2.1. Synthesis and characterization of new element organic frameworks (EOF-3, -4, and -5)
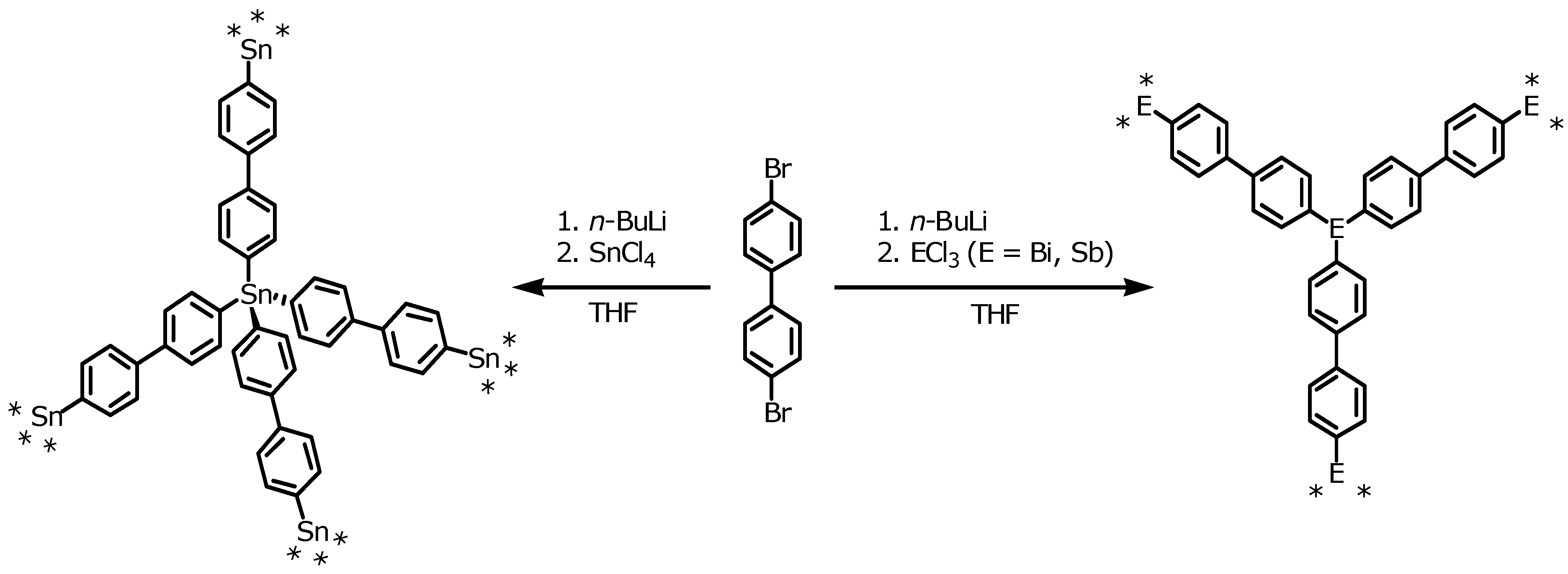
The synthesis of three new element organic frameworks based on Sn (EOF-3), Sb (EOF-4), and Bi (EOF-5) atoms as connectors between bifunctional organic linker molecules by direct element-carbon bond is shown in
Scheme 1.
Scheme 1.
Synthesis of EOF-3 (left), EOF-4, and EOF-5 (right).
Scheme 1.
Synthesis of EOF-3 (left), EOF-4, and EOF-5 (right).
With the two-fold lithiation of 4,4’-dibromobiphenyl and subsequent reaction with the respective element chloride, the EOFs can be synthesized in a one-step procedure. The resulting white to pale-grey powders of all three materials are insoluble in common solvents indicating the formation of a three-dimensional highly cross-linked framework. The materials are thermally stable in air up to 473 K (DTA/TG see
Figure S1). The stability is a little bit lower than for EOFs containing silicon as connector such as EOF-2 but sufficient for thermal removal of remaining solvent molecules [
11].
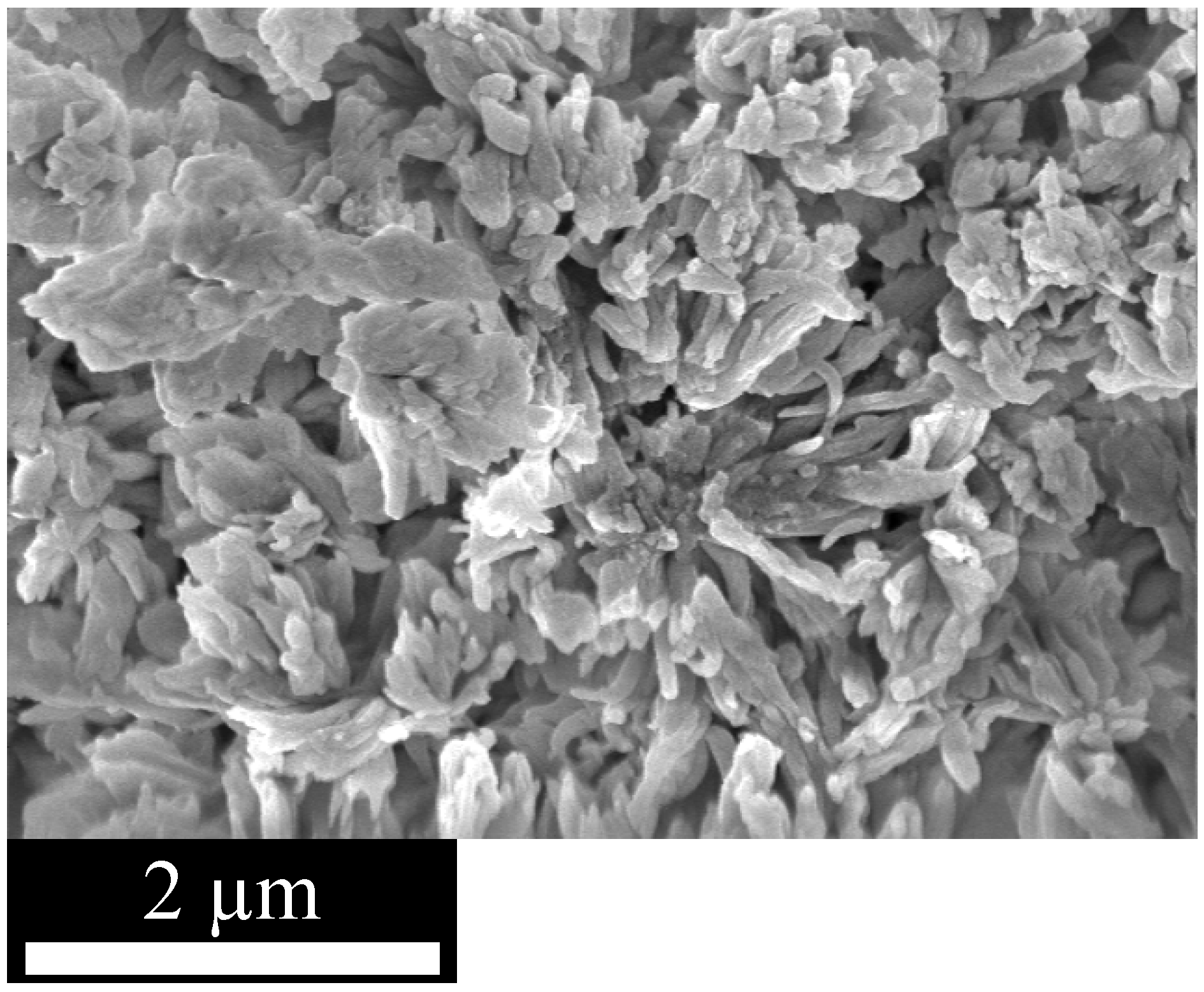
The morphology of the particles in the fine powders was investigated using scanning electron microscopy (SEM). The micrographs of all three materials look very similar (
Figure 1). They show a very heterogeneous distribution of the particle size in the lower micrometer range and relatively undefined particle shapes.
Figure 1.
SEM micrograph of EOF-5 shows very heterogeneous particle size and shape distribution. Micrographs of EOF-3 and -4 are not shown but look similar.
Figure 1.
SEM micrograph of EOF-5 shows very heterogeneous particle size and shape distribution. Micrographs of EOF-3 and -4 are not shown but look similar.
The framework materials are X-ray amorphous (not shown), as expected due to the kinetic controlled reaction path including the formation of covalent bonds that are irreversible under the present synthesis conditions. The composition, short range order and the close vicinity of linkers and connectors was investigated using elemental analysis (C, H),
Fourier transformed infrared spectroscopy (FT-IR) and solid state nuclear magnetic resonance spectroscopy (NMR). Under ideal conditions, the products have a chemical composition of Sn(4,4’-biphenyl)
2, Sb
2(4,4’-biphenyl)
3 and Bi
2(4,4’-biphenyl)
3 (
Scheme 1). The results of the elemental analysis (
Table S2) nearly confirm the calculated weight percents of carbon and hydrogen. The differences could be a result of a partial substitution of tin, antimony, or bismuth with aliphatic or hydroxy groups. Further proof is given by FT-IR and solid state NMR analysis described in the following sections.
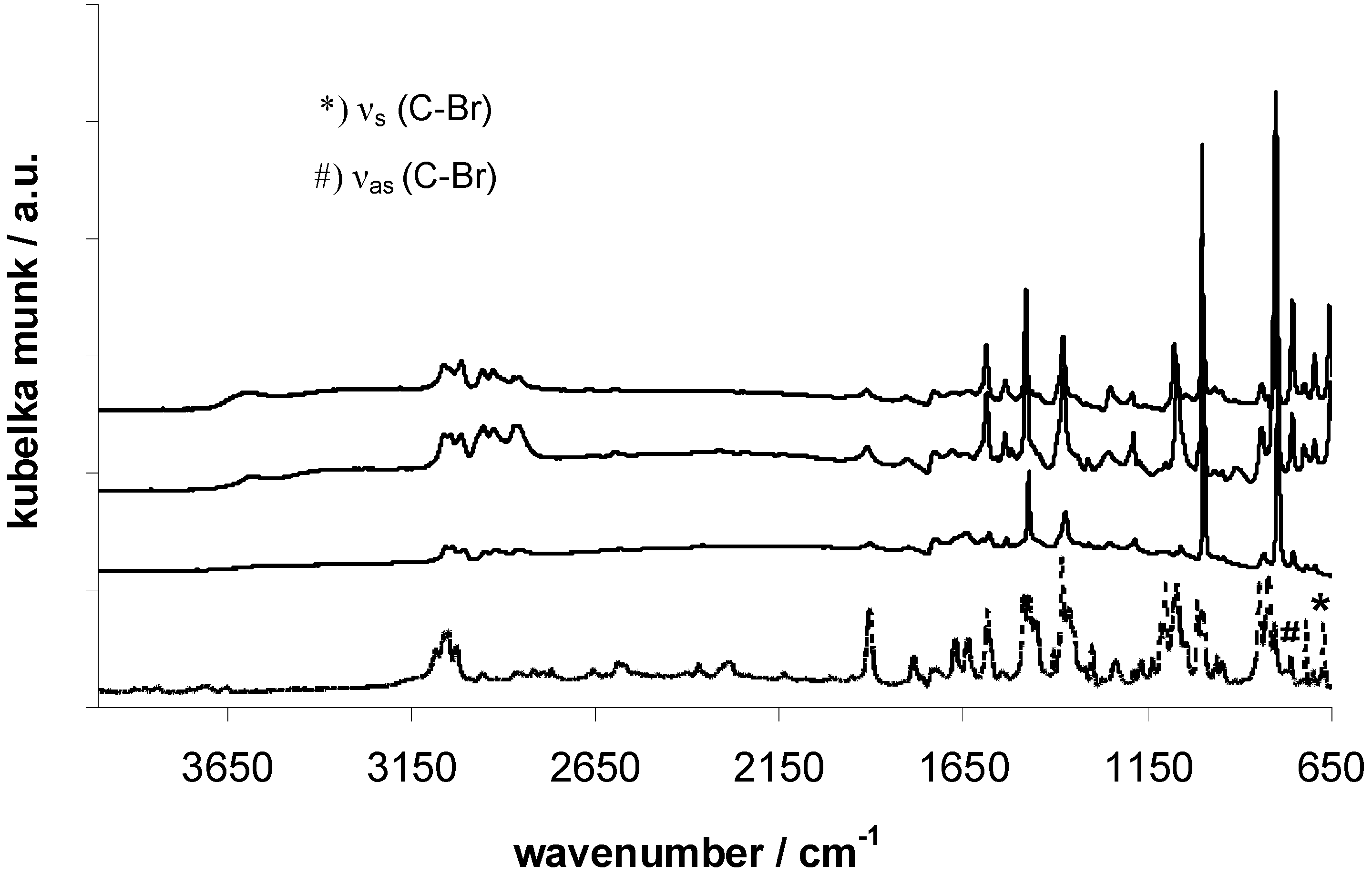
FT-IR analysis shows the successful halogen-metal-exchange of 4,4’-dibromobiphenyl in all three cases, according to the absence of ν
s (C-Br) at 671 cm
-1 and ν
as (C-Br) at 760 cm
-1 in the EOF-spectra (
Figure 2) [
17,
18]. The signals at 2950 cm
-1 indicate an aliphatic substitution of tin, antimony or bismuth as a result of the lithiation with
n-butyllithium and therefore residual reactive molecules at the moment of the framework formation. Another explanation for the aliphatic groups can be a decomposition reaction of buthyl lithium with the solvent tetrahydrofurane at higher reaction temperatures as mentioned in the literature before [
19] and resulting inclusion of aliphatic chains in the framework. But also the aromatic linker molecules show several signals between 3000 and 3100 cm
-1 as a result of aromatic C-H-group vibrations [
20]. The broad band at 3606 cm
-1 indicates the presence of Sn-OH and Sb-OH groups in the respective EOFs as a result of residual chlorine by reaction with water molecules.
The results of the
1H MAS NMR measurement (
Figure S2) confirm the presence of aliphatic groups by a signal at a chemical shift of around 0 ppm and the aromatic linkers with a signal maximum at 6.3 ppm. Additionally, the shoulder in the spectrum of EOF-4 at 3.5 ppm indicates the presence of metallic hydroxy groups, confirming the assumption from the FT-IR data presented in
Figure 2.
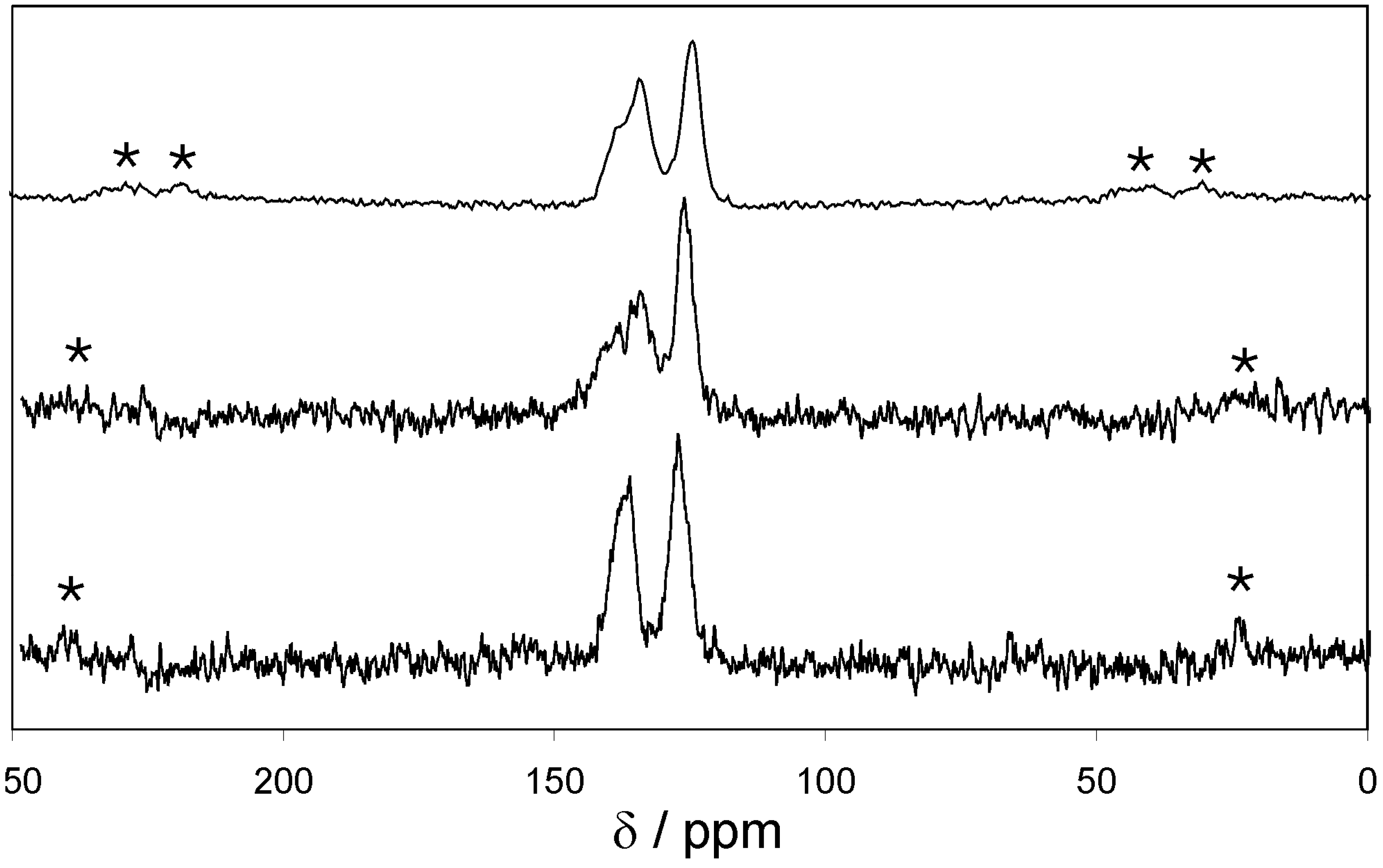
The
13C CP MAS NMR spectra of EOF-3 to -5 (
Figure 3) show two strong signals in a chemical shift region of 120–140 ppm resulting from the aromatic biphenyl units as the main compound of the organic frameworks. The ratio of aliphatic groups is too small to be detected by this method. Signals should appear below 40 ppm.
Figure 2.
FT-IR spectra of EOF-3 (top), -4 (middle) and -5 (bottom) in comparison with 4,4’-dibrombiphenyl (dashed line).
Figure 2.
FT-IR spectra of EOF-3 (top), -4 (middle) and -5 (bottom) in comparison with 4,4’-dibrombiphenyl (dashed line).
Figure 3.
13C CP MAS NMR spectra of EOF-3 (top), -4 (middle), and -5 (bottom) (* spinning sidebands).
Figure 3.
13C CP MAS NMR spectra of EOF-3 (top), -4 (middle), and -5 (bottom) (* spinning sidebands).
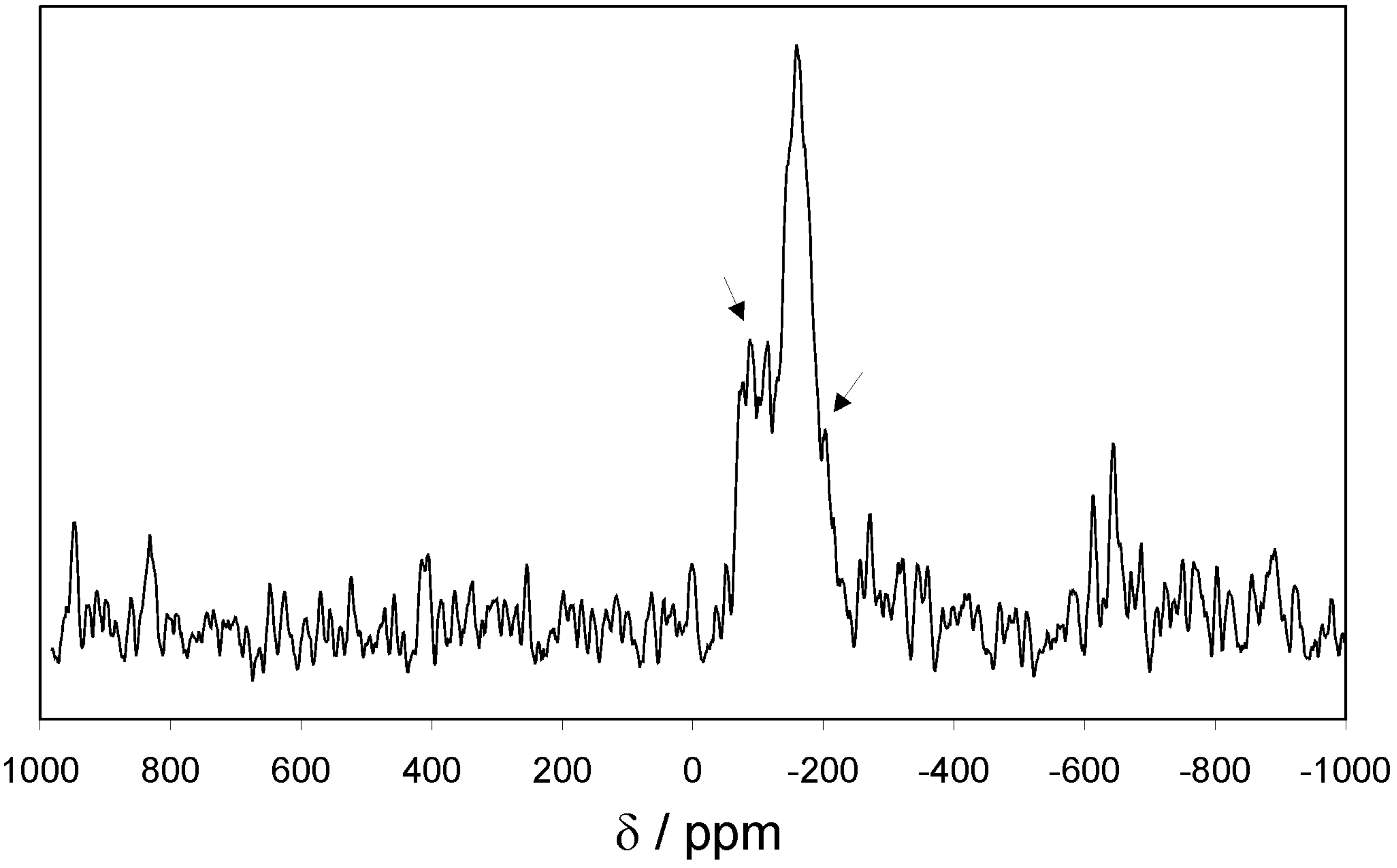
Additionally, the tin containing EOF-3 was investigated using
119Sn MAS NMR spectroscopy (
Figure 4). Only one strong signal at a chemical shift of -160 ppm can be observed, indicating a tin-phenyl-species. All the smaller signals in the close neighbourhood are satellites from the coupling with
117Sn. No signal indicating the substitution of tin by aliphatic or hydroxyl groups can be detected.
The main conclusion from the structural investigation of the frameworks by FT-IR and NMR spectroscopy is that the framework consists mainly of aromatic biphenyl units that are connected by the respective elements. Only a small amount of aliphatic substituents from side reactions with the buthyl lithium and maybe from decomposition reactions of buthyl lithium with the solvent tetrahydrofurane can be detected. Also small amounts of hydroxy groups connected to the elements were found (
Scheme 2).
Figure 4.
119Sn MAS NMR spectra of EOF-3 (→ satellites of 117Sn coupling).
Figure 4.
119Sn MAS NMR spectra of EOF-3 (→ satellites of 117Sn coupling).
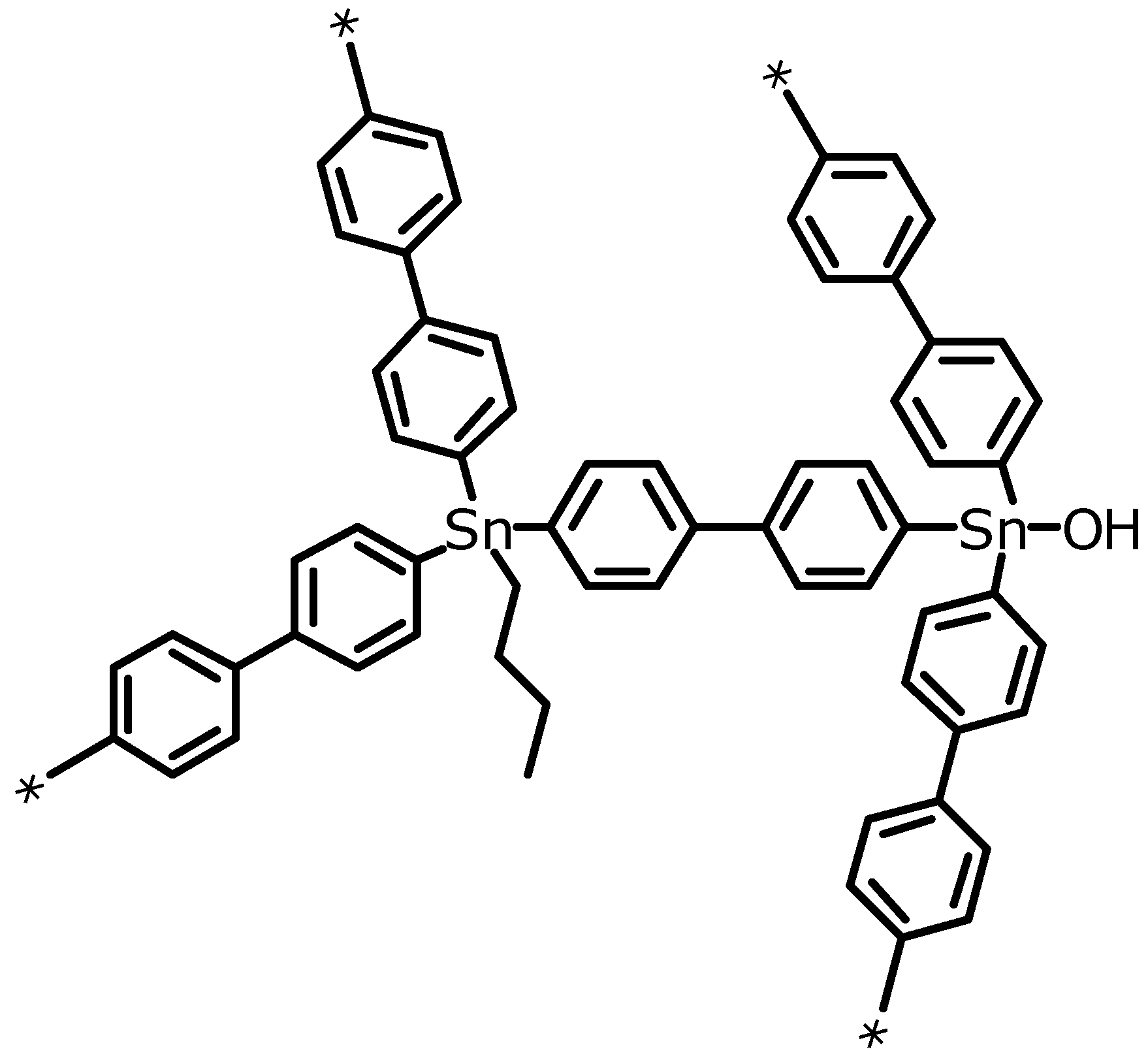
Scheme 2.
Schematic representation of the three-dimensional framework structure of EOF-3 including additional aliphatic and hydroxy groups.
Scheme 2.
Schematic representation of the three-dimensional framework structure of EOF-3 including additional aliphatic and hydroxy groups.
2.2. Characterization of physisorption properties
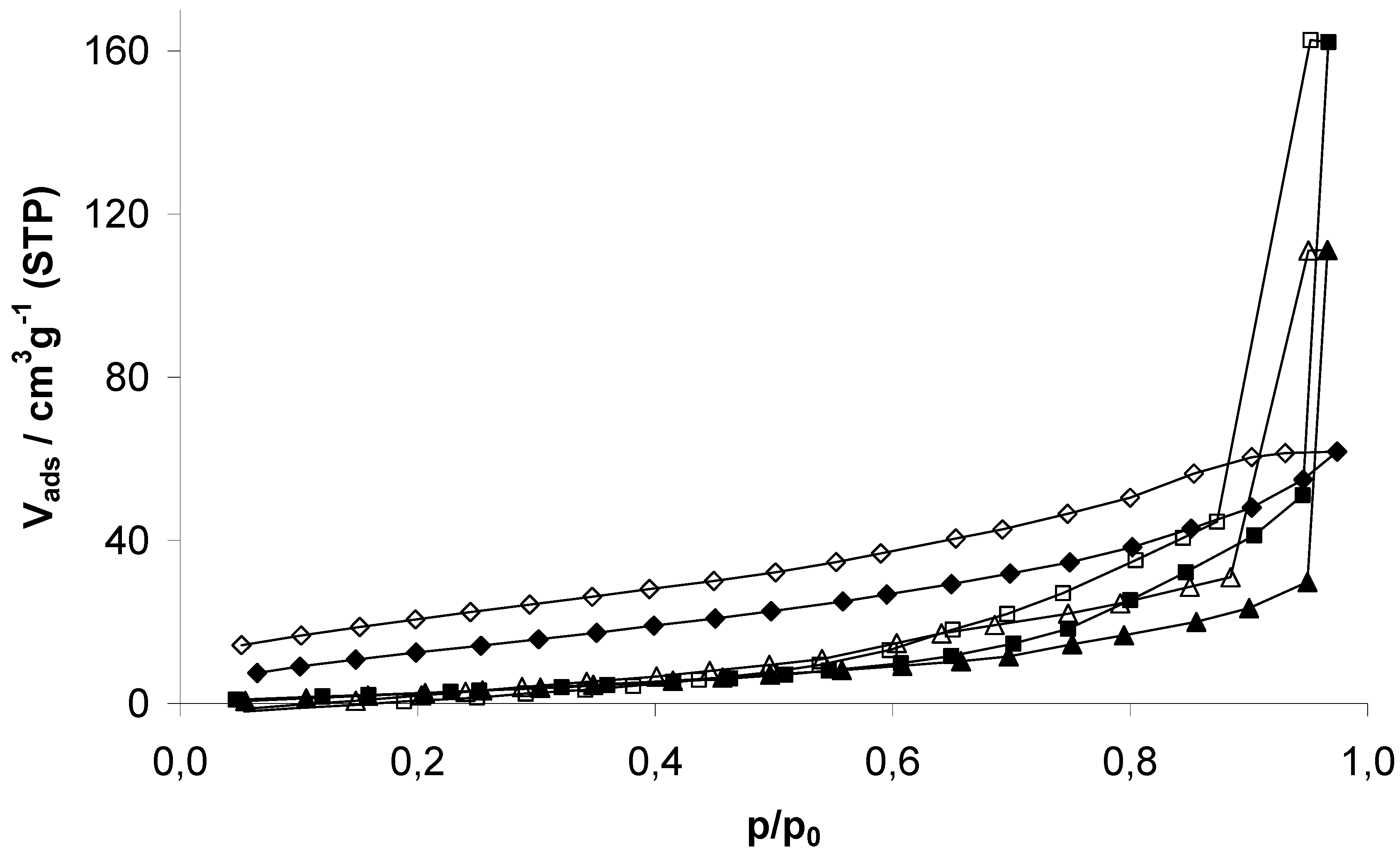
EOF-3, -4, and -5 show moderate porosity determined by nitrogen physisorption experiments at 77 K (
Figure 5). The isotherms shape can be described as a combination of type I and II according to the IUPAC classification. The steep increase in nitrogen uptake at very low pressures indicates the presence of micropores. Further continuous increase of the uptake is a result of the high external surface area due to the presence of very small particles, also indicated by the increasing capacity at p/p
0 > 0.9 by adsorption in interparticular voids. All isotherms show a hysteresis over the whole pressure range as a result of the amorphous flexible framework. This effect was already described in several publications dealing with porous polymers [
9,
10].
EOF-3 to -5 exhibit a specific surface area (SSA) of 445, 423 and 261 m
2g
-1 (BET), respectively. The t-plot method for determination of the micropore volume and differentiation between internal and external surface area shows a significant ratio of external surface area due to very small particles. All determined values concerning the porosity of the three materials are summarized in
Table 1.
The pore size distribution was investigated using the quenched solid density functional theory (QSDFT) equilibrium model for nitrogen on carbon at 77 K with slit pores. So far, there are no models available for materials other than carbon and silica. But for qualitative predictions and the comparison of similar materials other than carbon this method should be sufficient. Especially the organic framework structures should be comparable to porous carbon materials. One should be aware of the general limitations in quantitative determination of pore size distribution.
Figure 5.
Nitrogen physisorption isotherms of EOF-3 (diamonds), -4 (squares) and -5 (triangles) measured at 77 K.
Figure 5.
Nitrogen physisorption isotherms of EOF-3 (diamonds), -4 (squares) and -5 (triangles) measured at 77 K.
Table 1.
Porosity characterization results for EOF-3 to -5.
Table 1.
Porosity characterization results for EOF-3 to -5.
| | | EOF-3 | EOF-4 | EOF-5 |
|---|
| SSABET (p/p0 = 0.05-0.15) | m2g-1 | 445 | 423 | 261 |
| SSAmicro(t-Plot) | m2g-1 | 246 | 272 | 127 |
| SSAexternal(t-Plot) | m2g-1 | 140 | 152 | 134 |
| Vmicro (Gurvich, p/p0 = 0.2) | cm3g-1 | 0.19 | 0.18 | 0.11 |
| Vtotal (Gurvich, p/p0 = 0.9) | cm3g-1 | 0.27 | 0.27 | 0.19 |
| Vmicro (DFT) | cm3g-1 | 0.26 | 0.30 | 0.22 |
| Vmicro (t-Plot, p/p0 = 0.2-0.5) | cm3g-1 | 0.13 | 0.12 | 0.05 |
| VH2O(p/p0 = 0.95) | cm3g-1 | 0.044 | 0.041 | 0.024 |
| VH2O/Vmicro(N2,p/p0 = 0.2)*100 | % | 16 | 15 | 13 |
| Vads(H2, STP) | cm3g-1 | 60 | 63 | 29 |
| H2 uptake | wt % | 0.55 | 0.56 | 0.26 |
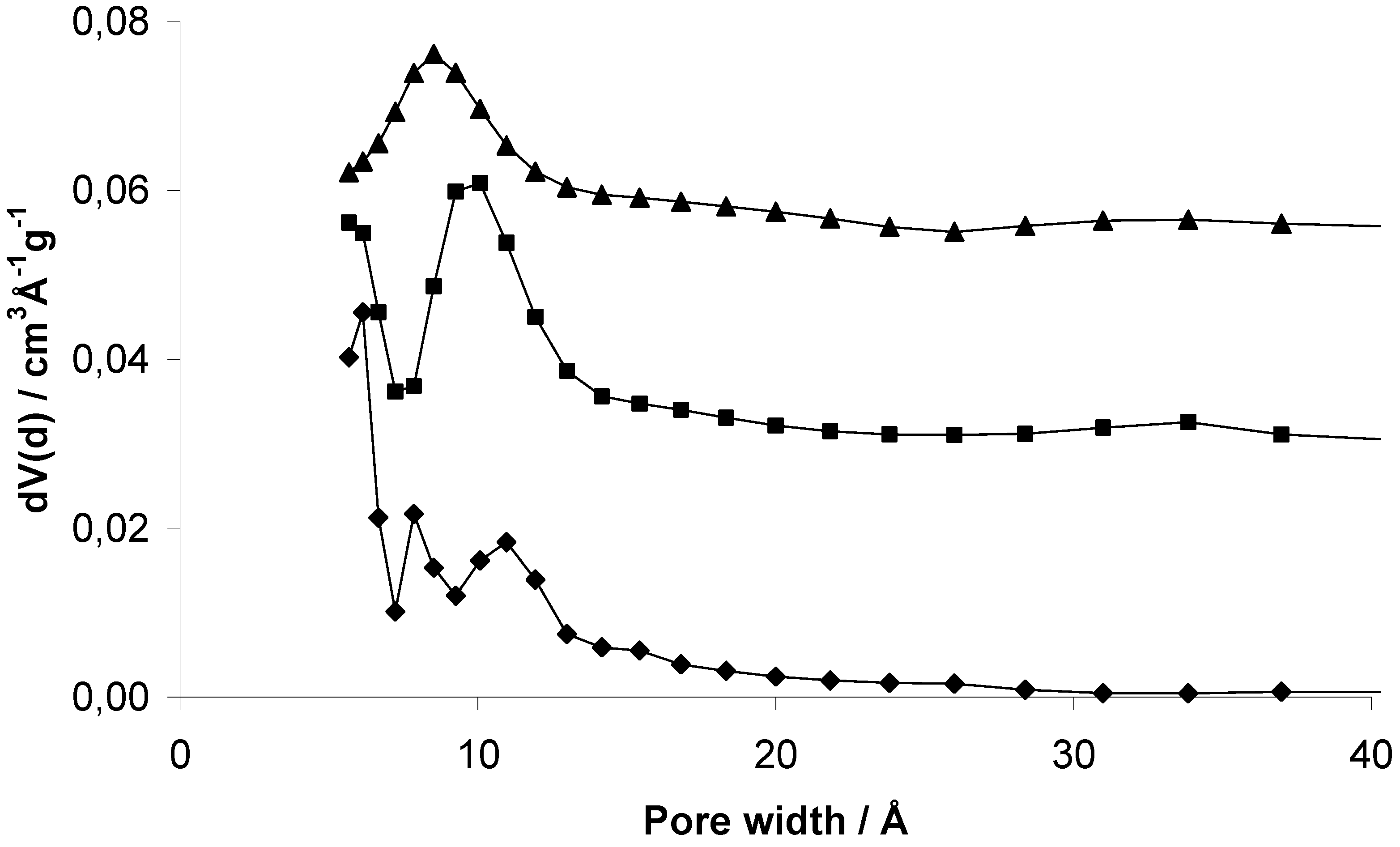
However, all three samples show a broad distribution mainly of micropores (
Figure 6). EOF-3 shows two maxima at 8 and 11 Å while EOF-4 and -5 exhibit one broad maximum at 10 and 9 Å, respectively. The comparison of the corresponding micropore volumes with different methods (
Table 1) leads to very different results. The values determined by DFT are much higher than the values determined by the t-plot method or by the method according to
Gurvich. The reason for this could be the high ratio of external surface area that is not taken into account using the DFT method.
Figure 6.
Pore size distribution of EOF-3 (diamonds), -4 (squares, offset: 0.03) and -5 (triangles, offset: 0.055) determined using the quenched solid density functional theory (QSDFT) equilibrium model for nitrogen on carbon at 77 K with slit pores.
Figure 6.
Pore size distribution of EOF-3 (diamonds), -4 (squares, offset: 0.03) and -5 (triangles, offset: 0.055) determined using the quenched solid density functional theory (QSDFT) equilibrium model for nitrogen on carbon at 77 K with slit pores.
Hydrogen uptake capacities were measured at 77 K up to a pressure of 1 bar (
Figure S3). EOF-3 and -4 show very similar values of 0.55 and 0.56 wt %, respectively. The slightly higher capacity of EOF-4, which has a lower total SSA, can be explained by the higher ratio of internal micropore surface area in comparison to the external surface area determined by the t-plot method (
Table 1). As expected, the significantly lower SSA of EOF-5 results in a much lower hydrogen capacity of 0.26 wt %.
Water vapor physisorption was measured at 298 K to investigate the polarity of the surface (
Figure 7). All three materials show very low adsorption at low relative pressure, which is slightly increasing with increasing humidity. At high relative pressure >0.95 a steep increase in water uptake can be observed for EOF-4 and -5, indicating water condensation in the cell maybe due to the high external surface area. The convex shape of the isotherms in relation to the x-axis indicates very weak interactions between the water molecules and the adsorbent surface. A semi-quantitative approach for polarity characterization is the comparison of the pore volumes at defined relative pressures from the water vapor and nitrogen physisorption isotherms. The ratio of these two values can be interpreted as a pore filling degree. Assuming the adsorption begins on more polar sites in the framework, e.g., like Sn-OH groups, water clusters start growing. But the highly non-polar surface on the organic linkers shows too low interaction with water molecules for adsorption. Therefore, remaining voids in the pores that are not filled during water adsorption can be identified. In case of our materials the ratio of the pore volume from water adsorption at p/p
0 = 0.95 (assumption: all pores are filled and before water condensation in the cell) and from nitrogen adsorption in micropores at p/p
0 = 0.2 was determined (
Table 1). For all three EOFs pore filling degrees between 10-20% were observed, indicating a highly non-polar surface area.
Figure 7.
Water vapor physisorption isotherms of EOF-3 (diamonds), -4 (squares) and -5 (triangles) measured at 298 K.
Figure 7.
Water vapor physisorption isotherms of EOF-3 (diamonds), -4 (squares) and -5 (triangles) measured at 298 K.
2.3. Catalysis
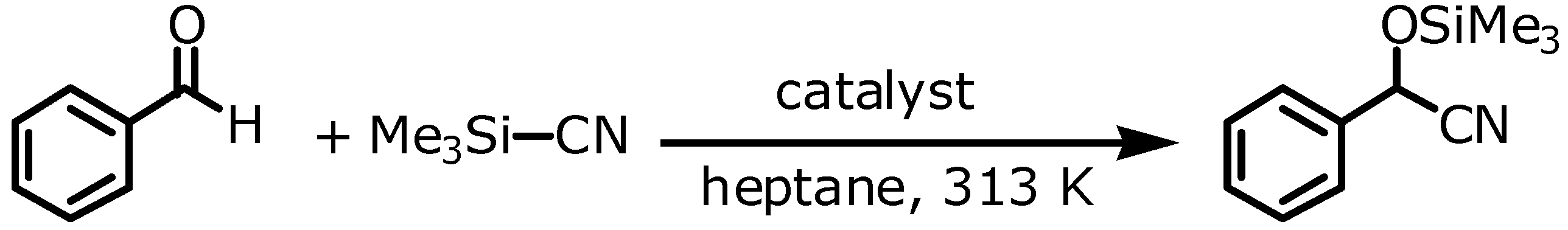
The cyanosilylation of aldehydes or ketones is a convenient route to cyanohydrin derivates using molecular or solid Lewis acid or base catalysts (
Scheme 3). Mukaiyama
et al. found that trimethylsilylcyanide reacts smoothly with aldeydes in the presence of Lewis base catalysts such as tributylphosphine or triphenylantimony in excellent yields [
21]. Also the homogeneously catalyzed cyanosilylation of benzaldehyde with triphenylbismuthine was successful [
22].
Scheme 3.
Cyanosilylation of benzaldehyde.
Scheme 3.
Cyanosilylation of benzaldehyde.
We used the addition of trimethylsilylcyanide to benzaldehyde as a test reaction to evaluate the catalytic activity of the element organic frameworks (EOF-3 to -5) described in the foregoing sections. Furthermore, the heterogeneity of the reaction mechanism was investigated. For all tests, the respective EOF was activated in vacuum at 353 K overnight. Subsequently, the reactants and the solvent were added and the reaction was monitored using GC-MS analysis. All three materials, EOF-3 as well as EOF-4 and EOF-5, are catalytically active in cyanosilylation of benzaldehyde (
Figure 8).
Figure 8.
Product concentration during the cyanosilylation of benzaldehyde using EOF-3 (circles), EOF-4 (triangles) and EOF-5 (diamonds) as catalysts.
Figure 8.
Product concentration during the cyanosilylation of benzaldehyde using EOF-3 (circles), EOF-4 (triangles) and EOF-5 (diamonds) as catalysts.
Using EOF-3 and EOF-4 as catalyst, all benzaldehyde is converted after 24 h. With the catalyst EOF-5, a nearly complete consumption is already detected after 12 h. The higher catalytic activity of the bismuth containing EOF-5 in comparison to the antimony analogon EOF-4 confirms the
1H MAS NMR data (
Figure S2). The shoulder at 3.5 ppm in the spectrum of EOF-4 indicates the presence of metallic hydroxy groups, suggesting a lower number of active centers in EOF-4 compared to EOF-5.
According to the results presented here, all three EOFs have a higher activity towards the cyanosilylation of benzaldehyde as compared to other porous materials such as the metal-organic framework HKUST-1 showing only moderate yields of 57% after a duration of 72 h [
15]. Comparing the catalytic activity with MIL-101 [
13], the EOFs show an inferior performance but with regard to the toxicity of chromium the use of MIL-101 is limited.
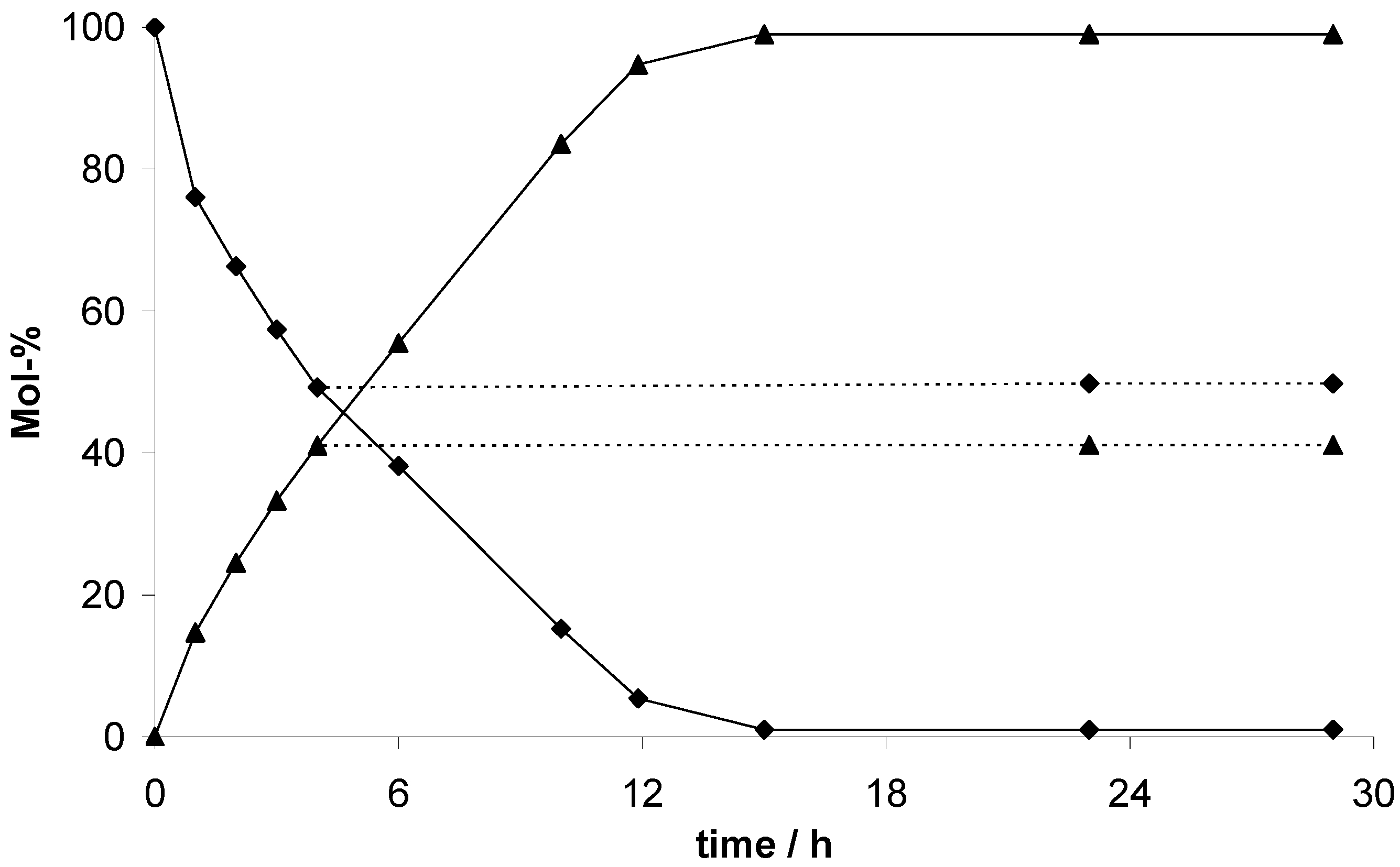
To demonstrate the heterogeneous mechanism of the cyanosilylation catalyzed by the EOFs, the catalyst was filtered off after 4 h (EOF-5) or 6 h (EOF-3 and -4) reaction time and stirring of the filtrate was continued under the same conditions. The reaction mixture was examined with GC-MS analysis again and the concentration of benzaldehyde and the product are shown in
Figure 9. The filtration test evidences no change of compound concentration with proceeding reaction time proving the mechanism to be heterogeneous.
Figure 9.
Consumption of benzaldehyde (diamonds) and product yield (triangles) during the EOF-5 catalyzed cyanosilylation with filtration test (dotted lines).
Figure 9.
Consumption of benzaldehyde (diamonds) and product yield (triangles) during the EOF-5 catalyzed cyanosilylation with filtration test (dotted lines).
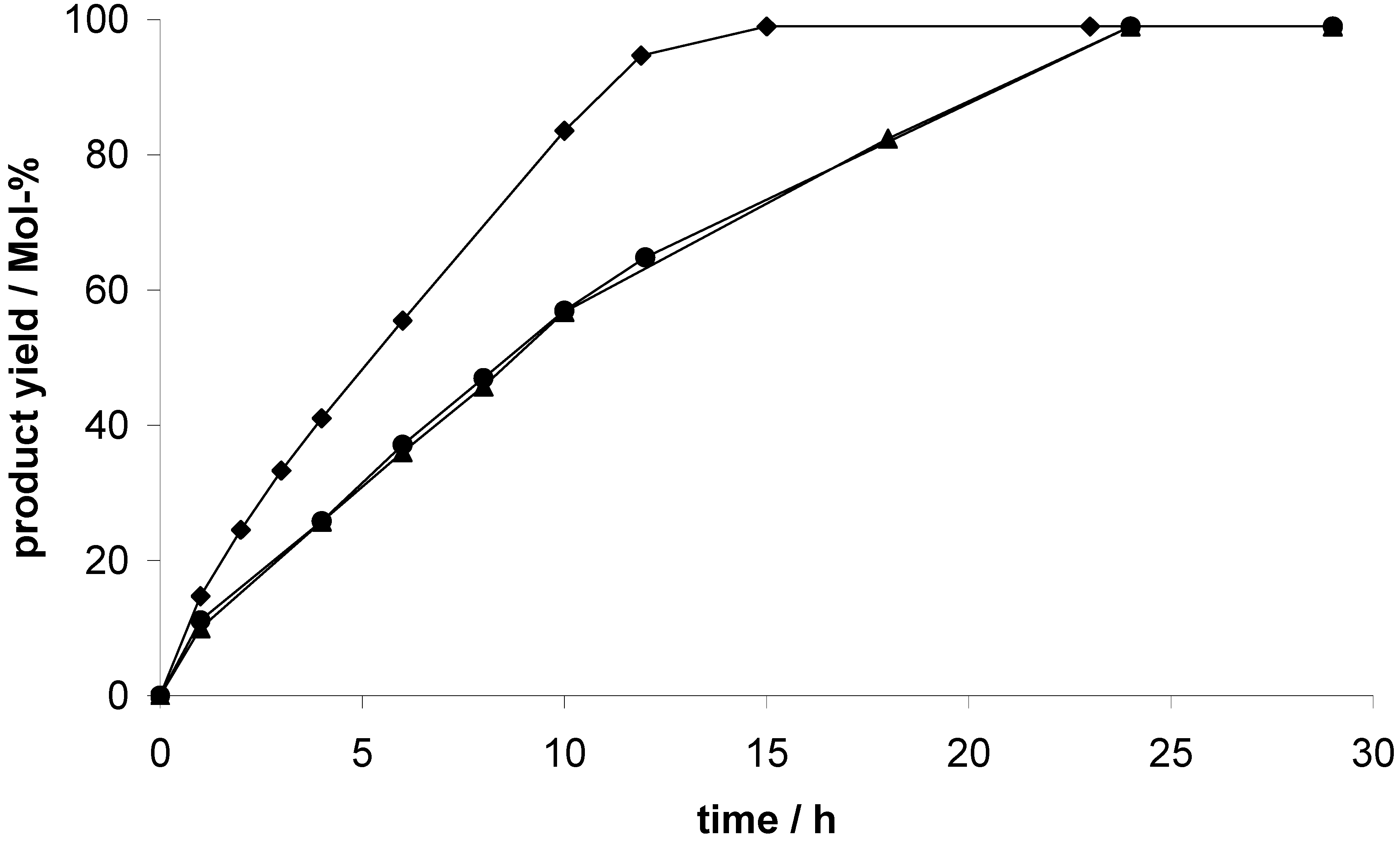
Considering the recyclability of the catalysts, a recycling test with three consecutive runs was performed. After each run, the catalyst was activated again in vacuum at 353 K overnight. Exemplified for EOF-5, the results of the recycling test are shown in
Figure 10. As mentioned before, a product yield of 99% is achieved after 12 h in the first run. In the second and the third run, an almost complete conversion to the product is detected after 15 h. A slight decrease of the reaction rate from the first to the second run is observed but after the second run there is no further loss of activity. Consequently, the EOFs can be reused several times as catalyst in the cyanosilylation of benzaldehyde with faintly reduced activity.
Figure 10.
Product concentration during the EOF-5 catalyzed cyanosilylation of benzaldehyde, three consecutive runs were monitored: 1st run (circles), 2nd run (triangles), 3rd run (diamonds).
Figure 10.
Product concentration during the EOF-5 catalyzed cyanosilylation of benzaldehyde, three consecutive runs were monitored: 1st run (circles), 2nd run (triangles), 3rd run (diamonds).