, 147–153. [Google Scholar]
- Blaschke, C.; Volz, U. Soft and hard tissue response to zirconium dioxide implants - a clinical study in man. Neuro. Endocrinol. Lett. 2006, 27, 69–72. [Google Scholar] [PubMed]
- Oliva, J.; Oliva, X.; Oliva, J.D. One-year follow-up of first consecutive 100 zirconia dental implants in humans: A comparison of 2 different rough surfaces. Int. J. Oral Maxillofac. Implants 2007, 22, 430–435. [Google Scholar] [PubMed]
- Volz, U.; Blaschke, C. Metal-free reconstructions with zirconia implants and zirconia crowns. Quintessence J. Dent. Technol. 2004, 2, 324–330. [Google Scholar]
- Kohal, R.; Knauf, M.; Butz, F.; Larsson, B. Clinical evaluation of a zirconia oral implant. A 1-year follow-up. Clin. Oral Implants Res. 2008, 19 (abstract 044), 848–849. [Google Scholar]
- Sandhaus, S. Tecnica e strumentario dell'impianto C.B.S. (Crystalline Bone Screw). Inf. Odontostomatol. 1968, 4, 19–24. [Google Scholar] [PubMed]
- d'Hoedt, B. 10 Jahre Tübinger Implantat aus Frialit. Eine Zwischenauswertung der Implantatdatei. Z. Zahnärztl. Implantol. 1986, 2, 6–10. [Google Scholar]
- Müller, W.; Piesold, J.; Glien, W. Eigenschaften und klinische Anwendung von Kieferimplantaten aus Aluminiumoxidkeramik Bionit. Stomatol. DDR 1988, 38, 673–678. [Google Scholar] [PubMed]
- Brinkmann, E. Das keramik-anker-implantat nach mutschelknauss. Zahnärztl. Prax. 1978, 29, 148–150. [Google Scholar] [PubMed]
- Akagawa, Y.; Hashimoto, M.; Kondo, N.; Satomi, K.; Takata, T.; Tsuru, H. Initial bone-implant interfaces of submergible and supramergible endosseous single-crystal sapphire implants. J. Prosthet. Dent. 1986, 55, 96–100. [Google Scholar] [CrossRef] [PubMed]
- McKinney, R.V.J.; Koth, D.L. The single-crystal sapphire endosteal dental implant: Material characteristics and 18-month experimental animal trials. J. Prosthet. Dent. 1982, 47, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Steflik, D.E.; Koth, D.L.; McKinney, R.V., Jr. Human clinical trials with the single crystal sapphire endosteal dental implant: Three year results, statistical analysis, and validation of an evaluation protocol. J. Oral Implantol. 1987, 13, 39–53. [Google Scholar] [PubMed]
- Koth, D.L.; McKinney, R.V.; Steflik, D.E.; Davis, Q.B. Clinical and statistical analyses of human clinical trials with the single crystal aluminum oxide endosteal dental implant: Five-year results. J. Prosthet. Dent. 1988, 60, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Klaus, G.; Strub, J.R. Zirconia-implant-supported all-ceramic crowns withstand long-term load: A pilot investigation. Clin. Oral Implants Res. 2006, 17, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.R.; Coelho, P.G.; Fernandes, C.A.; Navarro, J.M.; Dias, R.A.; Thompson, V.P. Reliability of one-piece ceramic implant. J. Biomed. Mater. Res. Part B: Appl. Biom. 2009, 88, 419–426. [Google Scholar] [CrossRef]
- Andreiotelli, M.; Kohal, R.J. Fracture strength of zirconia implants after artificial aging. Clin. Implant Dent. Relat. Res. 2009, 11, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.R.; Nourian, P.; Coelho, P.G.; Rekow, E.D.; Thompson, V.P. Impact fracture resistance of two titanium-abutment systems versus a single-piece ceramic implant. Clin. Implant Dent. Relat. Res. 2009. [Google Scholar] [CrossRef]
- Kohal, R.J.; Att, W.; Bächle, M.; Butz, F. Ceramic abutments and ceramic oral implants. An update. Periodontol. 2000 2008, 47, 224–243. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, Y.; Ichikawa, Y.; Nikai, H.; Tsuru, H. Interface histology of unloaded and early loaded partially stabilized zirconia endosseous implant in initial bone healing. J. Prosthet. Dent. 1993, 69, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, Y.; Hosokawa, R.; Sato, Y.; Kamayama, K. Comparison between freestanding and tooth-connected partially stabilized zirconia implants after two years' function in monkeys: A clinical and histologic study. J. Prosthet. Dent. 1998, 80, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Di Carlo, F.; Quaranta, M.; Piattelli, A. Bone response to zirconia ceramic implants: An experimental study in rabbits. J. Oral Implantol. 2003, 29, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Sennerby, L.; Dasmah, A.; Larsson, B.; Iverhed, M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin. Implant Dent. Relat. Res. 2005, 7 (Suppl. 1), S13–S20. [Google Scholar] [CrossRef]
- Hoffmann, O.; Angelov, N.; Gallez, F.; Jung, R.E.; Weber, F.E. The zirconia implant-bone interface: A preliminary histologic evaluation in rabbits. Int. J. Oral Maxillofac. Implants 2008, 23, 691–695. [Google Scholar] [PubMed]
- Depprich, R.; Zipprich, H.; Ommerborn, M.; Naujoks, C.; Wiesmann, H.P.; Kiattavorncharoen, S.; Lauer, H.C.; Meyer, U.; Kübler, N.R.; Handschel, J. Osseointegration of zirconia implants compared with titanium: An in vivo study. Head Face Med. 2008, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sieweke, J.H.; Rodriguez, N.A.; Schüpbach, P.; Lindström, H.; Susin, C.; Wikesjö, U.M. Evaluation of nano-technology-modified zirconia oral implants: A study in rabbits. J. Clin. Periodontol. 2009, 36, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Wolkewitz, M.; Hinze, M.; Han, J.S.; Bächle, M.; Butz, F. Biomechanical and histological behavior of zirconia implants: An experiment in the rat. Clin. Oral Implants Res. 2009, 20, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Rocchietta, I.; Fontana, F.; Addis, A.; Schüpbach, P.; Simion, M. Surface-modified zirconia implants: Tissue response in rabbits. Clin. Oral Implants Res. 2009, 20, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Gahlert, M.; Gudehus, T.; Eichhorn, S.; Steinhauser, E.; Kniha, H.; Erhardt, W. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin. Oral Implants Res. 2007, 18, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Gahlert, M.; Röhling, S.; Wieland, M.; Eichhorn, S.; Küchenhoff, H.; Kniha, H. A comparison study of the osseointegration of zirconia and titanium dental implants. A biomechanical evaluation in the maxilla of pigs. Clin. Implant. Dent. Relat. Res. 2009. [Google Scholar] [CrossRef]
- Gahlert, M.; Röhling, S.; Wieland, M.; Sprecher, C.M.; Kniha, H.; Milz, S. Osseointegration of zirconia and titanium dental implants: A histological and histomorphometrical study in the maxilla of pigs. Clin. Oral Implants Res. 2009, 20, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Lambrich, M.; Iglhaut, G. Vergleich der überlebensrate von zirkondioxid- und titanimplantaten. Z. Zahnärztl. Implantol. 2008, 24, 182–191. [Google Scholar]
- Mellinghoff, J. Erste klinische ergebnisse zu dentalen schraubenimplantaten aus zirkonoxid. Z. Zahnärztl. Implantol. 2006, 22, 288–293. [Google Scholar]
- Kohal, R.J.; Weng, D.; Bächle, M.; Strub, J.R. Loaded custom-made zirconia and titanium implants show similar osseointegration: An animal experiment. J. Periodontol. 2004, 75, 1260–1266. [Google Scholar] [CrossRef]
- Russias, J.; Saiz, E.; Deville, S.; Gryn, K.; Liu, G.; Nalla, R.K.; Tomsia, A.P. Fabrication and in vivo characterization of three-dimensional organic/inorganic scaffolds by robocasting. J. Biomed. Mater. Res. A. 2007, 83, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kim, J.W. Graded structures for damage resistant and aesthetic all-ceramic restorations. Dent. Mater. 2009, 25, 781–790. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Silva, N.R.F.A.; Sailer, I.; Zhang, Y.; Coelho, P.G.; Guess, P.C.; Zembic, A.; Kohal, R.J. Performance of Zirconia for Dental Healthcare. Materials 2010, 3, 863-896. https://doi.org/10.3390/ma3020863
Silva NRFA, Sailer I, Zhang Y, Coelho PG, Guess PC, Zembic A, Kohal RJ. Performance of Zirconia for Dental Healthcare. Materials. 2010; 3(2):863-896. https://doi.org/10.3390/ma3020863
Chicago/Turabian StyleSilva, Nelson R.F.A., Irena Sailer, Yu Zhang, Paulo G. Coelho, Petra C. Guess, Anja Zembic, and Ralf J. Kohal. 2010. "Performance of Zirconia for Dental Healthcare" Materials 3, no. 2: 863-896. https://doi.org/10.3390/ma3020863





