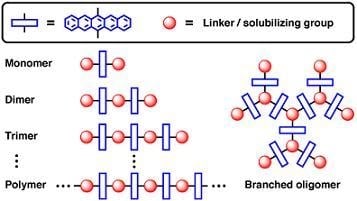
Oligomers and Polymers Based on Pentacene Building Blocks
Abstract
:1. Introduction

2. Non-Conjugated Oligomers and Polymers
2.1. Non-conjugated Pentacene Oligomers
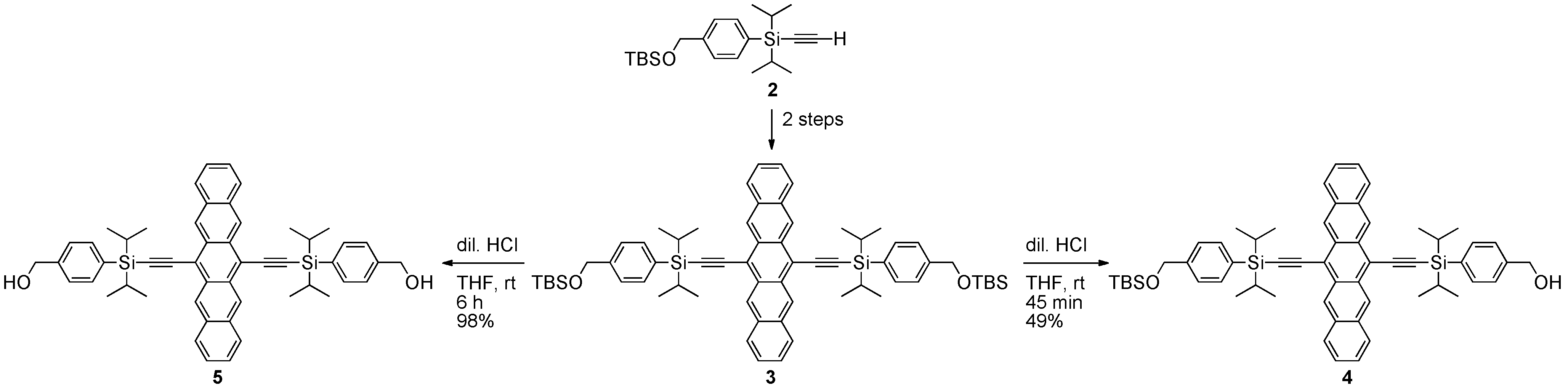
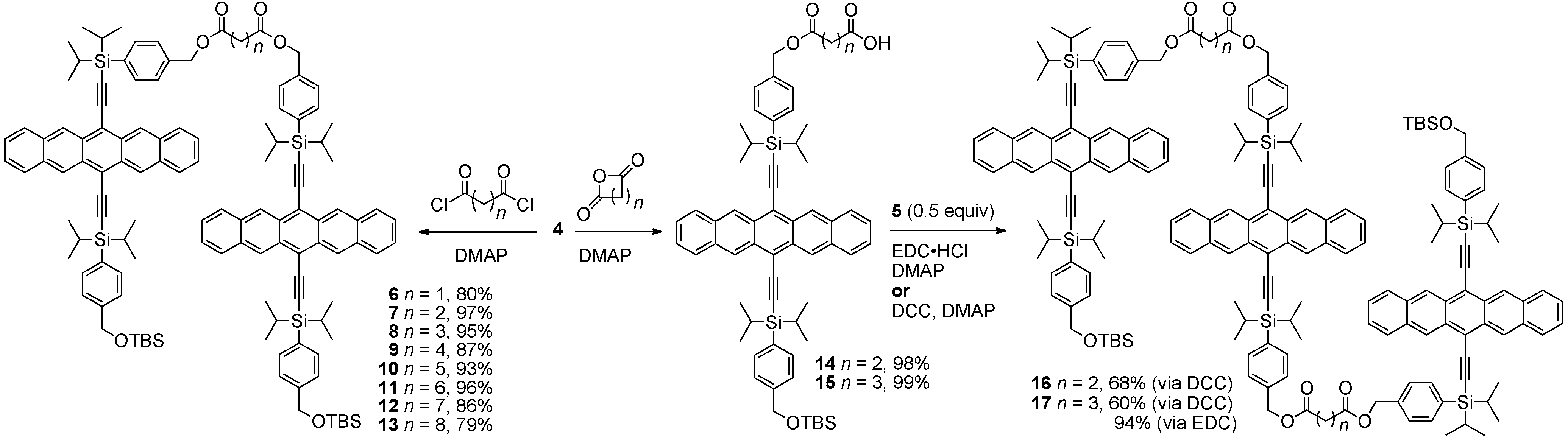
2.2. Non-conjugated Pentacene Polymers
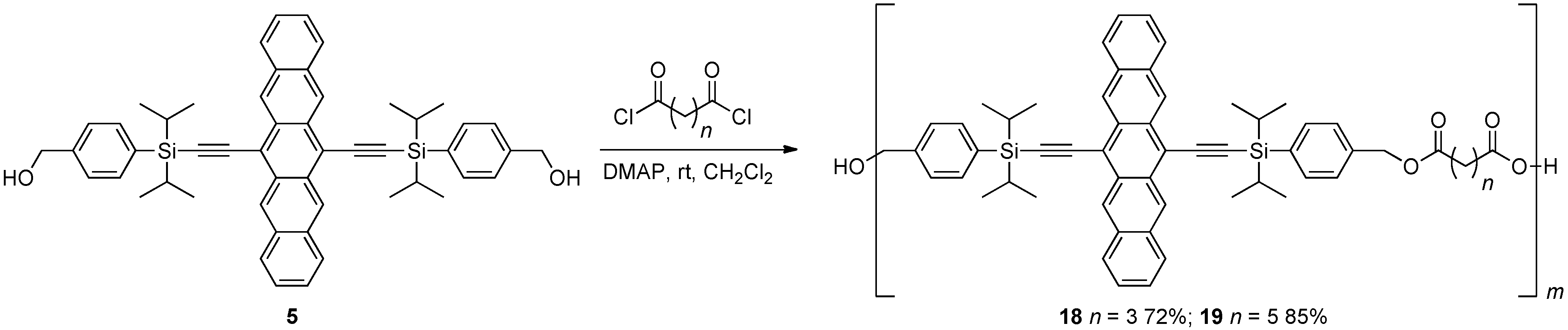
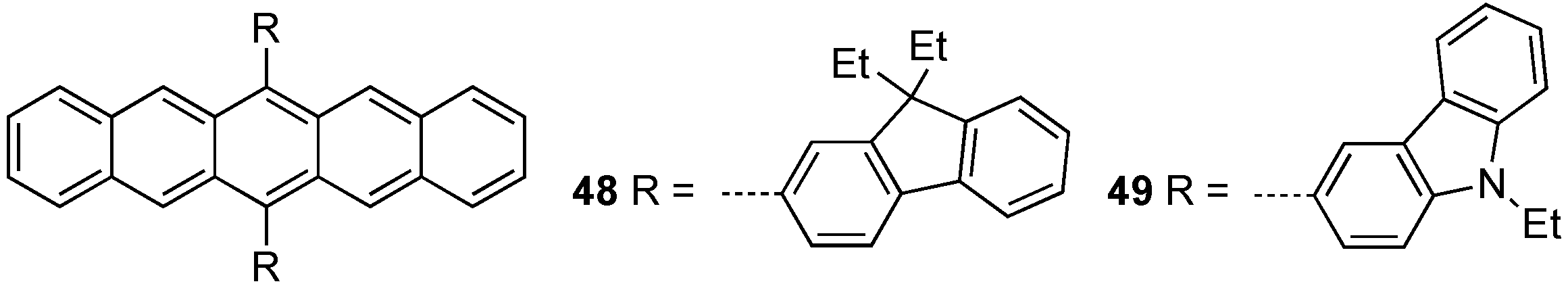
2.3. Non-Conjugated Pentacene Dendrimers
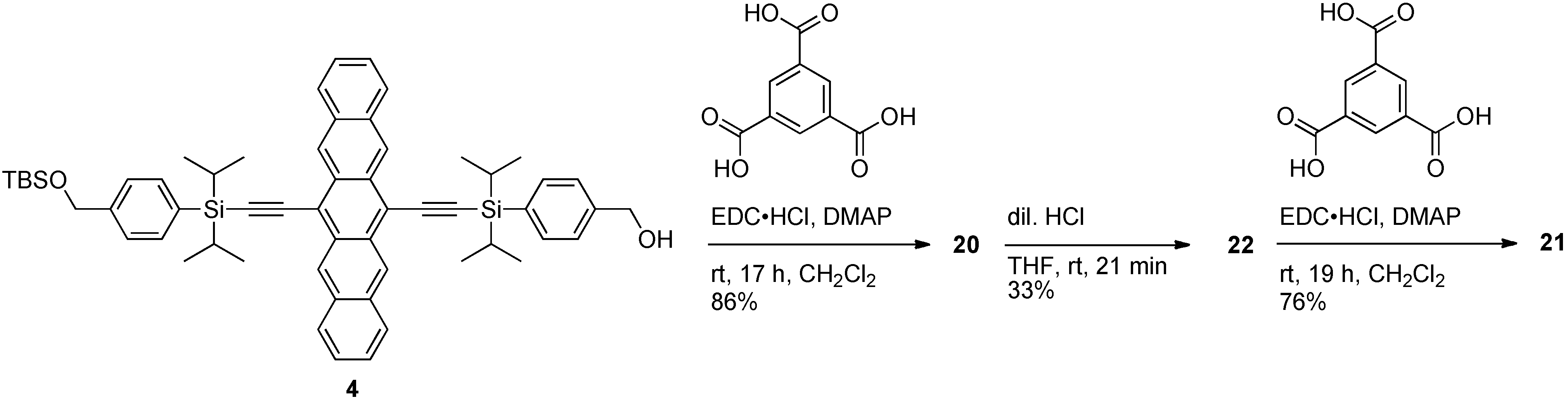
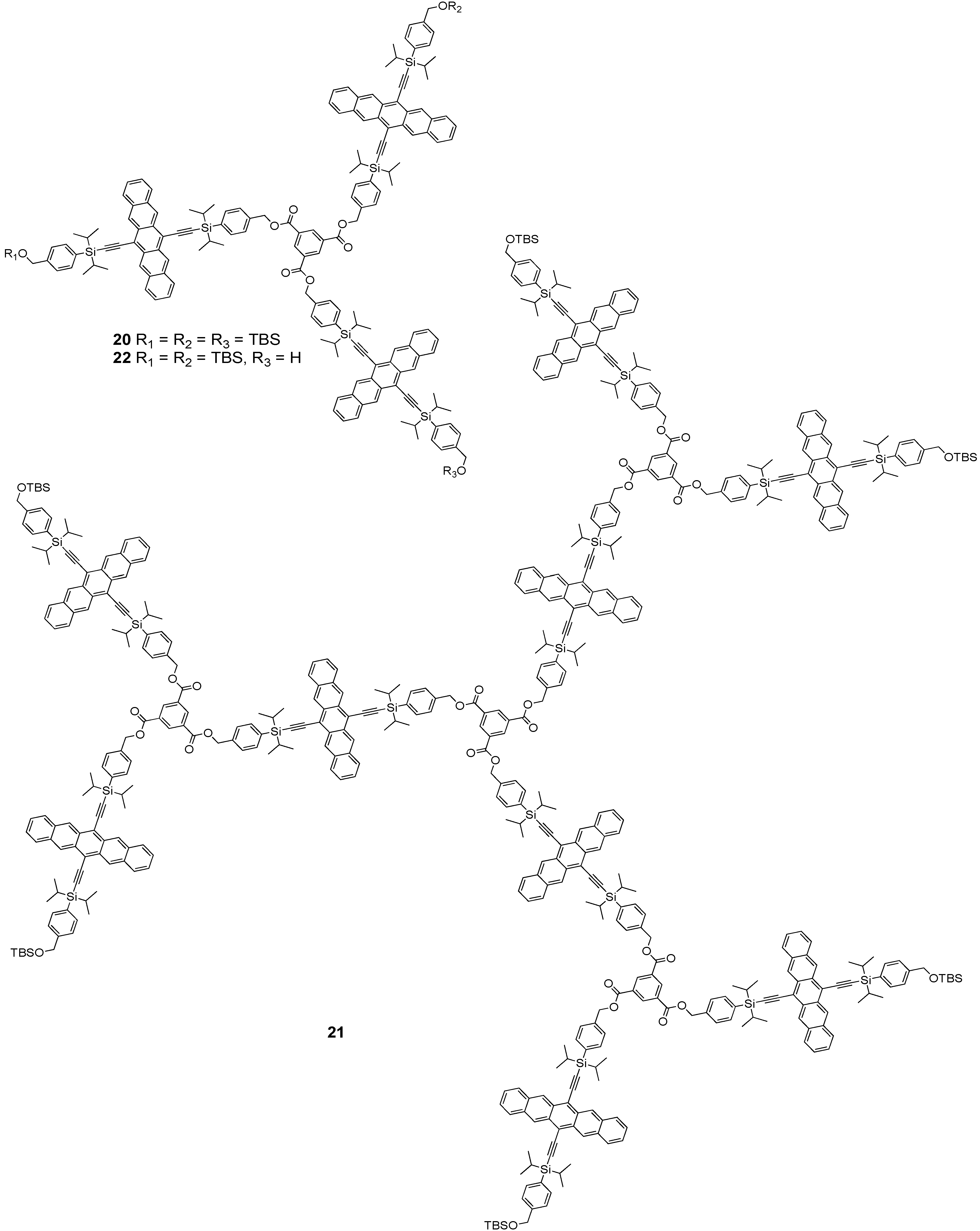
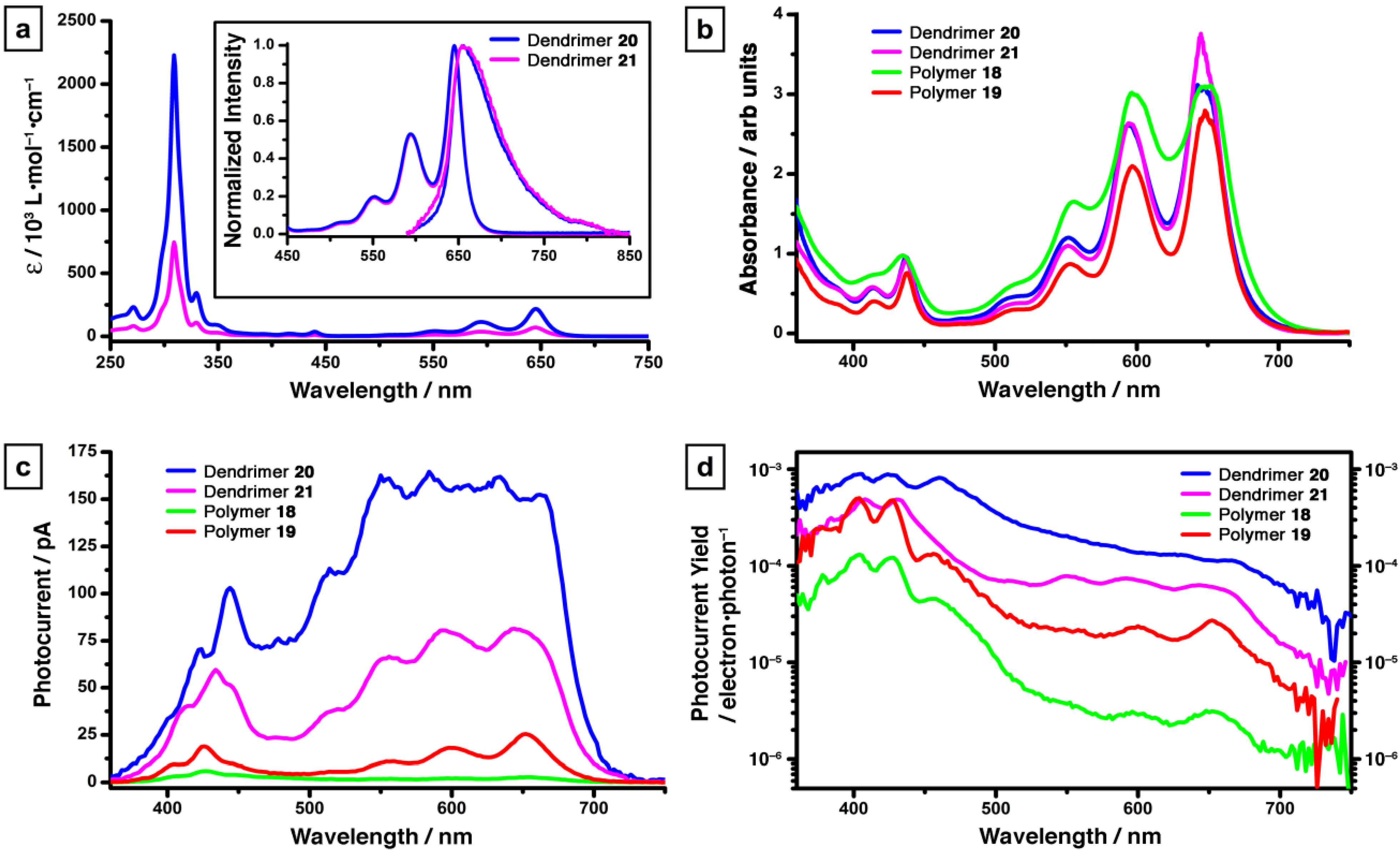
3. Pentacene-Based Polycyclic Aromatic Hydrocarbon Conjugates as Pseudo-Oligomers
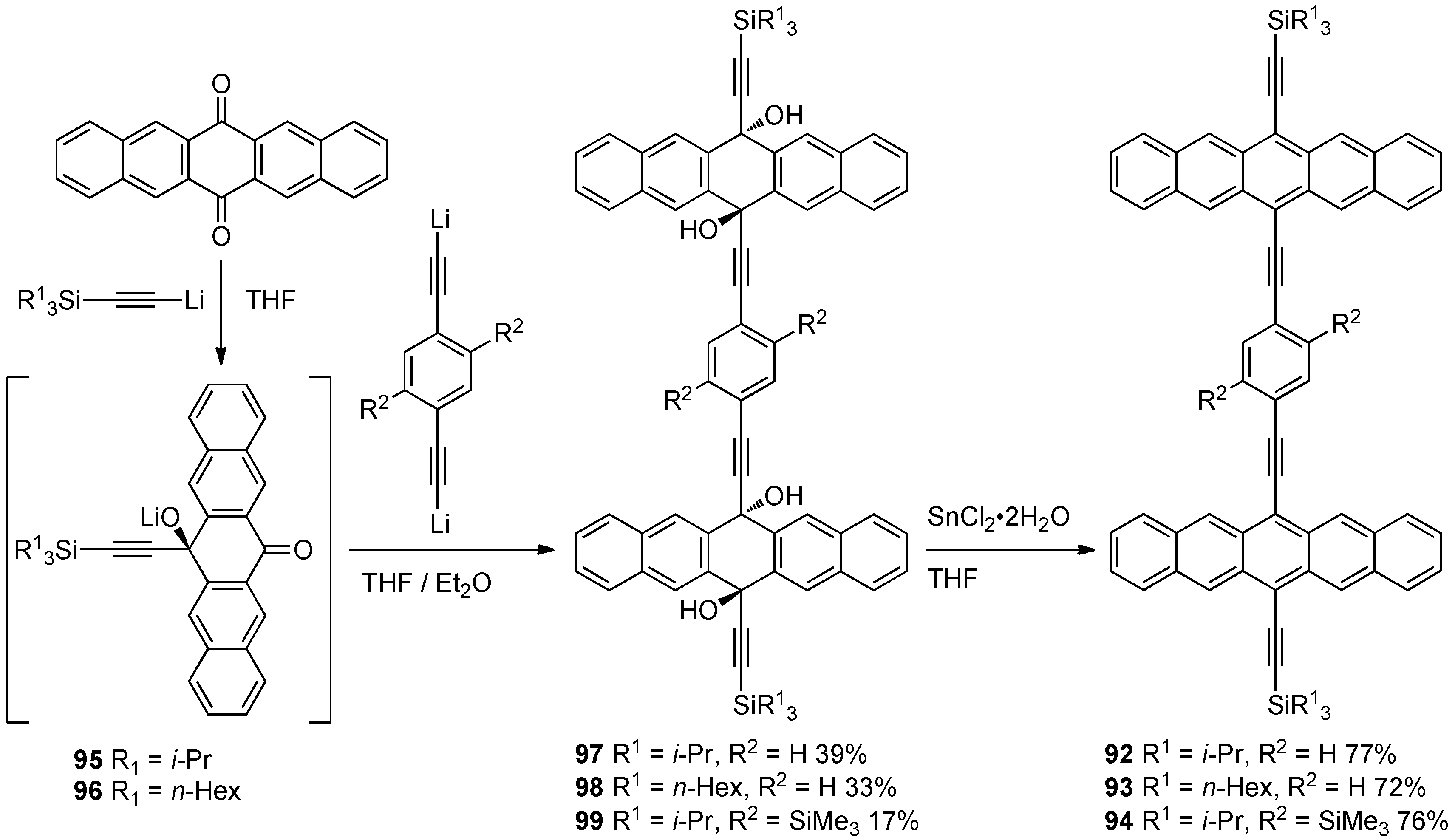
3.1. Arylated Pentacenes


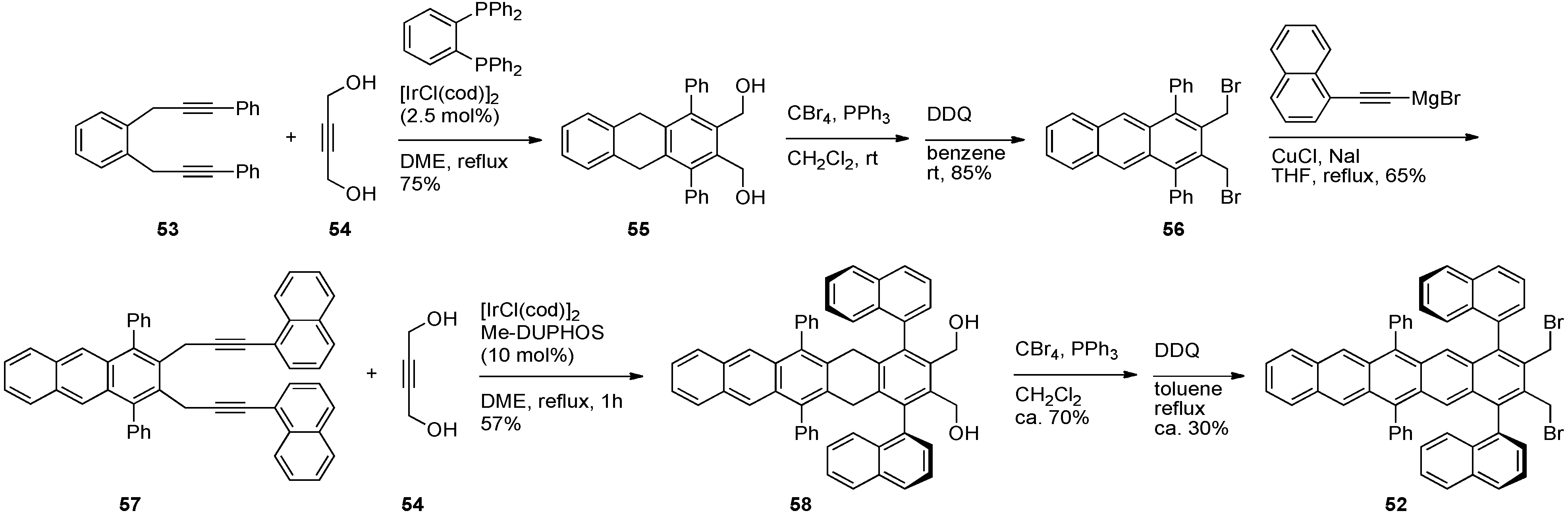
| Compound | λmax [a] (in toluene) /nm | λmax (thin film) /nm | Egopt [b]/eV | Egelectro [c]/eV | EHOMO [c]/eV | ELUMO [c]/eV | Td [d]/°C | t1/2 [e]/min |
|---|---|---|---|---|---|---|---|---|
| 27 | 620 | 622 | 1.97 | 2.02 | –5.26 | –3.24 | 300 | 12 |
| 59 | 635 | 625 | 1.94 | 1.98 | –5.24 | –3.26 | 350 | 75 |
| 60 | 639 | 633 | 1.93 | 1.75 | –5.16 | –3.41 | 340 | 54 |
| 61 | 628 | 633 | 1.96 | 2.08 | –5.22 | –3.14 | 360 | 39 |
| 62 | 638 | 673 | 1.93 | 1.89 | –5.16 | –3.27 | 375 | 97 |
| 63 | 643 | 686 | 1.92 | 1.79 | –5.14 | –3.35 | 380 | 50 |
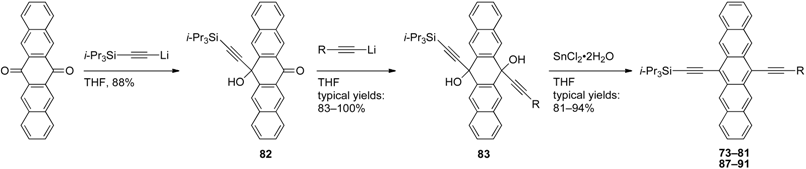
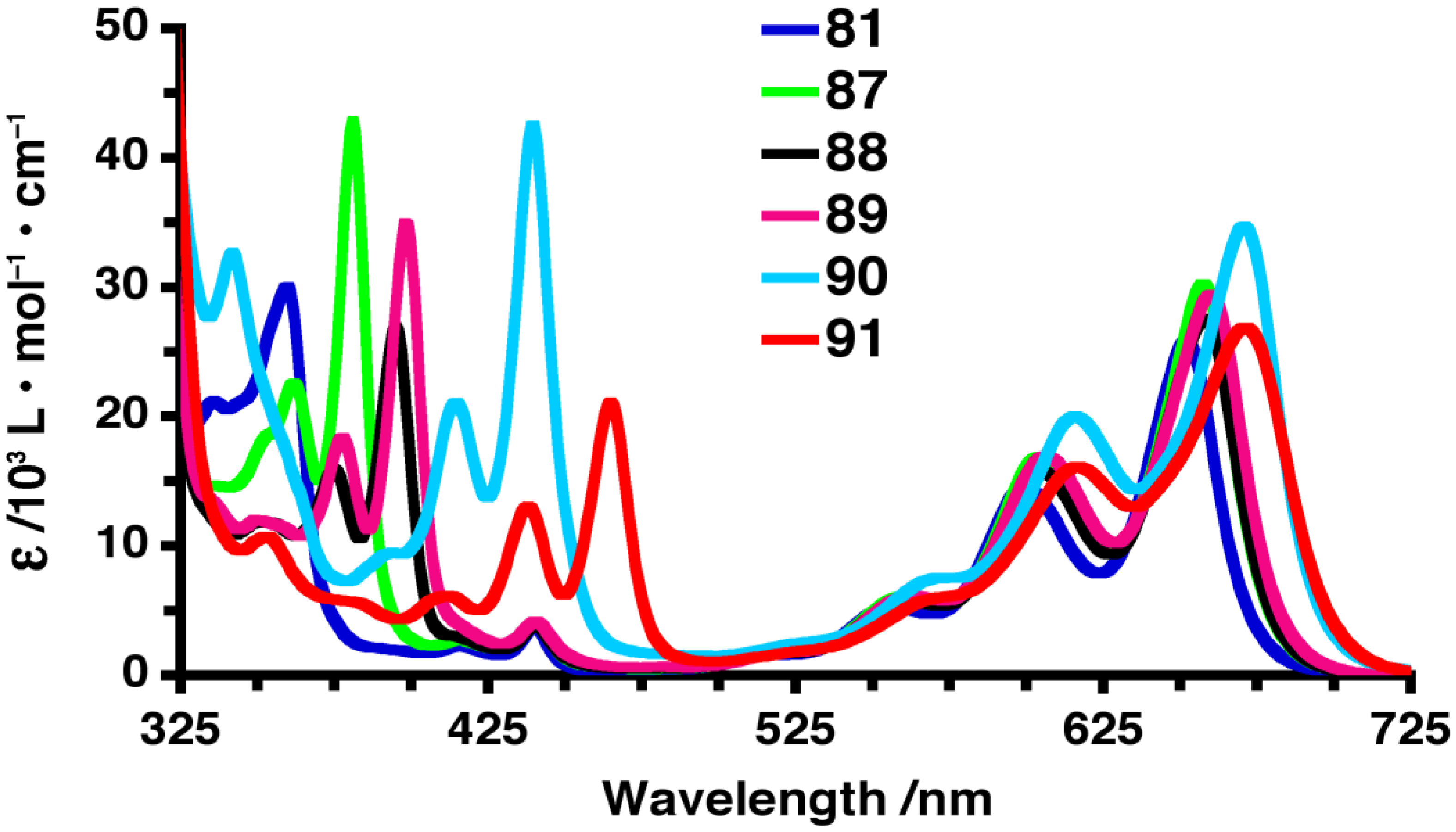
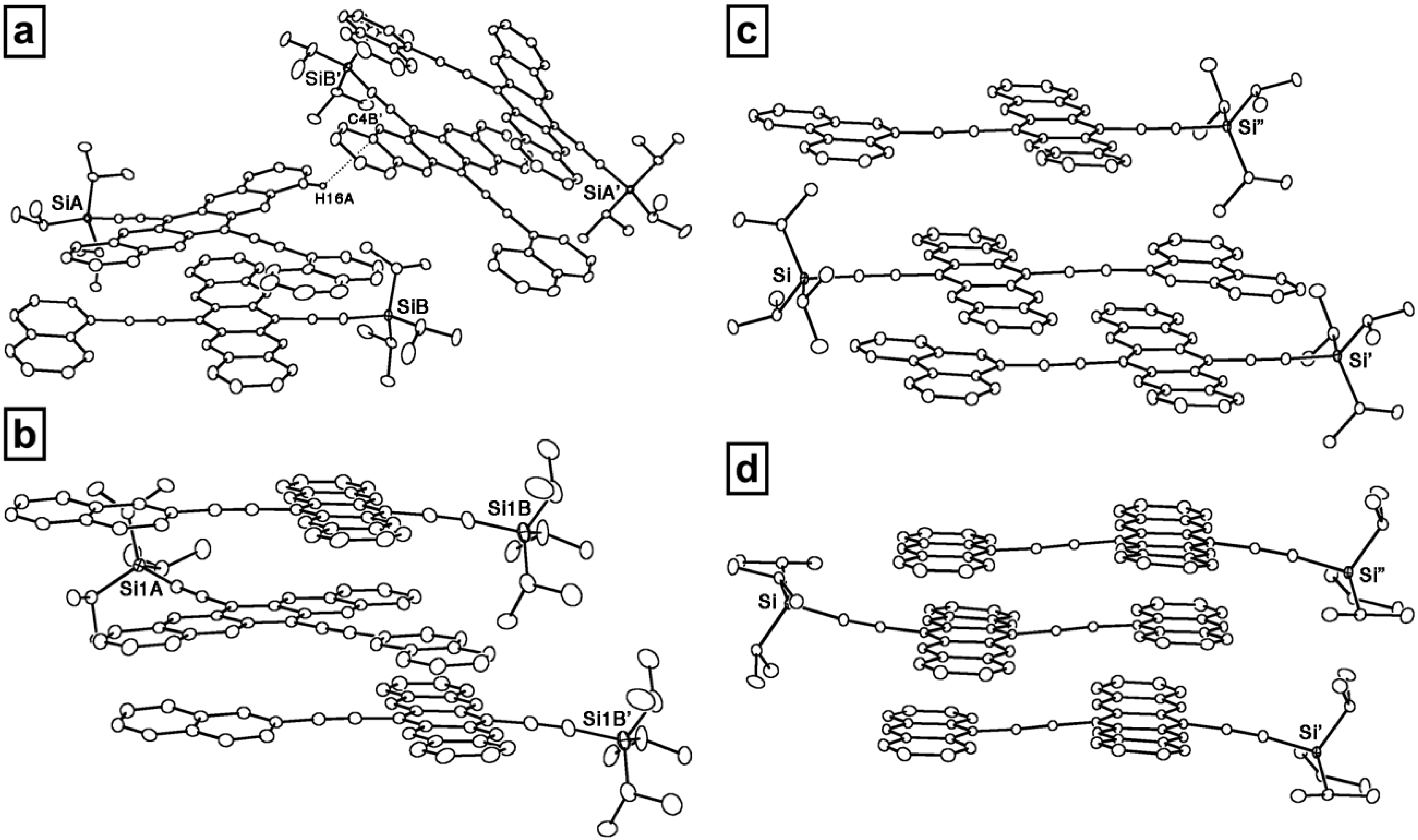
3.2. Unsymmetrically 6,13-Disubstituted Pentacenes
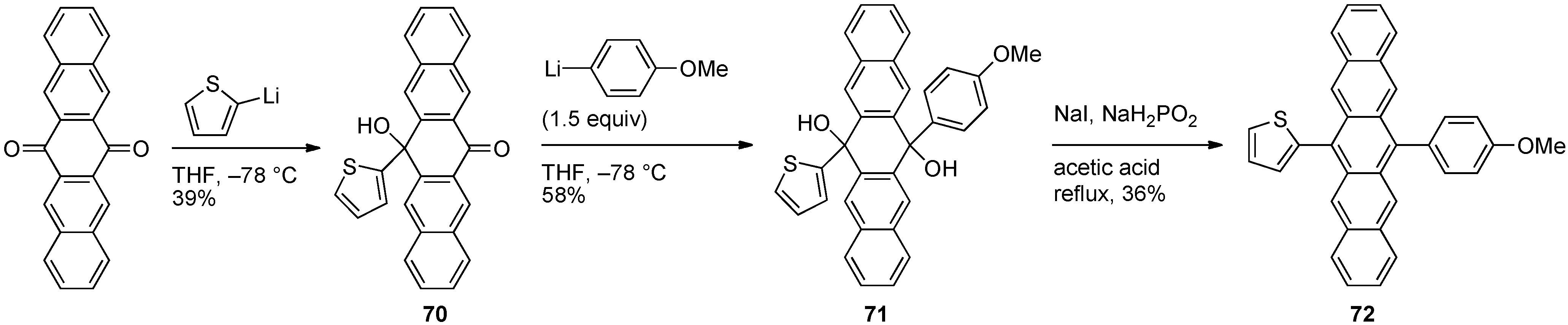
| Compound number and R = | λmid [a] (CH2Cl2)/nm | λmax [b] (CH2Cl2)/nm | λmax (film)/nm | λmax,em (CH2Cl2)/nm | ΦF [c] (CH2Cl2) | Egopt [d] (CH2Cl2)/eV | Egelectro [e] (3:1 PhH/MeCN) /eV | Td (DSC)/°C | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 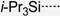 | 328 | 643 | – | 649 | 0.15 | 1.84 | – | 265 |
| 73 |  | 327 | 642 | – | 649 | 0.13 | 1.84 | – | 167 |
| 74 |  | 368 | 656 | – | 666 | 0.05 | 1.78 | – | 136 |
| 75 |  | 367 | 655 | – | 666 | 0.05 | 1.78 | – | 149 |
| 76 |  | 363 | 654 | – | 661 | 0.10 | 1.80 | – | 191 |
| 77 | 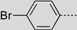 | 366 | 654 | – | 663 | 0.12 | 1.80 | – | 214 |
| 78 |  | 364 | 659 | – | 668 | 0.12 | 1.78 | – | 210 |
| 79 |  | 365 | 654 | – | 667 | 0.06 | 1.79 | – | 215 |
| 80 |  | 357 | 651 | – | 660 | 0.13 | 1.81 | – | 194 |
| 81 |  | 360 | 652 | 671 | 661 | 0.13 | 1.81 | 1.83 | 180 |
| 86 | (see Scheme 8) | 374 | 664 | – | 679 | 0.04 | 1.76 | – | 175 |
| 87 | 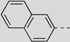 | 381 | 657 | 679 | 667 | 0.12 | 1.79 | 1.80 | 197 |
| 88 | 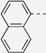 | 395 | 658 | 679 | 668 | 0.12 | 1.79 | 1.81 | 210 |
| 89 | 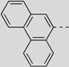 | 398 | 660 | 686 | 670 | 0.11 | 1.78 | 1.79 | 221 |
| 90 |  | 440 | 671 | 712 | 681 | 0.07 | 1.74 | 1.74 | 256 |
| 91 | 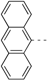 | 465 | 671 | 712 | 688 | 0.006 | 1.74 | 1.71 | 271 |
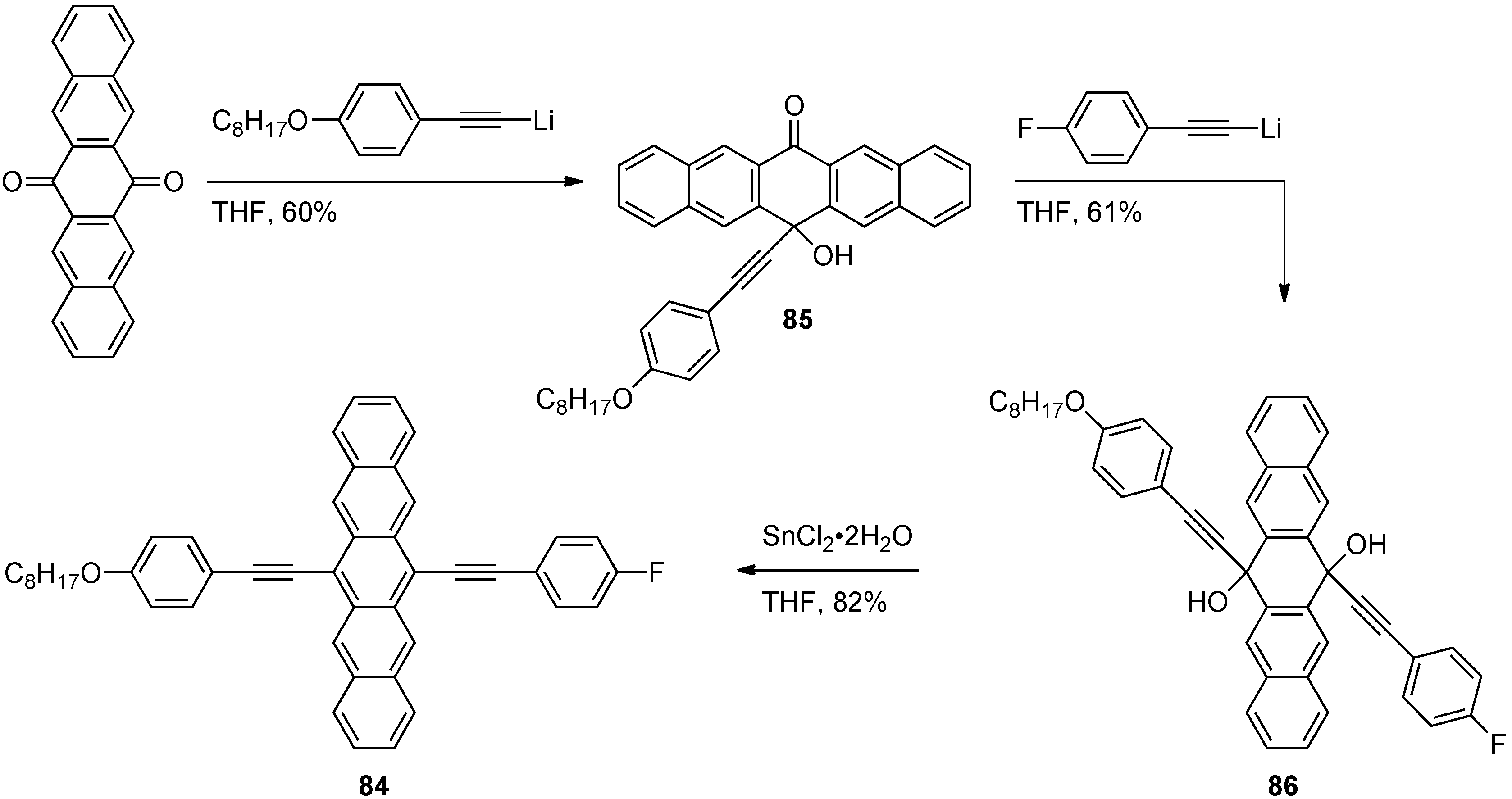
4. Conjugated Pentacene Oligomers
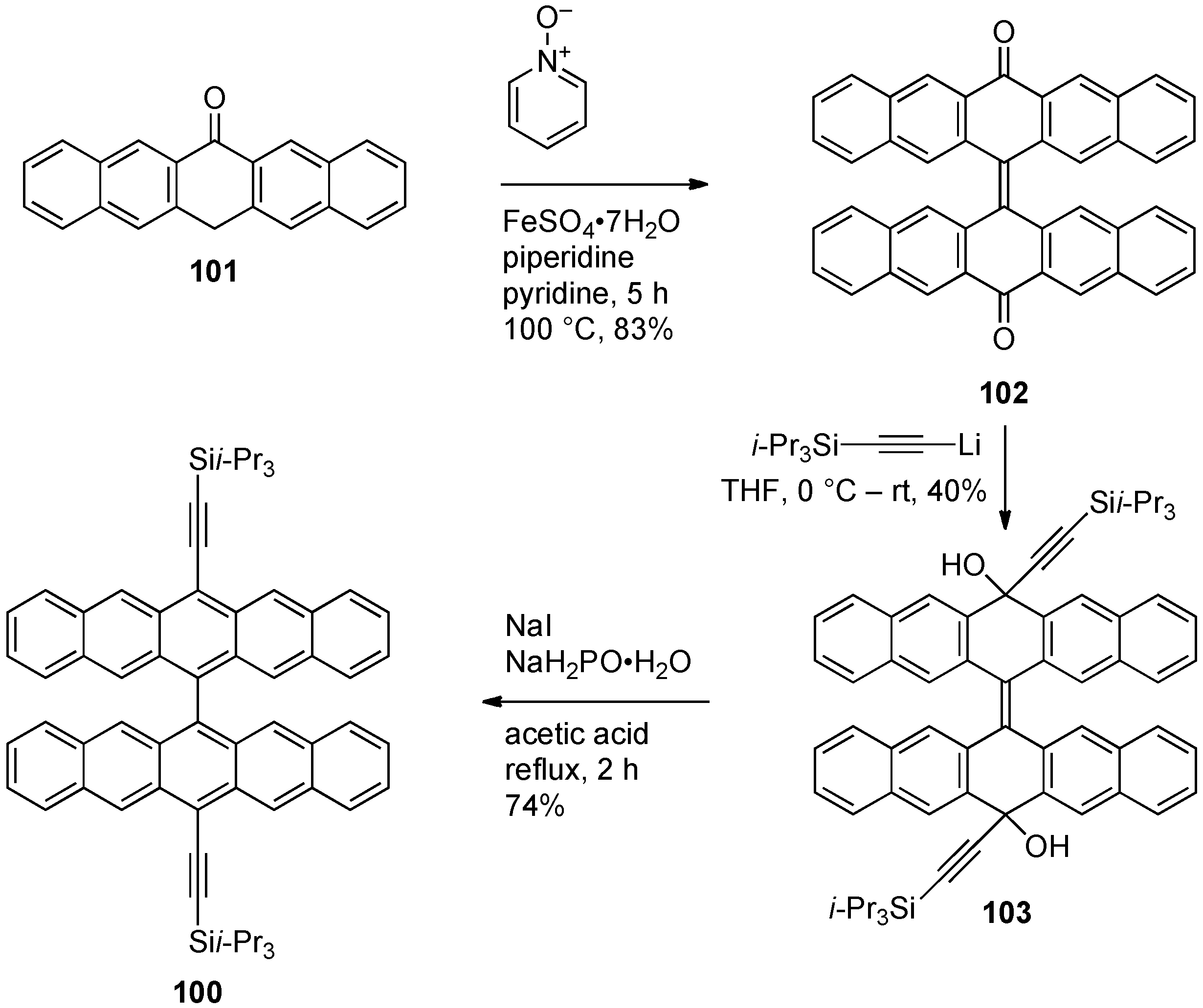
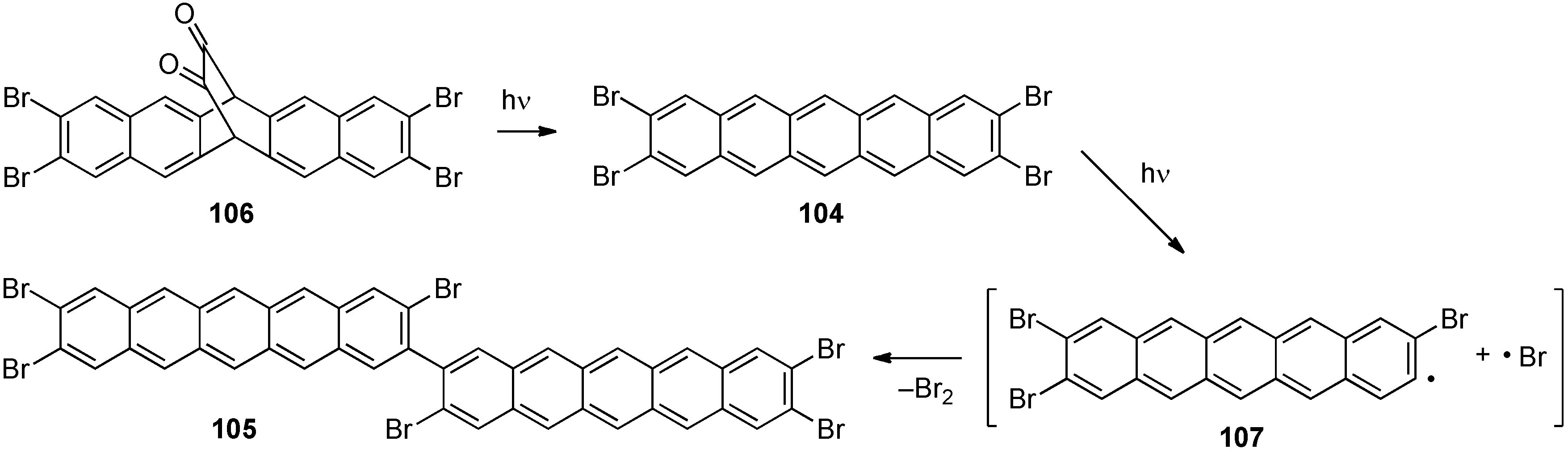
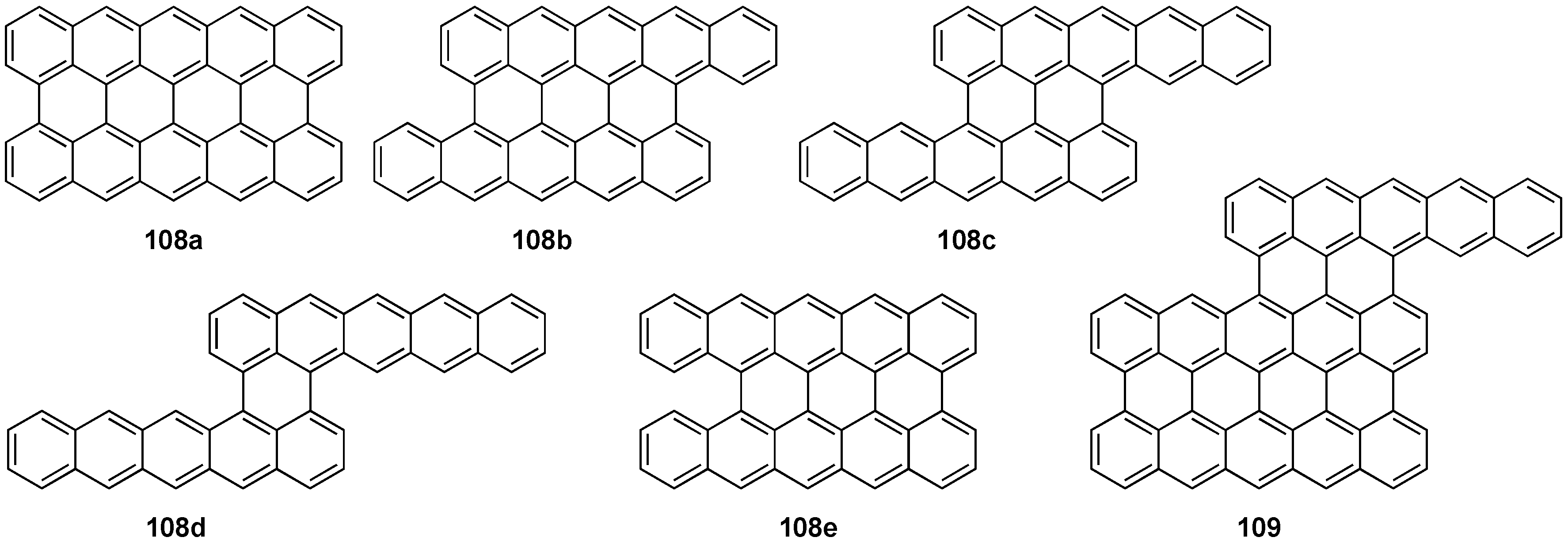
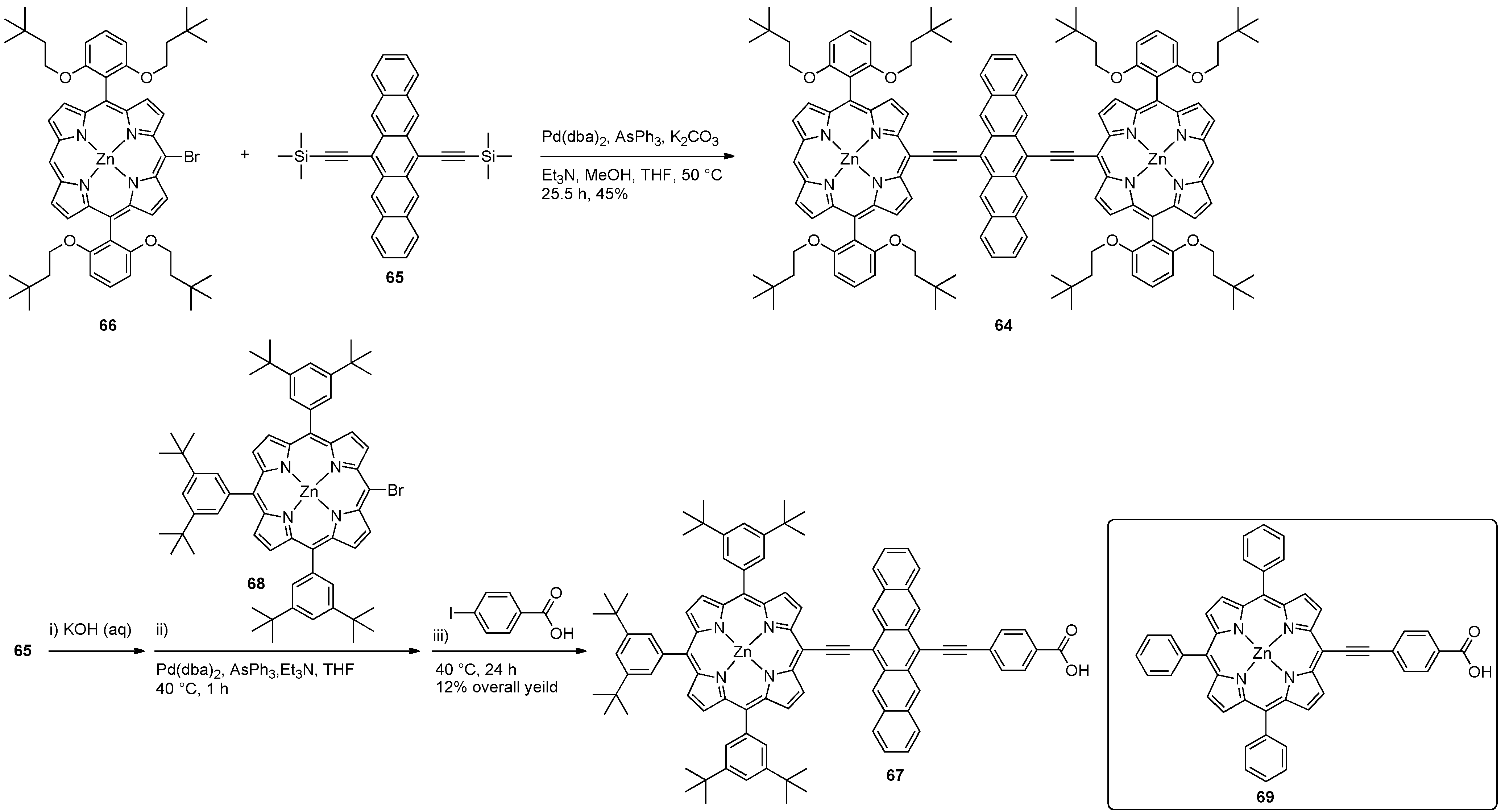
5. Conjugated Pentacene Polymers

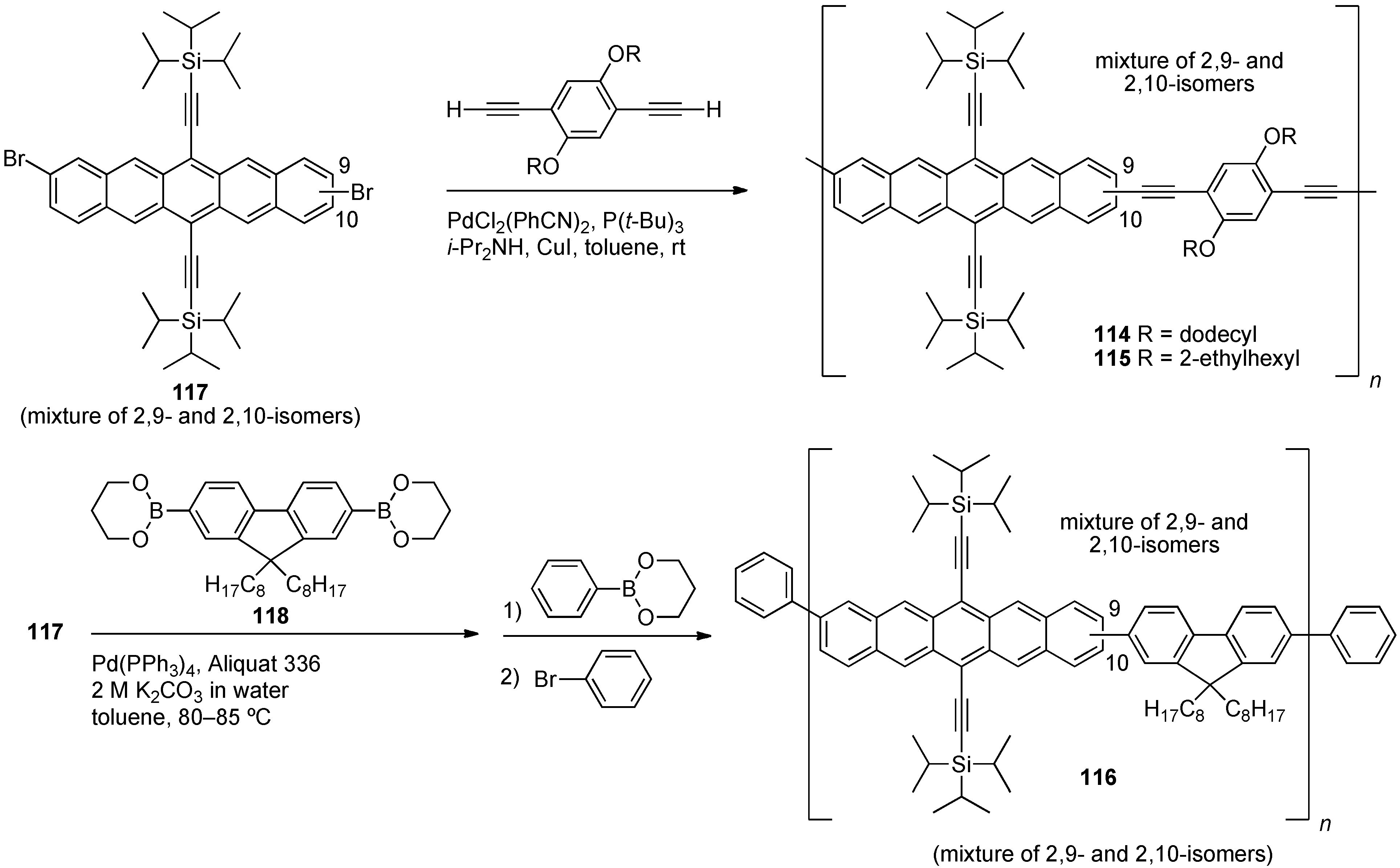
| Compound | Ered1onset[c]/V | Ered2onset[c]/V | Eoxonset[c]/V | λmax, λonset (o-DCB) /nm | λmax, λonset (film) /nm | λmax,em (o-DCB) /nm | Egelectro/eV | Egopt[c]/eV |
|---|---|---|---|---|---|---|---|---|
| 114 | –1.37 | –1.80 | 0.32 | 671, 693 | 672, 736 | 676[a] | 1.69 | 1.68 |
| 115 | –1.42 | –1.82 | 0.28 | 674, 695 | 674, 706 | 679[a] | 1.70 | 1.76 |
| 116 | –1.40 | –1.89 | 0.24 | 675, 695 | 670, 695 | 683[b] | 1.64 | 1.78 |
6. Conclusions
Acknowledgements
References and Notes
- Roncali, J. Conjugated poly(thiophenes): synthesis, functionalization, and applications. Chem. Rev. 1992, 92, 711–738. [Google Scholar] [CrossRef]
- Fichou, D. Handbook of Oligo- and Polythiophenes; Wiley-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Ito, K.; Suzuki, T.; Sakamoto, Y.; Kubota, D.; Inoue, Y.; Sato, F.; Tokito, S. Oligo(2,6-anthrylene)s: acene–oligomer approach for organic field-effect transistors. Angew. Chem. Int. Ed. 2003, 42, 1159–1162. [Google Scholar] [CrossRef]
- Taylor, M.S.; Swager, T.M. Poly(anthrylenebutadiynylene)s: precursor-based synthesis and band-gap tuning. Angew Chem. Int. Ed. 2007, 46, 8480–8483. [Google Scholar] [CrossRef]
- Dell'Aquila, A.; Marinelli, F.; Tey, J.; Keg, P.; Lam, Y.-M.; Kapitanchuk, O.L.; Mastrorilli, P.; Nobile, C.F.; Cosma, P.; Marchenko, A.; Fichou, D.; Mhaisalkar, S.G.; Suranna, G.P.; Torsi, L. 9,10-Ter-anthrylene-ethynylene: a new molecular architecture for solution processed anthracene-based thin film transistors. J. Mater. Chem. 2008, 18, 786–791. [Google Scholar] [CrossRef]
- Merlo, J.A.; Newman, C.R.; Gerlach, C.P.; Kelley, T.W.; Muyres, D.V.; Fritz, S.E.; Toney, M.F.; Frisbie, C.D. p-Channel organic semiconductors based on hybrid acene–thiophene molecules for thin-film transistor applications. J. Am. Chem. Soc. 2005, 127, 3997–4009. [Google Scholar] [CrossRef] [PubMed]
- Toyota, S.; Goichi, M.; Kotani, M. Macrocyclic 1,8-anthrylene–ethynylene oligomers: three-dimensional π-conjugated architectures. Angew. Chem. Int. Ed. 2004, 43, 2248–2251. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, X.; Jiang, X.; Tian, H.; Yan, D.; Geng, Y.; Jing, X.; Wang, F. Synthesis and characterization of soluble oligo(9,10-bisalkynylanthrylene)s. Org. Lett. 2006, 8, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Tang, Q.; Chan, H.S.; Xu, J.; Lo, K.Y.; Miao, Q. Transistors from a conjugated macrocycle molecule: field and photo effects. Chem. Commun. 2008, 4324–4326. [Google Scholar] [CrossRef]
- Park, J.-H.; Chung, D.S.; Lee, D.H.; Kong, H.; Jung, I.H.; Park, M.-J.; Cho, N.S.; Park, C.E.; Shim, H.-K. New anthracene-thiophene-based copolymers that absorb across the entire UV-vis spectrum for application in organic solar cells. Chem. Commun. 2010, 1863–1865. [Google Scholar] [CrossRef]
- Egbe, D.A.M.; Türk, S.; Rathgeber, S.; Kühnlenz, F.; Jadhav, R.; Wild, A.; Birckner, E.; Adam, G.; Pivrikas, A.; Cimrova, V.; Knör, G.; Sariciftci, N.S.; Hoppe, H. Anthracene based conjugated polymers: correlation between π–π-stacking ability, photophysical properties, charge carrier mobility, and photovoltaic performance. Macromolecules 2010, 43, 1261–1269. [Google Scholar] [CrossRef]
- Zaumseil, J.; Sirringhaus, H. Electron and ambipolar transport in organic field-effect transistors. Chem. Rev. 2007, 107, 1296–1323. [Google Scholar] [CrossRef] [PubMed]
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Dos Santos, D.A.; Brédas, J.L.; Lögdlund, M.; Salaneck, W.R. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Thomas III, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.R.; Fréchet, J.M.J. Organic semiconducting oligomers for use in thin film transistors. Chem. Rev. 2007, 107, 1066–1096. [Google Scholar] [CrossRef] [PubMed]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Anthony, J.E. Functionalized acenes and heteroacenes for organic electronics. Chem. Rev. 2006, 106, 5028–5048. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.E. The larger acenes: verstile organic semiconductors. Angew. Chem. Int. Ed. 2008, 47, 452–483. [Google Scholar] [CrossRef]
- Bendikov, M.; Wudl, F.; Perepichka, D.F. Tetrathiafulvalenes, oligoacenenes, and their buckminsterfullerene derivatives: the brick and mortar of organic electronics. Chem. Rev. 2004, 104, 4891–4945. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.E.; Brooks, J.S.; Eaton, D.L.; Parkin, S.R. Functionalized pentacene: improved electronic properties from control of solid-state order. J. Am. Chem. Soc. 2001, 123, 9482–9483. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.E.; Eaton, D.L.; Parkin, S.R. A road map to stable, soluble, easily crystallized pentacene derivatives. Org. Lett. 2002, 4, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.M.; Parkin, S.R.; Anthony, J.E. Functionalized higher acenes: hexacene and heptacene. J. Am. Chem. Soc. 2005, 127, 8028–8029. [Google Scholar] [CrossRef] [PubMed]
- Chun, D.; Cheng, Y.; Wudl, F. The most stable and fully characterized functionalized heptacene. Angew. Chem. Int. Ed. 2008, 47, 8380–8385. [Google Scholar] [CrossRef]
- Odom, S.A.; Parkin, S.R.; Anthony, J.E. Tetracene derivatives as potential red emitters for organic LEDs. Org. Lett. 2003, 5, 4245–4248. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.S.; Park, J.W.; Park, J.-H.; Moon, D.; Kim, G.H.; Lee, H.-S.; Lee, D.H.; Shim, H.-K.; Kwon, S.-K.; Park, C.E. High mobility organic single crystal transistors based on soluble triisopropylsilylethynyl anthracene derivatives. J. Mater. Chem. 2010, 20, 524–530. [Google Scholar] [CrossRef]
- Subramanian, S.; Park, S.K.; Parkin, S.R.; Podzorov, V.; Jackson, T.N.; Anthony, J.E. Chromophore fluorination enhances crystallization and stability of soluble anthradithiophene semiconductors. J. Am. Chem. Soc. 2008, 130, 2706–2707. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.L.; Oh, J.H.; Reichardt, A.D.; Bao, Z. Chlorination: a general route toward electron transport in organic semiconductors. J. Am. Chem. Soc. 2009, 131, 3733–3740. [Google Scholar] [CrossRef] [PubMed]
- Appleton, A.L.; Miao, S.; Brombosz, S.M.; Berger, N.J.; Barlow, S.; Marder, S.R.; Lawrence, B.M.; Hardcastle, K.I.; Bunz, U.H.F. Alkynylated aceneo[2,1,3]thiadiazoles. Org. Lett. 2009, 11, 5222–5225. [Google Scholar] [CrossRef] [PubMed]
- Lehnherr, D.; Tykwinski, R.R. Pentacene oligomers and polymers: functionalization of pentacene to afford mono-, di-, tri-, and polymeric materials. Org. Lett. 2007, 9, 4583–4586. [Google Scholar] [CrossRef] [PubMed]
- Lehnherr, D.; McDonald, R.; Ferguson, M.J.; Tykwinski, R.R. Synthesis of soluble oligo- and polymeric pentacene-based materials. Tetrahedron 2008, 64, 11449–11461. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications–reflections on the field. Adv. Drug Delivery Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Astruc, D.; Chardac, F. Dendritic catalysts and dendrimers in catalysis. Chem. Rev. 2001, 101, 2991–3023. [Google Scholar] [CrossRef] [PubMed]
- Adronov, A.; Fréchet, J.M.J. Light harvesting dendrimers. Chem. Commun. 2000, 1701–1710. [Google Scholar] [CrossRef]
- Balzani, V.; Bergamini, G.; Ceroni, P.; Vögtle, F. Electronic spectroscopy of metal complexes with dendritic ligands. Coord. Chem. Rev. 2007, 251, 525–535. [Google Scholar] [CrossRef]
- Lo, S.-C.; Burn, P.L. Development of dendrimers: macromolecules for use in organic light-emitting diodes and solar cells. Chem. Rev. 2007, 107, 1097–1116. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.G.; Miller, L.L.; Tomalia, D.A. An electrically conducting dendrimer. J. Am. Chem. Soc. 1995, 117, 10783–10784. [Google Scholar] [CrossRef]
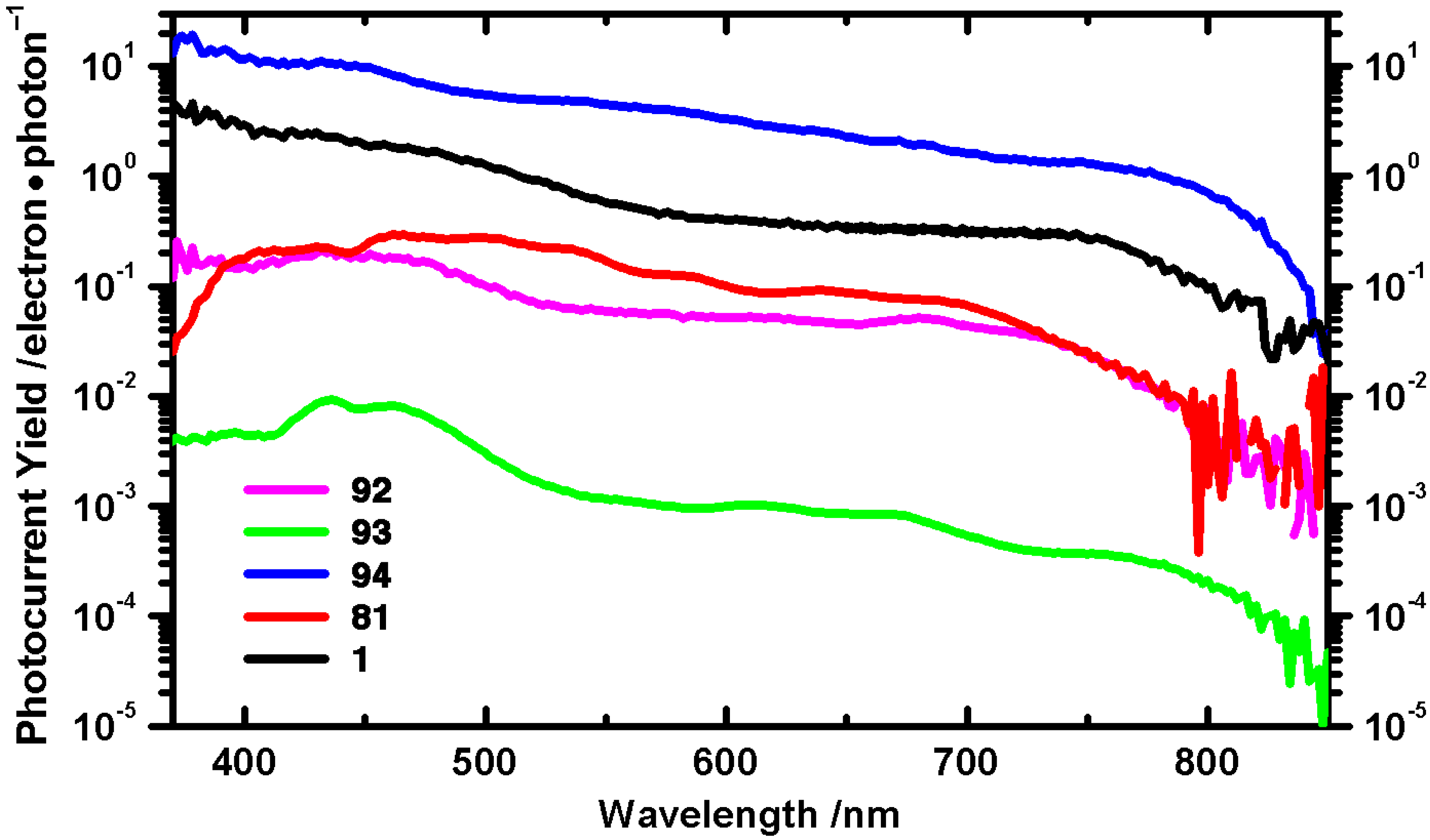
- Lehnherr, D.; Gao, J.; Hegmann, F.A.; Tykwinski, R.R. Pentacene-based dendrimers: synthesis and thin film photoconductivity measurements of branched pentacene oligomers. J. Org. Chem. 2009, 74, 5017–5024. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.W. Glossary of terms used in photochemistry. Pure Appl. Chem. 1996, 68, 2223–2286. [Google Scholar] [CrossRef]
- Ostroverkhova, O.; Cooke, D.G.; Shcherbyna, S.; Egerton, R.F.; Hegmann, F.A.; Tykwinski, R.R.; Anthony, J.E. Bandlike transport in pentacene and functionalized pentacene thin films revealed by subpicosecond transient photoconductivity measurements. Phys. Rev. B 2005, 71, 035204. [Google Scholar] [CrossRef]
- Onsager, L. Initial recombination of ions. Phys. Rev. 1938, 54, 554–557. [Google Scholar] [CrossRef]
- Najafov, H.; Biaggio, I.; Podzorov, V.; Calhoun, M.F.; Gershenson, M.E. Primary photoexcitations and the origin of the photocurrent in rubrene single crystals. Phys. Rev. Lett. 2006, 96, 056604. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.F.H.; Bell, A. Action of Grignard reagents on certain pentacenequinones, 6,13-diphenylpentacene. J. Am. Chem. Soc. 1942, 64, 1253–1260. [Google Scholar] [CrossRef]
- Maulding, D.R.; Roberts, B.G. Electronic absorption and fluorescence of phenylethynyl-substituted acenes. J. Org. Chem. 1969, 34, 1734–1736. [Google Scholar] [CrossRef]
- Vets, N.; Smet, M.; Dehaen, W. Reduction versus rearrangement of 6,13-dihydro-6,13-diarylpentacene-6,13-diols affording 6,13- and 13,13'-substituted pentacene derivatives. Synlett 2005, 217–222. [Google Scholar]
- Gorodetsky, A.A.; Cox, M.; Tremblay, J.; Kymissis, I.; Nuckolls, C. Solar cells from a solution processable pentacene with improved air stability. Chem. Mater. 2009, 21, 4090–4092. [Google Scholar] [CrossRef]
- Vets, N.; Diliën, H.; Toppet, S.; Dehaen, W. Synthesis of 5,7,12,14-tetraarylpentacenes from pentacene-5,7,12,14-tetrone and characterisation of the tetrol intermediates. Synlett 2006, 1359–1362. [Google Scholar]
- Wolak, M.A.; Jang, B.-B.; Palilis, L.C.; Kafafi, Z.H. Functionalized pentacene derivatives for use as red emitters in organic light-emitting diodes. J. Phys. Chem. B 2004, 108, 5492–5499. [Google Scholar] [CrossRef]
- Kaur, I.; Jia, W.; Kopreski, R.P.; Selvarasah, S.; Dokmeci, M.R.; Pramanik, C.; McGruer, N.E.; Miller, G.P. Substituent effects in pentacenes: gaining control over HOMO–LUMO gaps and photooxidative resistances. J. Am. Chem. Soc. 2008, 130, 16274–16286. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.-B.; Lee, S.H.; Kafafi, Z.H. Asymmetric pentacene derivatives for organic light-emitting diodes. Chem. Mater. 2006, 18, 449–457. [Google Scholar] [CrossRef]
- Zhao, Y.; Mondal, R.; Neckers, D.C. Photochemical formation of substituted pentacenes. J. Org. Chem. 2008, 73, 5506–5513. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, S.; Yamada, H.; Okujima, T.; Uno, H. Photochemical synthesis of tetraaryl-substituted pentacenes. Tetrahedron Lett. 2010, 51, 1397–1400. [Google Scholar] [CrossRef]
- Miller has previously formed and reacted pentacene 39 in situ with C60, see: Rainbolt, J.E.; Miller, G.P. 4,7-Diphenylisobenzofuran: a useful intermediate for the construction of phenyl-substituted acenes. J. Org. Chem. 2007, 72, 3020–3030.
- Miao, Q.; Chi, X.; Xiao, S.; Zeis, R.; Lefenfeld, M.; Siegrist, T.; Steigerwald, M.L.; Nuckolls, C. Organization of acenes with a cruciform assembly motif. J. Am. Chem. Soc. 2006, 128, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ho, D.M.; Vogelaar, N.J.; Kraml, C.M.; Pascal, R.A., Jr. A pentacene with a 144° twist. J. Am. Chem. Soc. 2004, 126, 11168–11169. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ho, D.M.; Vogelaar, N.J.; Kraml, C.M.; Bernhard, S.; Byrne, N.; Kim, L.R.; Pascal, R.A., Jr. Synthesis, structure, and resolution of exceptionally twisted pentacenes. J. Am. Chem. Soc. 2006, 128, 17043–17050. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Kaafarani, B.R.; Neckers, D.C. Design, synthesis and properties of new derivatives of pentacene. J. Org. Chem. 2006, 71, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-F.; Shu, Y.; Parkin, S.R.; Anthony, J.E. Soluble n-type pentacene derivatives as novel acceptors for organic solar cells. J. Mater. Chem. 2009, 19, 3049–3056. [Google Scholar] [CrossRef]
- Bénard, C.P.; Geng, Z.; Heuft, M.A.; VanCrey, K.; Fallis, A.G. Double Diels–Alder strategies to soluble 2,9- and 2,9,6,13-tetraethynylpentacenes, photolytic [4 + 4] cycloadditions, and pentacene crystal packing. J. Org. Chem. 2007, 72, 7229–7236. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Totani, H.; Hiei, T.; Yoshino, A.; Saito, K.; Eguchi, K.; Tomura, M.; Nishida, J.-I.; Yamashita, Y. Photooxidation and reproduction of pentacene derivatives substituted by aromatic groups. Tetrahedron 2007, 63, 9699–9704. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Fu, N.-Y.; Chan, S.-H.; Lee, H.-K.; Wong, H.N.C. Synthesis, characterization, and reactions of 6,13-disubstituted 2,3,9,10-tetrakis(trimethylsilyl)pentacene derivatives. Tetrahedron 2007, 63, 8586–8597. [Google Scholar] [CrossRef]
- Stone, M.T.; Anderson, H.L. Three-step synthesis of end-substituted pentacenes. J. Org. Chem. 2007, 72, 9776–9778. [Google Scholar] [CrossRef] [PubMed]
- Mosurkal, R.; Samuelson, L.A.; Kumar, J.; Waller, D.; Gaudiana, R. Synthesis of a soluble pentacene derivative: 6,13-bis(m-trifluoromethyl phenyl ethenyl) pentacene. J. Macromol. Sci., A: Pure Appl. Chem. 2008, 45, 948–951. [Google Scholar]
- Schmidt, R.; Göttling, S.; Leusser, D.; Stalke, D.; Krause, A.-M.; Würthner, F. Highly soluble acenes as semiconductors for thin film transistors. J. Mater. Chem. 2006, 16, 3708–3714. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Liu, P.; Prostran, Z.; Gardner, S.; Ong, B.S. Stable solution-processed high-mobility substituted pentacene semiconductors. Chem. Mater. 2007, 19, 418–423. [Google Scholar] [CrossRef]
- Northrop, B.H.; Houk, K.N.; Maliakal, A. Photostability of pentacene and 6,13-disubstituted pentacene derivatives: a theoretical and experimental mechanistic study. Photochem. Photobiol. Sci. 2008, 7, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Maliakal, A.; Raghavachari, K.; Katz, H.; Chandross, E.; Siegrist, T. Photochemical stability of pentacene and substituted pentacene in solution and in thin films. Chem. Mater. 2004, 16, 4980–4986. [Google Scholar] [CrossRef]
- Hwang, E.-J.; Kim, Y.-E.; Lee, C.-J.; Park, J.-W. Synthesis and luminescent properties of pentacene derivatives having a chromophore. Thin Film Solids 2006, 499, 185–191. [Google Scholar] [CrossRef]
- For absorption characteristics of pentacene, see: reference [43].
- Picciolo, L.C.; Murata, H.; Kafafi, Z.H. Organic light-emitting devices with saturated red emission using 6,13-diphenylpentacene. Appl. Phys. Lett. 2001, 78, 2378–2380. [Google Scholar] [CrossRef]
- Kim, Y.-E.; Park, J.-W. Luminescent properties of pentacene derivatives with naphthalene moiety. Mol. Cryst. Liq. Cryst. 2006, 444, 137–143. [Google Scholar] [CrossRef]
- Son, J.-H.; Kang, I.-N.; Oh, S.-Y.; Park, J.-W. Structure effects on organic thin-film transistor properties of dinaphthyl substituted pentacene derivatives. Bull. Korean Chem. Soc. 2007, 28, 995–998. [Google Scholar] [CrossRef]
- Kim, Y.E.; Park, J.W.; Kim, K.S.; Lee, S.D. Electroluminescent properties of pentacene derivatives having a naphthalene moiety. Mol. Cryst. Liq. Cryst. 2006, 459, 57–63. [Google Scholar]
- Shibata, T.; Yoshida, S.; Arai, Y.; Otsuka, M.; Endo, K. Iridium-catalyzed consecutive and enantioselective [2+2+2] cycloaddition of tetraynes and hexaynes for the construction of an axially chiral biaryl system. Tetrahedron 2008, 64, 821–830. [Google Scholar] [CrossRef]
- Takahashi, T.; Kitamura, M.; Shen, B.; Nakajima, K. Straightforward method for synthesis of highly alkyl-substituted naphthacene and pentacene derivatives by homologation. J. Am. Chem. Soc. 2000, 122, 12876–12877. [Google Scholar] [CrossRef]
- Takahashi, T.; Li, S.; Huang, W.; Kong, F.; Nakajima, K.; Shen, B.; Ohe, T.; Kanno, K.-i. Homologation method for preparation of substituted pentacenes and naphthacenes. J. Org. Chem. 2006, 71, 7967–7977. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Liu, Y.-Y.; Song, C.-L.; Shi, Z.-F.; Peng, J.-B.; Zhang, H.-L.; Cao, X.-P. New oligothiophene-pentacene hybrids as highly stable and soluble organic semiconductors. Org. Lett. 2009, 11, 2563–2566. [Google Scholar] [CrossRef] [PubMed]
- Susumu, K.; Duncan, T.V.; Therien, M.J. Potentiometric, electronic structural, and ground- and excited-state optical properties of conjugated bis[(porphinato)zinc(II)] compounds featuring proquinoidal spacer units. J. Am. Chem. Soc. 2005, 127, 5186–5195. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Wang, Y.-C.; Hsu, S.-J.; Lo, C.-F.; Diau, E.W.G. Preparation and spectral, electrochemical, and photovoltaic properties of acene-modified zinc porphyrins. J. Phys. Chem. C 2010, 114, 687–693. [Google Scholar] [CrossRef]
- Lehnherr, D.; McDonald, R.; Tykwinski, R.R. Exploring electronically polarized pentacenes. Org. Lett. 2008, 10, 4163–4166. [Google Scholar] [CrossRef] [PubMed]
- Lehnherr, D.; Gao, J.; Hegmann, F.A.; Tykwinski, R.R. Synthesis and electronic properties of conjugated pentacene dimers. Org. Lett. 2008, 10, 4779–4782. [Google Scholar] [CrossRef] [PubMed]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C.J. Design rules for donors in bulk-heterojunction solar cells–towards 10% energy-conversion efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Brabec, C.J.; Sariciftci, N.S.; Hummelen, J.C. Plastic solar cells. Adv. Funct. Mater. 2001, 11, 15–26. [Google Scholar] [CrossRef]
- Isak, S.J.; Eyring, E.M. Fluorescence quantum yield of cresyl violet perchlorate in methanol and water as a function of concentration. J. Phys. Chem. 1992, 96, 1738–1742. [Google Scholar] [CrossRef]
- Lehnherr, D.; Murray, A.H.; McDonald, R.; Ferguson, M.J.; Tykwinski, R.R. Pentacene-based polycyclic aromatic hydrocarbon dyads with cofacial solid-state π-stacking. Chem. Eur. J. 2009, 15, 12580–12584. [Google Scholar] [CrossRef] [PubMed]
- Brédas, J.-L.; Beljonne, D.; Coropceanu, V.; Cornil, J. Charge-transfer and energy-transfer processes in π-conjugated oligomers and polymers: a molecular picture. Chem. Rev. 2004, 104, 4971–5003. [Google Scholar] [CrossRef] [PubMed]
- Mondal, R.; Tönshoff, C.; Khon, D.; Neckers, D.C.; Bettinger, H.F. Synthesis, stability, and photochemistry of pentacene, hexacene, and heptacene: a matrix isolation study. J. Am. Chem. Soc. 2009, 131, 14281–14289. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Stein, N.N.; Kopreski, R.P.; Miller, G.P. Exploiting substituent effects for the synthesis of a photooxidatively resistant heptacene derivative. J. Am. Chem. Soc. 2009, 131, 3424–3425. [Google Scholar] [CrossRef] [PubMed]
- Mondal, R.; Shah, B.K.; Neckers, D.C. Photogeneration of heptacene in a polymer matrix. J. Am. Chem. Soc. 2006, 128, 9612–9613. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Jazdzyk, M.; Stein, N.N.; Prusevich, P.; Miller, G.P. Design, synthesis, and characterization of a persistent nonacene derivative. J. Am. Chem. Soc. 2010, 132, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.M.; Odom, S.A.; Parkin, S.R.; Anthony, J.E. Stable, crystalline acenedithiophenes with up to seven linearly fused rings. Org. Lett. 2004, 6, 3325–3328. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.L.; Mannsfeld, S.C.B.; Sun, Y.-S.; Becerril, H.A.; Bao, Z. Pentaceno[2,3-b]thiophene, a hexacene analogue for organic thin film transistors. J. Am. Chem. Soc. 2009, 131, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Goodings, E.P.; Mitchard, D.A.; Owen, G. Synthesis, structure, and electrical properties of naphthacene, pentacene, and hexacene sulphides. J. Chem. Soc., Perkin Trans. 1 1972, 1310–1314. [Google Scholar] [CrossRef]
- Briseno, A.L.; Miao, Q.; Ling, M.-M.; Reese, C.; Meng, H.; Bao, Z.; Wudl, F. Hexathiapentacene: structure, molecular packing, and thin-film transistors. J. Am. Chem. Soc. 2006, 128, 15576–15577. [Google Scholar] [CrossRef] [PubMed]
- Briseno, A.L.; Mannsfeld, S.C.B.; Lu, X.; Xiong, Y.; Jenekhe, S.A.; Bao, Z.; Xia, Y. Fabrication of field-effect transistors from hexathiapentacene single-crystal nanowires. Nano Lett. 2007, 7, 668–675. [Google Scholar] [CrossRef] [PubMed]
- For a discussion of the mechanisms of photoconductive gain in organic materials, see: Campbell, I.H.; Crone, B.K. Bulk photoconductive gain in poly(phenylene vinylene) based diodes. J. Appl. Phys. 2007, 101, 024502. [Google Scholar]
- Gao, J.; Hegmann, F.A. Bulk photoconductive gain in pentacene thin films. Appl. Phys. Lett. 2008, 93, 223306. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.; Luo, J.; Chi, C.; Chen, H.; Wu, J. A cruciform 6,6'-dipentacenyl: synthesis, solid-state packing and application in thin film transistors. Chem. Eur. J. 2010, 16, 464–468. [Google Scholar] [CrossRef] [PubMed]
- A theoretical study of conjugated oligomers endcapped with pentacene moieties has been explored toward understanding bridge-mediated electronic coupling, see: Eng, M.P.; Albinsson, B. Non-exponential distance dependence of bridge-mediated electronic coupling. Angew. Chem. Int. Ed. 2006, 45, 5626–5629. [Google Scholar]
- Zhao, Y.; Cai, X.; Danilov, E.; Li, G.; Neckers, D.C. Photochemical synthesis of 2,3,9,10-tetraboromopentacene: its unusual photodimerization. Photochem. Photobiol. Sci. 2009, 8, 34–36. [Google Scholar] [CrossRef] [PubMed]
- For the thermal dimerization and polymerization of 2,3,9,10-tetrachloropentacene, see: Perepichka, D.F.; Bendikov, M.; Meng, H.; Wudl, F. A one-step synthesis of a poly(iptycene) through an unusual Diels–Alder cyclization/dechlorination of tetrachloropentacene. J. Am. Chem. Soc. 2003, 125, 10190–10191. [Google Scholar]
- Mattheus, C.C.; Baas, J.; Meetsma, A.; de Boer, J.L.; Kloc, C.; Siegrist, T.; Palstra, T.T.M. Acta Cryst., Sect. E: Struct. Rep. Online 2002, E58, o1229–o1231. [CrossRef]
- Roberson, L.B.; Kowalik, J.; Tolbert, L.M.; Kloc, C.; Zeis, R.; Chi, X.; Fleming, R.; Wilkins, C. Pentacene disproportionation during sublimation for field-effect transistors. J. Am. Chem. Soc. 2005, 127, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Northrop, B.H.; Norton, J.E.; Houk, K.N. On the mechanism of peripentacene formation from pentacene: computational studies of a prototype for graphene formation form smaller acenes. J. Am. Chem. Soc. 2007, 129, 6536–6546. [Google Scholar] [CrossRef] [PubMed]
- Tokito, S.; Weinfurtner, K.-H.; Fujikawa, H.; Tsutsui, T.; Taga, Y. Acene containing polyfluorenes for red, green and blue emission in organic light-emitting diodes. Proc. SPIE–Int. Opt. Soc. Eng. 2001, 4105, 69–74. [Google Scholar]
- Okamoto, T.; Bao, Z. Synthesis of solution-soluble pentacene-containing conjugated copolymers. J. Am. Chem. Soc. 2007, 129, 10308–10309. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Jiang, Y.; Qu, F.; Mayer, A.C.; Parmer, J.E.; McGehee, M.D.; Bao, Z. Synthesis and characterization of pentacene– and anthradithiophene–fluorene conjugated copolymers synthesized by Suzuki reactions. Macromolecules 2008, 41, 6977–6980. [Google Scholar] [CrossRef]
- Mack, J.; Miller, G.P. Synthesis and characterization of a C60-pentacene monoadduct. Fullerene Sci. Tech. 1997, 5, 607–614. [Google Scholar] [CrossRef]
- Miller, G.P.; Mack, J. Completely regioselective, highly stereoselective syntheses of cis-bisfullerene[60] adducts of 6,13-disubstituted pentacenes. Org. Lett. 2000, 2, 3979–3982. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.P.; Mack, J.; Briggs, J. π-Stacking interactions in cis-bisfullerene[60] adducts of 6,13-disubstituted pentacenes. Org. Lett. 2000, 2, 3983–3986. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.P.; Briggs, J.; Mack, J.; Lord, P.A.; Olmstead, M.M.; Balch, A.L. Fullerene–acene chemistry: single-crystal X-ray structure for a [60]fullerene–pentacene monoadduct and a cis-bis[60]fullerene adduct of 6,13-diphenylpentacene. Org. Lett. 2003, 5, 4199–4202. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.B.; Miller, G.P. [60]Fullerene–acene chemistry: a review. C. R. Chim. 2006, 9, 916–927. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lehnherr, D.; Tykwinski, R.R. Oligomers and Polymers Based on Pentacene Building Blocks. Materials 2010, 3, 2772-2800. https://doi.org/10.3390/ma3042772
Lehnherr D, Tykwinski RR. Oligomers and Polymers Based on Pentacene Building Blocks. Materials. 2010; 3(4):2772-2800. https://doi.org/10.3390/ma3042772
Chicago/Turabian StyleLehnherr, Dan, and Rik R. Tykwinski. 2010. "Oligomers and Polymers Based on Pentacene Building Blocks" Materials 3, no. 4: 2772-2800. https://doi.org/10.3390/ma3042772
APA StyleLehnherr, D., & Tykwinski, R. R. (2010). Oligomers and Polymers Based on Pentacene Building Blocks. Materials, 3(4), 2772-2800. https://doi.org/10.3390/ma3042772