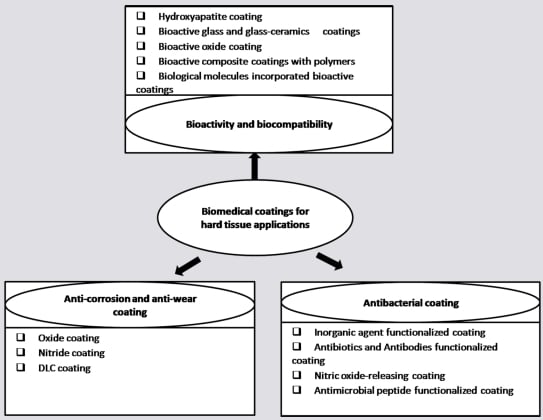
Functional Coatings or Films for Hard-Tissue Applications
Abstract
:1. Introduction
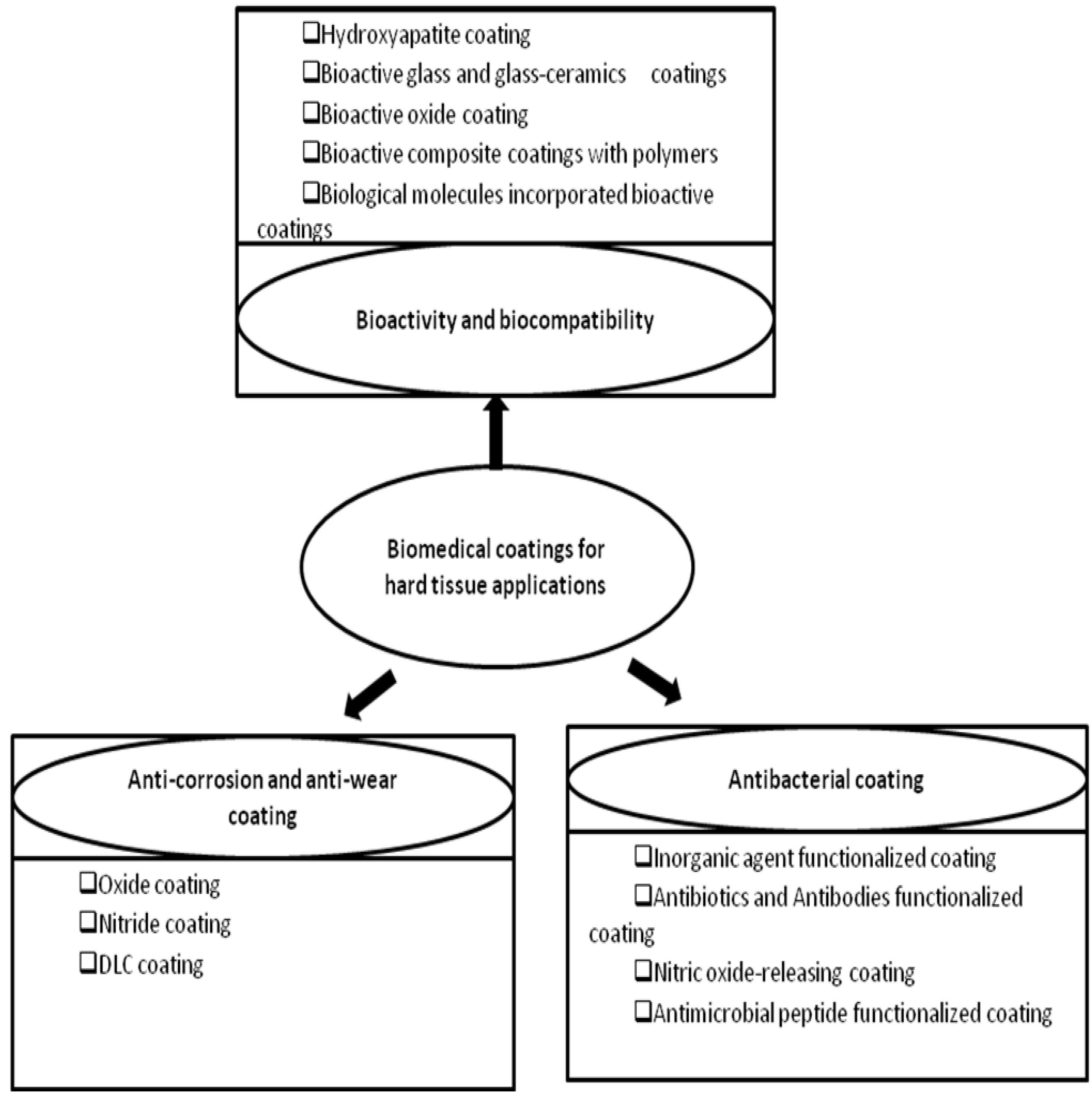
2. Anti-corrosion and Anti-wear Coatings
2.1. Oxide Coatings
2.1.1. Thermal oxidation technique (TO)
2.1.2. Microarc oxidation (MAO)
2.1.3. Oxygen ion implantation
2.1.4. Sol-gel method
2.1.5. Thermal spraying technique
- A
- Composite with other materials: Al2O3 is an attractive material for wear-resistance due to its high hardness and high thermal conductivity. However, brittleness is its main problem limiting its application in some fields. The addition of TiO2 and ZrO2 can improve the fracture toughness of Al2O3 but also lower its hardness [50,51]. Optimizing the appropriate proportion of alumina and zirconia to achieve a composite with improved wear resistance remains to be a challenge.
- B
- Nanostructured coatings: Grain size is another important factor influencing the wear resistance of materials. The relationship between wear resistance and the grain size follows the type of Hall-Petch law [52]. When the grain size is reduced to nanosize, higher external stress is required to induce grain boundary cracking and pulling-out of grains, hence, the nanostructured coatings shows better plastic deformation ability than traditional coating during sliding wear. Moreover, an improved hardness and toughness are also observed for the nanostructured coating [53]. Therefore, nanosized grains are expected to be able to improve the wear resistance of the coatings [54]. Chen et al. [55] compared the friction and wear properties of plasma sprayed nanostructured and conventional zirconia coating against stainless steel with a sliding, reciprocating and vibrating test machine under water-lubricated conditions. It was found that plasma sprayed nanostructured zirconia coatings possessed better wear resistance than traditional coatings in that the wear rate of the former was in the range from one-fourth to four-fifths of the latter under loads ranging from 20 to 50N. The great effects of nanostructured coating on wear resistance are also improved by other researchers using other coating systems like TiO2 [47], Al2O3-ZrO2 [56] and Al2O3-TiO2 [57].
- C
- Post-treatments: There are two major problems with plasma spraying. The primary problem is the poor bonding strength between the coating and substrates, which causes the sprayed material to peel off under high bending stress. The second problem is the high porosity of the coating, which usually reduces the anti-corrosion and anti-wear performance. To overcome these drawbacks, post-treatments by laser are often used. Post-treatments like laser remelting can significantly reduce the porosity and roughness of plasma sprayed coating, and apparently improve the bonding strength, thus enhancing the wear resistance of the as-sprayed coating [58]. For plasma sprayed zirconia coating, laser remelting could change the main wear mechanism from spallation to ploughing and gouging [58]. For Al2O3-13 wt. % TiO2 coating, the enhanced wear resistance after laser melting were ascribed to not only the improvement of the microstructures, but also the transformation from metastable phase γ-Al2O3 to stable phase α-Al2O3 [59].
2.2. Nitride Coatings
| Composition | Substrates | Methods | Electrolyte | Ref. |
|---|---|---|---|---|
| ZrN/Zr | biomedical AZ91 magnesium alloy | filtered cathodic arc deposition | simulated body fluids (SBF) | [60] |
| Zr, ZrN and ZrN/Zr | AISI 304 stainless steel | Hollow cathode discharge ion plating | 0.5 M H2SO4 containing 0.05M KSCN | [64] |
| ZrN0.83/Zr | NiTi shape-memory alloy | plasma immersion ion implantation and deposition | Hank’s Solution | [75] |
| ZrN,TiN and Ti/TiN | 316 L stainless steel | reactive magnetron sputtering | pH 5.6 acetic acid and sodium acetate buffer solution. | [62] |
| ZrN and ZrN-Ag nanocomposite | AISI 316 L surgical steel, and medical grade Ti-Al-V | reactive unbalanced magnetron sputtering | 3.5% NaCl solution | [65] |
| TiN and ZrN | Plain carbon steel | an unbalanced magnetron sputtering technique/low or mild energetic ion bombardment with high flux | sulfuric acid solution (1N) | [66] |
| Ref. | Substrate | Methods | Electrolyte |
|---|---|---|---|
| [62] | 316-L stainless steel | reactive magnetron sputtering | pH 5.6 acetic acid and sodium acetate buffer solution |
| [66] | Plain carbon stee (Ck35) | unbalanced magnetron sputtering technique | 1 N sulfuric acid solution |
| [69] | Biomedical NiTi shape memory alloy | plasma immersion ion implantation and deposition (PIIID) | Hank’s solution |
| [70] | 1Cr11Ni2W2MoV Martensitic stainless steel | hollow cathode ionic plating (HCIP) | 0.5 mol/L NaCl and 1mol/L H2SO4 diluted aqueous solution |
| [71] | Ti-6Al-4V | plasma assisted electron beam PVD technique | 0.5 N NaCl solution |
| [73] | NiTi coated Si | dc magnetron sputtering | 1 mol/L NaCl solution |
| [74] | Biomedical AISI 316L stainless steel | arc ion plating | neutral Troyde’s simulated body fluid |
2.3. Diamond-like Carbon (DLC) Films
- A
- sp3/sp2 ratio: Two types of carbon-carbon interatomic bond exist in the diamond-like carbon (DLC) films, one is sp2 hybridization, as in graphite; the other one is sp3 hybridization, as in diamond. The sp3/sp2 ratio in different DLC films varies significantly depending on the type of the applied deposition techniques and the used procedure parameters. Usually, films with a high proportion of sp2-bonded carbon atoms tend to be relatively soft and behave more like graphite during tribological tests, while films with more sp3-bonded carbons are more like diamond, and hence they are superhard and provide impressive tribological properties. The review written by Bhushan states that sp3/sp2 frictions are in the decreasing order for cathodic arc deposition, pulsed laser vaporization, direct ion beam deposition, plasma-enhanced chemical vapor deposition (PECVD), ion beam sputtering and DC/RF sputtering [103]. In this review paper, it was also proposed that the deposition of sp3-bonded carbon required the depositing species to have a kinetic energy in the order of 100ev or higher. Excess energy, such as that from substrate heating, is detrimental to the achievement of high sp3 friction.
- B
- Hydrogen content: DLC films sputtered with the addition of H2 or derived from a hydrocarbon source, such as acetylene or methane possess a large amount of hydrogen in the films. It is interesting that there is still about 10% hydrogen present in the DLC films sputtered in 100% Ar by direct current (DC) magnetron sputtering [107]. Hydrogen causes the shift of C-C bonds from sp2 to sp3, and generation of a larger number of C-H bonds which relieve the internal stress and produces a softer polymer-like materials. Compared with hydrogen-free DLC films, such films with a high degree of hydrogenation have low friction and wear especially when tests are performed in inert or vacuum test environments [101]. But in the moisture or water environments, their friction increases substantially as the condensed water molecules can give rise to capillary forces [101]. Ronkainen et al. [108] evaluated the tribological performance of different DLC films in water-lubricated conditions. Their results showed that the amorphous hydrogenated carbon films could not survive in the water-lubricated conditions, and was worn through during the test, while the hydrogen-free DLC films fabricated by vacuum arc discharge exhibited the best wear resistance. However, the wear resistance of hydrogenated DLC films can be improved by doping with Si, W and Cr or by interlayers [108].
- C
- Surface roughness: Surface roughness of the DLC films and its underlying substrates has a decisive influence on the wear of the counterface, especially in the case of a soft material such as ultra high molecular weight polyenthylene (UHMWPE). It was reported that even single scratches in the film, which may be undetectable by an average surface roughness measurement, are capable of increasing the wear rate of UHMWPE by a factor of 30–70 [109]. The effect of the substrate surface roughness on the wear behavior of DLC films was investigated on a ball-on-disk wear rig in dry air by Jiang et al. [110]. The wear rate of the films increased significantly with the increase in the substrate surface roughness, while the frictional behavior was not apparently affected. Roughness of 0.93 μm was found to be the critical substrate surface roughness, above which the dominant wear mechanism changed from adhesion to chip/flask formation and fragmentation [110].
- D
- Film thickness: Thick films are preferred for protecting metal from corrosion and wear. However, the compressive stress limits the maximum thickness of the adhesive films and may cause delamination during wearing. Therefore, various methods are used to improve the adhesion strength and reduce the compressive stress. Firstly, cleaning the surface of the substrates with Ar ion bombardment before film deposition is good for the availability of high interfacial adhesion strength. Secondly, forming a mixed interface between film and substrate in the first stage of deposition can also increase the adhesion strength. Thirdly, doping with metal or non-metal elements to reduce the internal stress of the film is also an effective way to obtain high adhesion strength. It was reported that Si doping could improve adhesion strength and reduce internal stress [111], thus increasing the thermal stability of the film as well as the insensitivity of the coefficient of friction to the humidity [112]. Doping metals such as Ta, W, Ti, Nb and Zr in the hydrogenated DLC film also decreased internal stress and lower the dependence of the friction coefficient of the film on humidity [113]. Fourthly, a multilayer approach using alternate soft layer is another effective way to reduce compressive stress in DLC film. Film fabricated by this method showed good friction and wear performance [114]. Finally, diamond-like nanocomposite (DLN) film. DLN film is a new class of materials with reduced compressive stress and increased adhesion strength. This kind of film is composed of two interpenetrating amorphous random network, one is a DLC (α-C:H) network and the other is a glass-like α-Si:O network [113]. Its advantages also include higher temperature stability and a low coefficient of friction.
3. Biocompatibility and Bioactivity
3.1. Hydroxyapatite (HA) Coatings
| Ca/P ratio | Compound | Formula | Solubility at 25oC, –log(Ksp) | Solubility at 37 oC, –log(Ksp) | pH stability range in aqueous solution at 25 oC |
|---|---|---|---|---|---|
| 0.5 | monocalcium phosphate monohydrate (MCPM) | Ca(H2PO4)2·H2O | 1.14 | no data | 0.0–2.0 |
| 0.5 | monocalcium phosphate anhydrate | Ca(H2PO4)2 | 1.14 | no data | [d] |
| 1.0 | diacalcium phosphate dehydrate (DCPD, “brushite”) | CaHPO4·2H2O | 6.59 | 6.63 | 2.0–6.0 |
| 1.0 | diacalcium phosphate anhydrate (DCPA, “monetite”) | CaHPO4 | 6.90 | 7.02 | [d] |
| 1.33 | octacalcium phosphate (OCP) | Ca8(HPO4)2(PO4)4·5H2O | 96.6 | 95.9 | 5.5–7.0 |
| 1.5 | α-tricalcium phosphate (α-TCP) | α-Ca3(PO4)2 | 25.5 | 25.5 | [b] |
| 1.5 | β-tricalcium phosphate (β -TCP) | β-Ca3(PO4)2 | 28.9 | 29.5 | [b] |
| 1.2-2.2 | amorphous calcium phosphate (ACP) | Cax(PO4)y·nH2O | [c] | [c] | [c] |
| 1.5–1.67 | Calcium-deficient hydroxyapatite (CDHA) | Ca10-x(HPO4)x(PO4)6-x(OH)2-x (0 < x < 1) | ≈ 85.1 | ≈ 85.1 | ≈ 6.5–9.5 |
| 1.67 | hydroxyapatite | Ca10(PO4)6(OH)2 | 116.8 | 117.2 | 9.5–12 |
| 2.0 | tetracalcium phosphate (TTCP) | Ca4(PO4)2O | 38–44 | 37–42 | [b] |
| Methods | Characteristics |
|---|---|
| Dip and immersion coating | High temperature for post-sintering HA layer can degrade the strength of the metal and impair the interfacial adhesion and cause the decomposition of HA |
| Electrophoresis deposition | Low bond strength and non-uniform thickness of the coating |
| Hot isostatic pressing | Difficult to seal borders on implants with complex shapes, high temperature during the process may denature HA |
| Solution deposition | A low temperature deposition method resulting in a pure, highly crystalline, firmly adherent coating |
| Sputtering deposition | A line-of-sight technique with low deposition rate and high cost, but the coatings are dense and with uniform thickness on flat substrates |
| Thermal spraying | A line-of-sight technique with high deposition rates and low cost; high temperature may cause decomposition of HA; high cooling rate may result in the formation of nanostructure, coatings usually have micro-rough surface |
| Sol-gel | Not a line-of-sight technique suitable for coating substrates with complex shapes; processing temperature is low; raw materials are expansive and sometimes including organic toxic solvent. |
| Biomimetic coating | Low processing temperature technique capable of coating complex-shaped substrates; time-consuming |
| Laser deposition | Be capable to restore complex stoichiometries and to produce crystalline and highly adherent coatings, but process temperature may cause the oxidation of metal or alloy substrates. |
- A
- Crytallinility: Crystallinity of plasma sprayed HA coating varies from 50% to 90%. Currently, there is no agreement on what the optimum crystallinity should be. However, it is generally agreed that HA coatings with low crystallinity have higher tendency to dissolve in the body fluid thus giving rise to a faster bone growth rate compared to those with high crystallinity. However, high dissolution rate of the HA coating may lead to mechanical degradation, deterioration of the interfacial adhesion, which would finally lead to the loss of the fixation and delamination of the coating. In addition, the debris from the coating may cause undesired inflammatory reaction, thereby compromising the fixation of the implant to bone [3].
- B
- Phase composition: High temperature process of plasma spraying usually causes the decomposition of certain amount of HA phase into an amorphous and tricalcium phosphate (α and/or β-TCP), tetracalcium phosphate (Ca4P2O9; i.e., TTCP) and calcium oxide (CaO). The dissolution rates of these decomposition products are much higher than that of HA, and are in the order of TTCP >> α-TCP > β-TCP >> HA [128]. The fast dissolution of these Ca & P compounds can easily produce supersaturated environment for precipitation of apatite on the coating surface, leading to an enhanced bone growth. It should be stressed that calcium oxide is not biocompatible and should be avoided although it has a high dissolution rate [128]. The side effect of the decomposition of HA is that the fast dissolution of the newly formed Ca & P compound may cause the undesirable fast degradation of the coating. Therefore, both the cystallinity and phase composition should be well designed or controlled for the biomedical use of HA coatings.
- C
- Microstructure and porosity: Microstructure and porosity of the HA coatings, depending on the process parameters, particle size and size distribution of the feedstock powders, can control the specific surface area of the coating thus influencing the physiochemical interactions at the implant-host interface [138].
- D
- Surface roughness: Surface roughness of the HA coating has a significant effect both on the initial mechanical stability of fixation and on osteo-integration. Plasma sprayed HA coating has a roughness of several micrometers, which is strongly influenced by the spray parameters [138], such as spray distance, spraying current, plasma forming gases, and powders conditions. Evidence suggests that rougher surface exhibits a greater mechanical fixation with the nature bone as they are more capable to enhance the adhesion of osteoblast cells and their subsequent proliferation and differentiation [139,140]. The proposed mechanism was that rough surface could induce the release of growth factors and cytokines in the adhering osteoblasts [141]. Additionally, rough surface also favors the precipitation of apatite. Firstly, high roughness allows a large contact area between the coating surface and the body fluid, resulting in an increased Ca & P release. Secondly, rough surface provides more nucleation sides with lower interface energy for bone-like apatite to anchor [142].
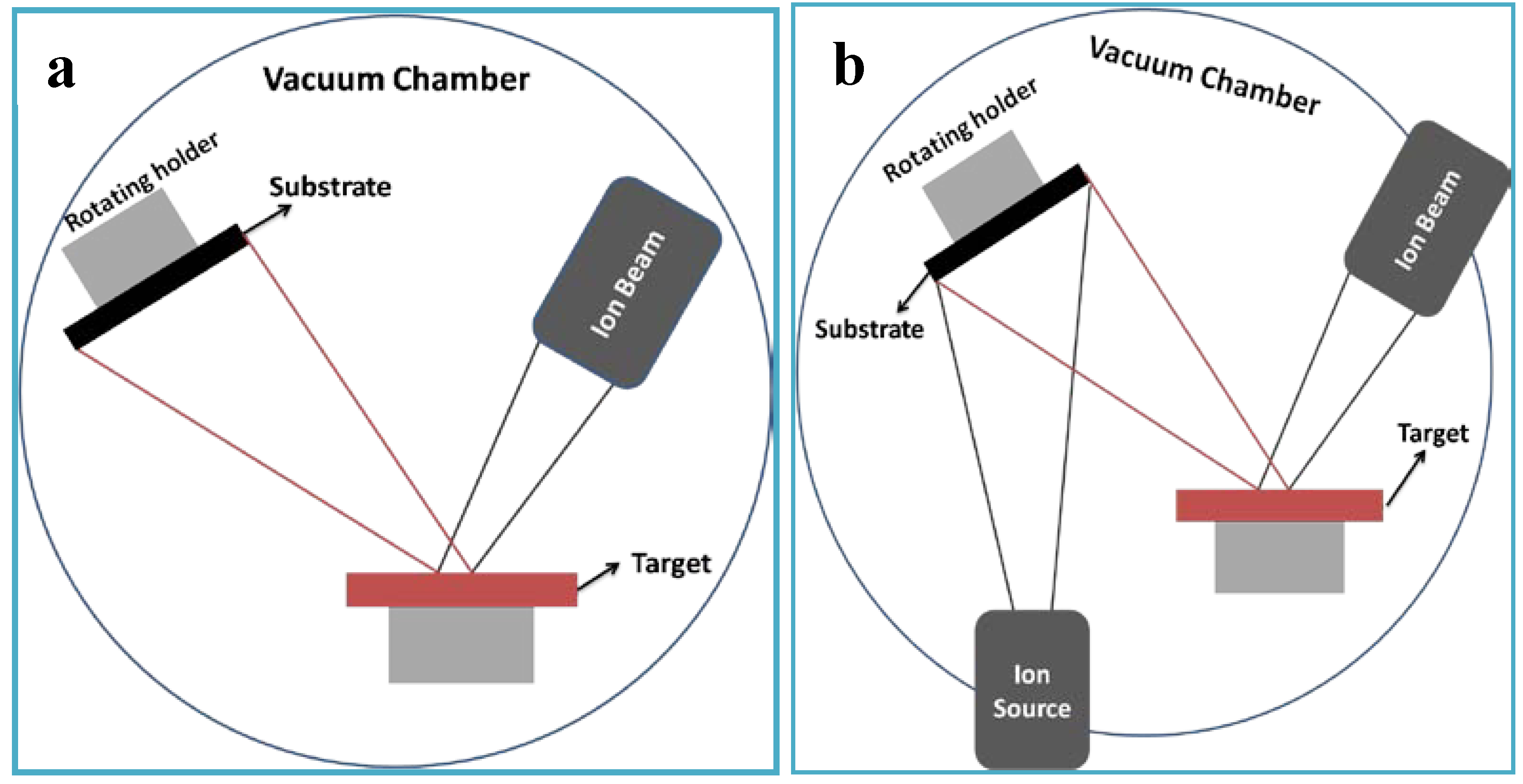
3.2. Bioactive Glass and Glass-ceramics Coatings
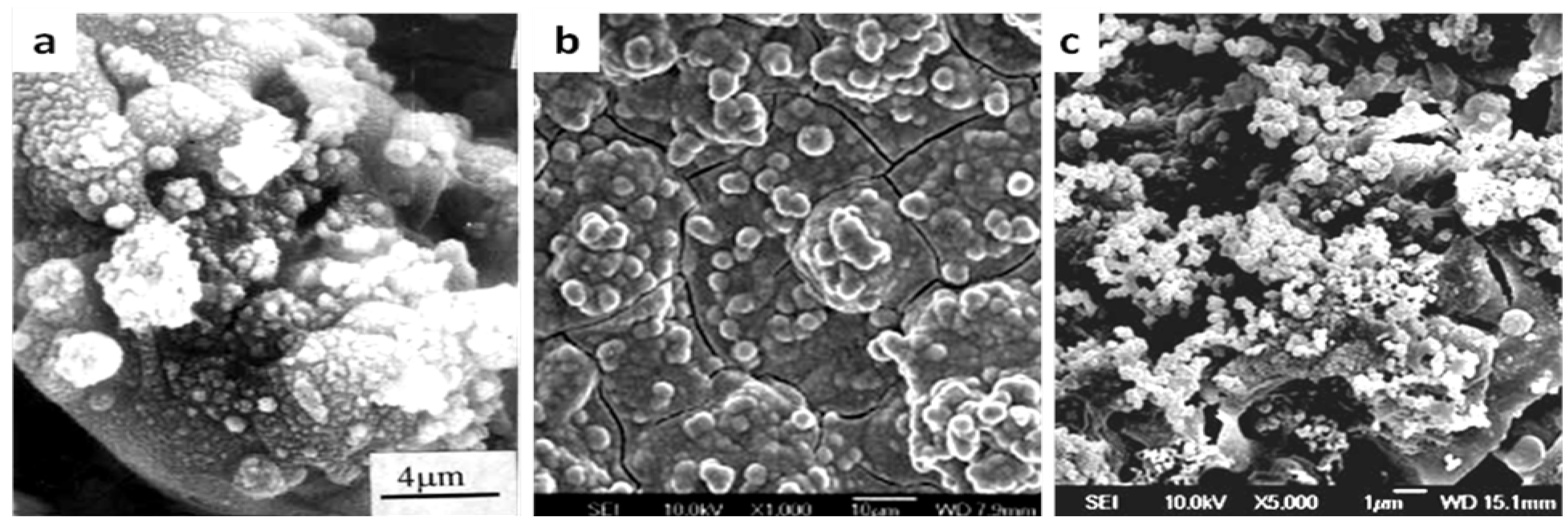
3.3 Bioactive Oxide Coatings
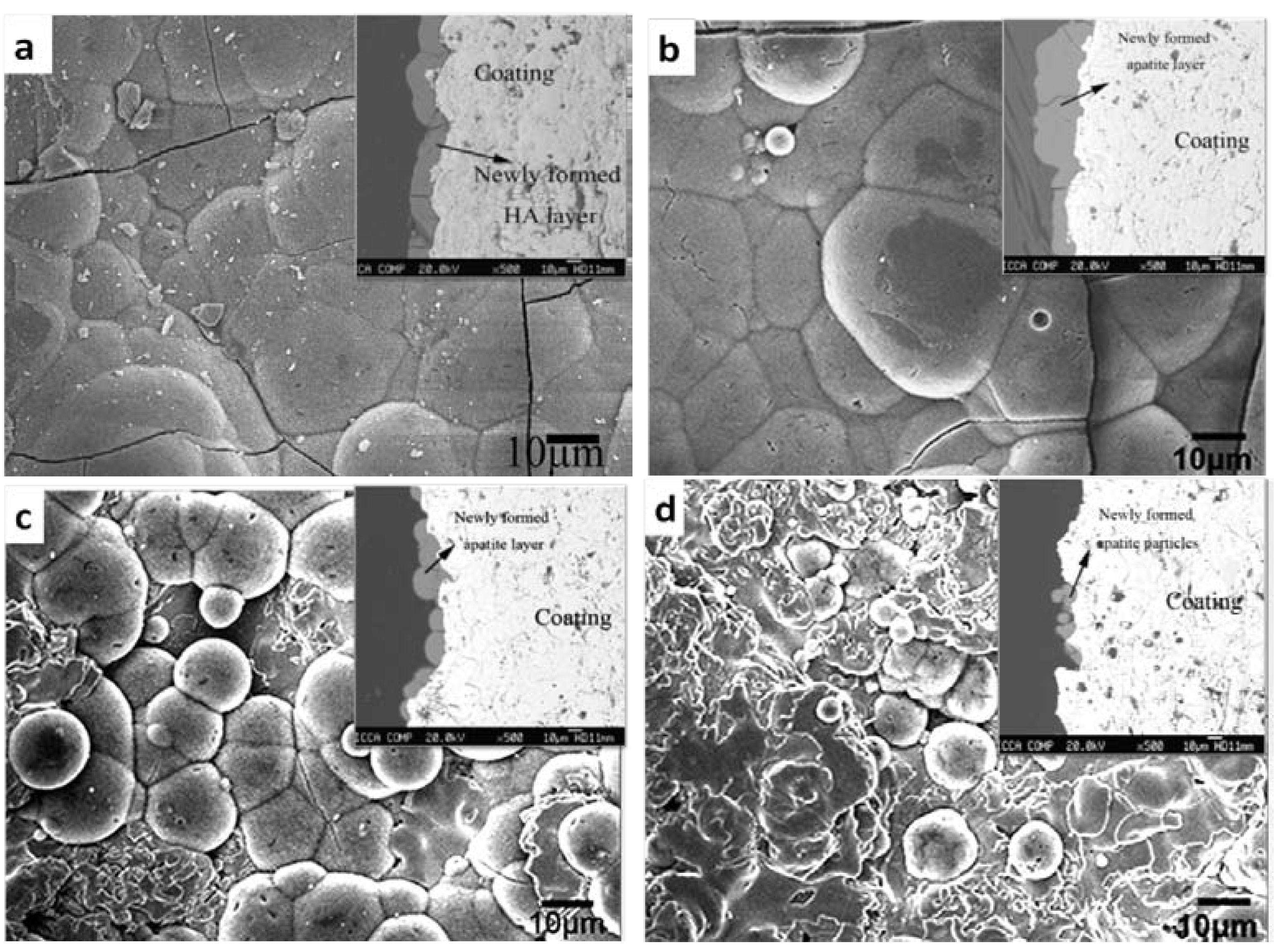
| Coating | Coating method | Ref. | Post-treatments | Phase | Influencing factors in bioactivity |
|---|---|---|---|---|---|
| TiO2 | Solution precursor plasma spray process | [234] | Chemically treated in 5M NaOH solution at 80 °C | Rutile | Formation of Ti-OH groups |
| Sol-gel | [235] | none | Anatase | Surface topography; charge; charge density | |
| [222] | 450 °C, 2 h | Anatase | Abundant Ti-OH groups and negatively charged surfaces | ||
| [223] | Heat-treatment | Anatase | Crystal structure: anatase show more ability to induce apatite formation in SBF than rutile | ||
| Plasma spraying & plasma immersion ion implantation (PIII) | [236] | Hydrogen incorporation by PIII | Rutile (bulk) & anatase (surface) | Combination of nanostructure and hydrogen incorporation can endow the coating with bioactivity | |
| Cathodic electrolytic deposition | [237] | None | Anatase (subcrystalline) | Crystal structure | |
| Below 300 °C | Anatase | ||||
| Above 500 °C | Rutile | ||||
| Anodic oxidation | [238] | H2SO4 and Na2SO4 solutions | rutile or rutile/anatase | Crystal structure: amorphous titania cannot induce apatite formation in SBF solution | |
| CH3COOH and H3PO4 solutions | amorphous titania | ||||
| ZrO2 | Plasma spraying | [219,224] | None | Tetragonal (CaO-ZrO2) | Nanostructured surface; crystal structure |
| None | Monoclinic (undoped ZrO2) | ||||
| Cathodic arc deposition | [239] | None | Tetragonal (undoped ZrO2) | Nanostructured surface | |
| Micro-arc oxidation | [225,227] | None | Monoclinic and small amount of tetragonal ZrO2 | Basic Zr-OH group | |
| NaOH treatment | |||||
| [226] | Ultraviolet (UV) irradiation | Monoclinic and small amount of tetragonal ZrO2 | |||
| SiO2 | Sol-gel | [222] | Heat-treatment at 400 °C for 2 h | amorphous silica | Silanol group (Si-OH) |
3.4. Bioactive Composite Coatings with Polymers
3.5. Biological Molecules Incorporated Bioactive Coatings
4. Antibacterial Coatings
| Immobilization method | Biological molecule | Substrate and pre-treatment | Results | Ref. | |
|---|---|---|---|---|---|
| Adsorption | Bone morphogenetic protein-3 (BMP-3) | Corundum-blasted Titanium alloy; Hydroxyapatite coated Titanium alloy; Ti coated Titanium alloy | BMP-3 coated samples showed more ability to induce new bone formation compared to those without BMP-3 | [251] | |
| Covalent immobilization | by chemical pretreatment | Synthetic receptor binding motif mimicking BMP-2 | 3-aminopropyltriethoxysilane (APTES) coated Titanium | enhance the rate of bone healing as compared with untreated Ti surfaces | [252] |
| Laminin and human epidermal growth factors (EGF) | Silanized TiO2-film Silanisation by reaction of GPTS1 | Significantly reduce the amount of irreversibly adsorbed salivary proteins | [253] | ||
| Heparin | Silanized and oxidized Titanium Oxidization by H2SO4/30% H2O2 or annealing at 750 °C; Silanisation by being boiled in APMS2 contained toluene soltution | The remaining activity of heparin is depending on the chain length of spacer | [243] | ||
| by plasma-based modification | Fibronectin | Plasma polymerization of HMDSO3 on Titanium | Enhanced adsorption of fibronectin | [254] | |
| BMP-4 | Plasma polymerization of allyl amine on Titanium alloy | Surfaces with BMP-4 are initially able to induce ALP activity in C3H10T1/2 cells, long term effect is depending on the concentration of surface amino group | [250] | ||
| Incorporation with carriers | Recombinant human BMP-2 (rhBMP-2) | Turned or surface etched Titanium dental implant Absorbable Collagen sponge (ACS) | rhBMP-2/ACS significantly enhances the effect of guided bore regeneration (GBR) | [255] | |
| BMP-2; insulin-like growth factor-1 and transforming growth factor-β1 | Titanium Kirschner wires incorporated with poly(D,L-lactide) (PDLLA) | Significantly accelerate the fracture healing | [256,257] | ||
4.1. Antibacterial Coatings with Inorganic Agents
| Inorganic agents | Coatings | Coating methods | Testing bacteria | Note | Ref. | |
|---|---|---|---|---|---|---|
| Ag-related agent | TiN/Ag multilayered films | ion beam assisted deposition | E. coli | Antibacterial activity is depending on the modulation period | [284] | |
| Silver doped perfluoropolyether-urethane coatings | Coating /evapration | P. aeruginosa A. baumannii S. epidermidis | Antibacterial activity is depending on the release of Ag ions | [278] | ||
| TiO2-Ag coating | Plasma electrolytic oxidation in Ag nanoparticle- contained electrolyte | S. aureus | Possibly, antibacterial activity is due to the close contact of bacteria with Ag particles and the release of Ag ions | [275] | ||
| polyethylene terephthalate implanted with Ag ion | Ion beam implantation | S. epidermidis | Ag exists in the form of Ag2CO3 and Ag2O | [281] | ||
| Poly(vinyl alcohol) / AgNO3 | Solution/evaporation | E. coli and S. aureus | Ag ions can release from the composite coating | [276] | ||
| Silver doped SiO2 film | Sol-gel | E. coli and S. aureus | Reduction of Ag+ ion is affected by the annealing temperature | [272] | ||
| Non-Ag agent | F | F--implanted titanium | Ion implantation | P. gingivalis and A. actinomycetemcomitans | Antibacterial activity was supposed to be caused by the formation of a metal fluoride complex on the surfaces | [282] |
| C | Carbon film | Plasma sputtering for H-free film Chemical vapor deposition for α-C:H film | E. coli | α-C:H film showed relatively poor antibacterial activity compared with hydrogen-free carbon films | [283] | |
| TiO2 | TiO2 film | plasma source ion implantation followed by annealing | A. actinomycetemcomitans F. nucleatum | Antibacterial activity is due to the photocatalytic bactericidal effect | [284] | |
| TiO2 film | A flame-assisted CVD to deposit SiO2, and thermal APCVD to deposit TiO2 | E. coli | [277] | |||
| DLC films containing TiO2 nanoparticles | plasma-enhanced chemical vapor deposition | E. coli | Enhanced antibacterial activity are contributed by the increased hydrophilicity and the decreased interfacial energy of bacteria adhesion | [285] | ||
| ZnO | ZnO coated glass | Ultrasonic irradiation | E. coli and S. aureus | The antibacterial activity is due to the generation of the reactive-oxygen-species (ROS) products | [286] | |
4.2. Antibacterial Coatings with Antibiotics and Antibodies
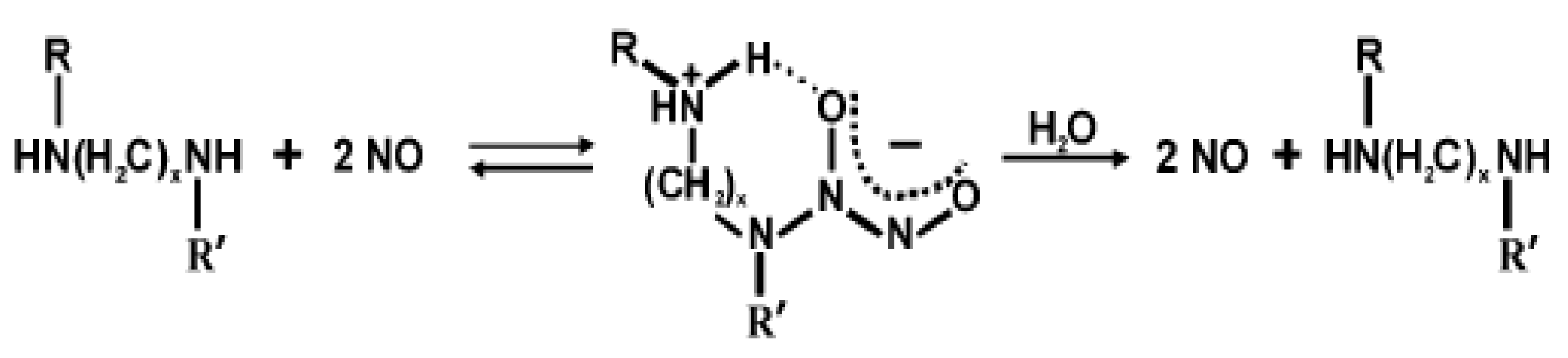
4.3. Nitric Oxide-releasing Antibacterial Coatings
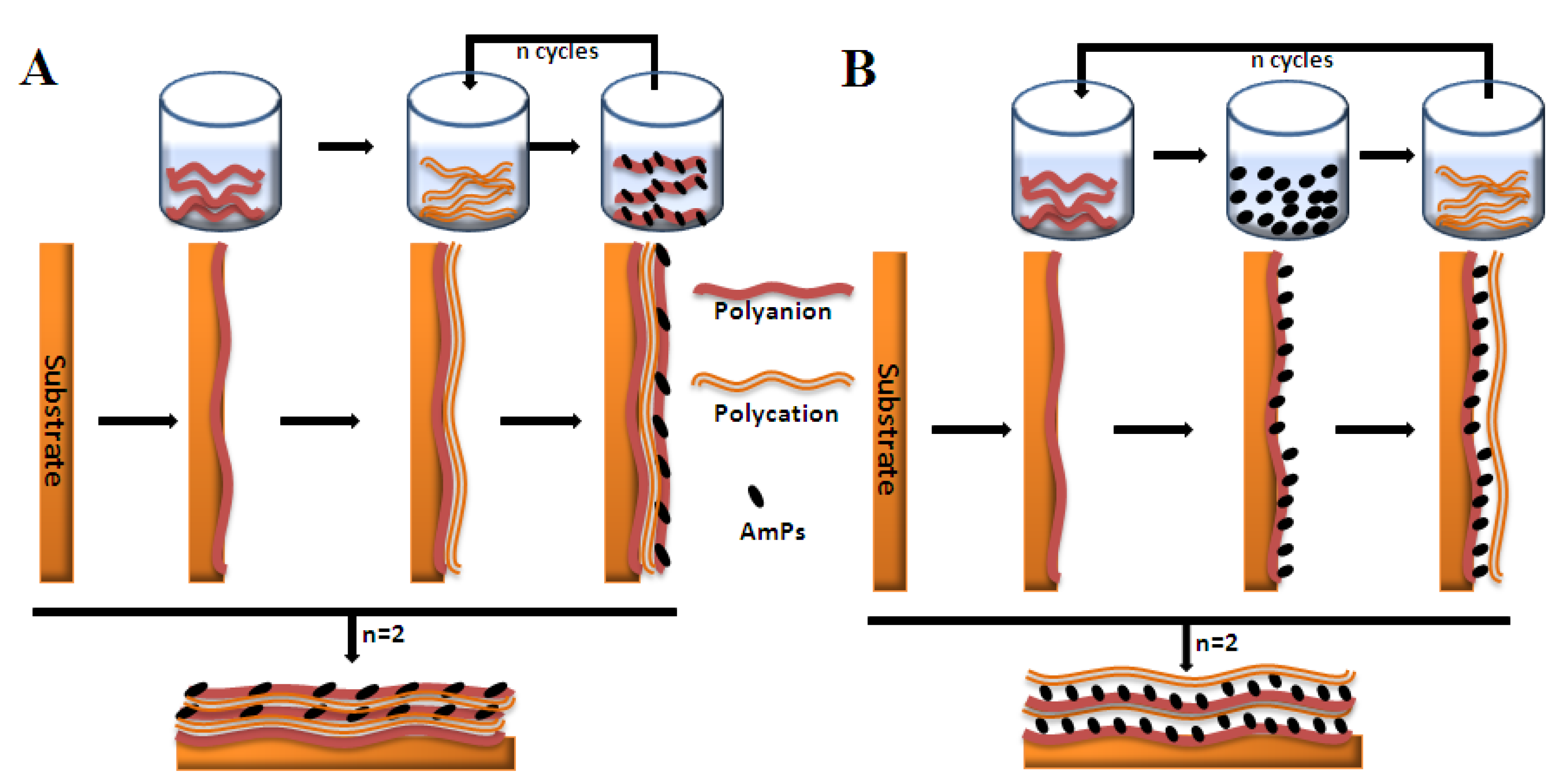
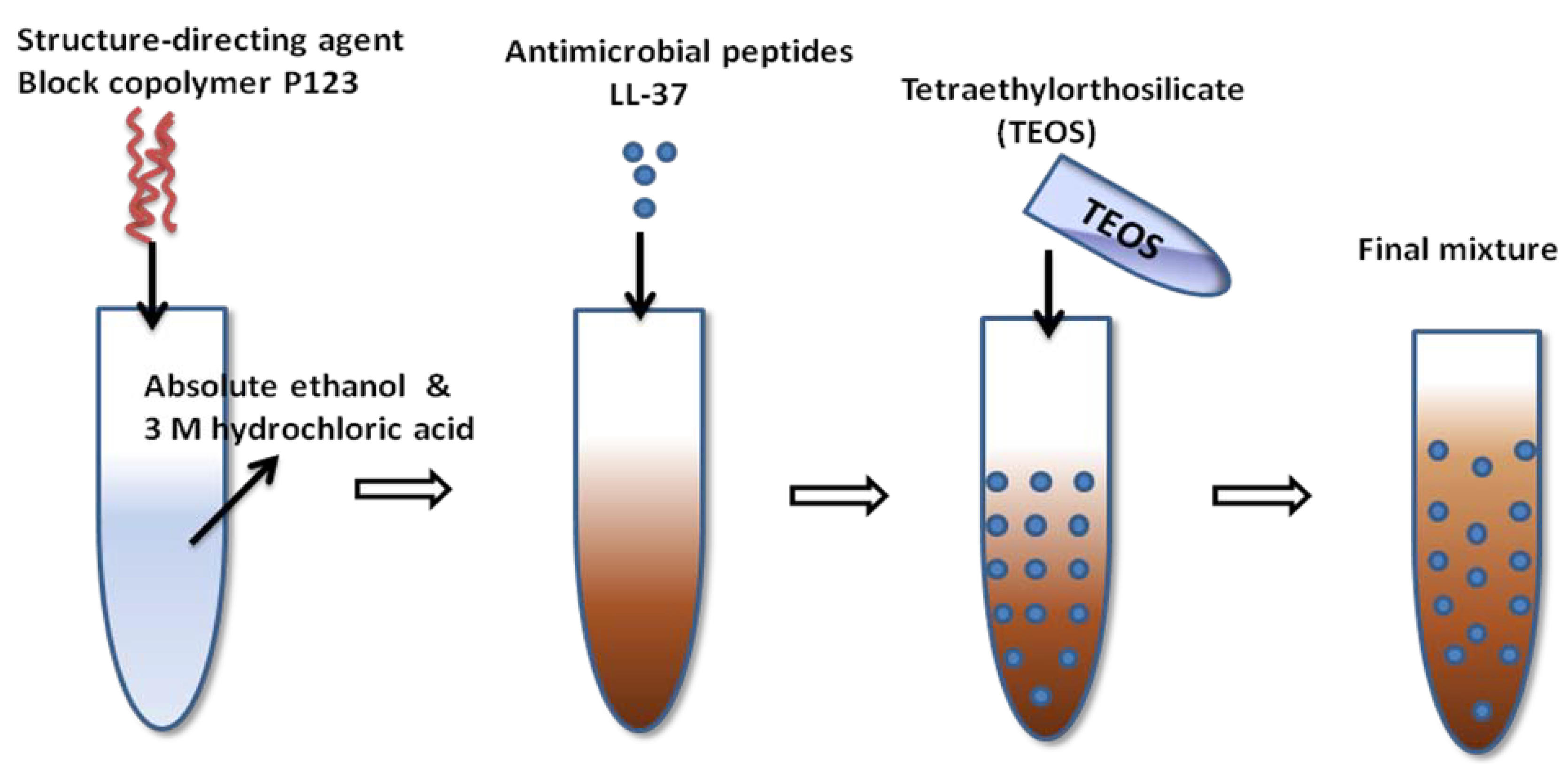
4.4. Antimicrobial Peptide Functionalized Films
| Antimicrobial peptides | Amino acid sequence | Coating methods | Ref. |
|---|---|---|---|
| Defensin | ATCDLASGFGVGSSLCAAHCIARRYRGGYCNSKAVCVCRN | LbL | [326] |
| Chromofungin | RILSILRHQNLLKELQDLAL | LbL | [327] |
| Magainin I | GIGLPLHSAGLPGLAPVGGIMLS | SAMs | [328] |
| Gramicidine A | VGALAVVVWLWLWLW | LbL | [321] |
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | One-pot EISA | [329] |
| Ponericin G1 | GWKDWAKKAGGWLKKKGPGMAKAALKAAMQ | LbL | [322] |
5. Summary
Acknowledgements
References
- Niinomi, M. Metallic biomaterials. J. Artif. Organs 2008, 11, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.P.; Daffner, R.H.; Gallo, R.A. Electrochemical corrosion of metal implants. AJR. Am. J. Roentgenol. 2005, 184, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Ingham, E.; Fisher, J. Biological reaction to wear debris in total joint replacement. Proc. Inst. Mech. Eng. H 2000, 214, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Spector, M. Biomaterial failure. Orthop. Clin. North. Am. 1992, 23, 211–217. [Google Scholar] [PubMed]
- Willmann, G. Coating of Implants with Hydroxyapatite—Material Connections between Bone and Metal. Adv. Eng. Mater. 1999, 1, 95–105. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.; Bronzino, J.D. Biomaterials, 2nd ed; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Peracchia, G.; Bobbio, P.; Vecchi, P. Effects of the introduction of a wire of radioactive tantalum (Ta-182) into the internal mammary artery. Experimental research. Boll. Soc. Ital. Biol. Sper. 1961, 37, 832–834. [Google Scholar] [PubMed]
- Mudali, U.K.; Sridhar, T.M.; RAJ, B. Corrosion of bio implants. Sadhana 2003, 28, 601–637. [Google Scholar] [CrossRef]
- Siva Rama Krishna, D.; Brama, Y.L.; Sun, Y. Thick rutile layer on titanium for tribological applications. Tribol. Int. 2007, 40, 329–334. [Google Scholar] [CrossRef]
- García-Alonso, M.C.; Saldaña, L.; Vallés, G.; González-Carrasco, J.L.; González-Cabrero, J.; Martínez, M.E.; Gil-Garay, E.; Munuera, L. In vitro corrosion behaviour and osteoblast response of thermally oxidised Ti6Al4V alloy. Biomaterials 2003, 24, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Dearnley, P.A.; Dahm, K.L.; Çimenoglu, H. The corrosion-wear behaviour of thermally oxidised CP-Ti and Ti-6Al-4V. Wear 2004, 256, 469–479. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Iozzelli, F.; Pradelli, G. Improvement of wear resistance of Ti-6Al-4V alloy by means of thermal oxidation. Mater. Lett. 2005, 59, 2159–2162. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Surface modification of a Ti-6Al-4V alloy by thermal oxidation. Surf. Coat. Tech. 2005, 192, 164–170. [Google Scholar] [CrossRef]
- Xia, J.; Li, C.X.; Dong, H.; Bell, T. Nanoindentation and nanoscratch properties of a thermal oxidation treated [gamma]-TiAl based alloy. Surf. Coat. Tech. 2006, 200, 4755–4762. [Google Scholar] [CrossRef]
- Siva Rama Krishna, D.; Sun, Y. Effect of thermal oxidation conditions on tribological behaviour of titanium films on 316L stainless steel. Surf. Coat. Tech. 2005, 198, 447–453. [Google Scholar] [CrossRef]
- Lavisse, L.; Grevey, D.; Langlade, C.; Vannes, B. The early stage of the laser-induced oxidation of titanium substrates. Appl. Surf. Sci. 2002, 186, 150–155. [Google Scholar] [CrossRef]
- Dong, H.; Bell, T. Enhanced wear resistance of titanium surfaces by a new thermal oxidation treatment. Wear 2000, 238, 131–137. [Google Scholar] [CrossRef]
- Güleryüz, H.; Çimenoglu, H. Effect of thermal oxidation on corrosion and corrosion-wear behaviour of a Ti-6Al-4V alloy. Biomaterials 2004, 25, 3325–3333. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, X.Y. Oxygen boost diffusion for the deep-case hardening of titanium alloys. Mater. Sci. Eng. A 2000, 280, 303–310. [Google Scholar] [CrossRef]
- Saldaña, L.; Vilaboa, N.; Vallés, G.; González-Cabrero, J.; Munuera, L. Osteoblast response to thermally oxidized Ti6Al4V alloy. J. Biomed. Mater. Res. A 2005, 73A, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Wang, C.; Chen, R.; Deng, Z. Structure and properties characterization of ceramic coatings produced on Ti-6Al-4V alloy by microarc oxidation in aluminate solution. Mater. Lett. 2002, 52, 435–441. [Google Scholar] [CrossRef]
- Rama Krishna, L.; Somaraju, K.R.C.; Sundararajan, G. The tribological performance of ultra-hard ceramic composite coatings obtained through microarc oxidation. Surf. Coat. Tech. 2003, 163-164, 484–490. [Google Scholar] [CrossRef]
- Liang, J.; Hu, L.; Hao, J. Improvement of corrosion properties of microarc oxidation coating on magnesium alloy by optimizing current density parameters. Appl. Surf. Sci. 2007, 253, 6939–6945. [Google Scholar] [CrossRef]
- Guo, H.F.; An, M.Z. Growth of ceramic coatings on AZ91D magnesium alloys by micro-arc oxidation in aluminate-fluoride solutions and evaluation of corrosion resistance. Appl. Surf. Sci. 2005, 246, 229–238. [Google Scholar] [CrossRef]
- Liang, J.; Guo, B.; Tian, J.; Liu, H.; Zhou, J.; Xu, T. Effect of potassium fluoride in electrolytic solution on the structure and properties of microarc oxidation coatings on magnesium alloy. Appl. Surf. Sci. 2005, 252, 345–351. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, H.; Yao, B.; Qin, Z.; Zhang, Q. Corrosion resistance property of the ceramic coating obtained through microarc oxidation on the AZ31 magnesium alloy surfaces. Surf. Coat. Tech. 2007, 201, 4905–4908. [Google Scholar] [CrossRef]
- Liang, J.; Hu, L.; Hao, J. Characterization of microarc oxidation coatings formed on AM60B magnesium alloy in silicate and phosphate electrolytes. Appl. Surf. Sci. 2007, 253, 4490–4496. [Google Scholar] [CrossRef]
- Guo, H.; An, M. Effect of surfactants on surface morphology of ceramic coatings fabricated on magnesium alloys by micro-arc oxidation. Thin Solid Films 2006, 500, 186–189. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, L.; Wei, B.; Liu, Q. Electrochemical performance of microarc oxidation films formed on AZ91D magnesium alloy in silicate and phosphate electrolytes. Surf. Coat. Tech. 2006, 200, 3727–3733. [Google Scholar] [CrossRef]
- Guo, H.; An, M.; Xu, S.; Huo, H. Microarc oxidation of corrosion resistant ceramic coating on a magnesium alloy. Mater. Lett. 2006, 60, 1538–1541. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng.: R 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Leng, Y.X.; Chen, J.Y.; Zeng, Z.M.; Tian, X.B.; Yang, P.; Huang, N.; Zhou, Z.R.; Chu, P.K. Properties of titanium oxide biomaterials synthesized by titanium plasma immersion ion implantation and reactive ion oxidation. Thin Solid Films 2000, 377-378, 573–577. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng.: R 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Tan, L.; Crone, W.C. Surface characterization of NiTi modified by plasma source ion implantation. Acta Mater. 2002, 50, 4449–4460. [Google Scholar] [CrossRef]
- Tan, L.; Dodd, R.A.; Crone, W.C. Corrosion and wear-corrosion behavior of NiTi modified by plasma source ion implantation. Biomaterials 2003, 24, 3931–3939. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Shaw, G.; Sridharan, K.; Crone, W.C. Effects of oxygen ion implantation on wear behavior of NiTi shape memory alloy. Mech. Mater. 2005, 37, 1059–1068. [Google Scholar] [CrossRef]
- Lima Neto, P.; Atik, M.; Avaca, L.A.; Aegerter, M.A. Sol-gel coatings for chemical protection of stainless steel. J. Sol-Gel Sci. Technol. 1994, 2, 529–534. [Google Scholar] [CrossRef]
- Liu, J.X.; Yang, D.Z.; Shi, F.; Cai, Y.J. Sol-gel deposited TiO2 film on NiTi surgical alloy for biocompatibility improvement. Thin Solid Films 2003, 429, 225–230. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Wang, C. Tribological behavior of sol-gel TiO2 films on glass. Wear 2002, 253, 377–384. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, Y.; Wu, Z.; Zhang, P. Tribological properties of anatase TiO2 sol-gel films controlled by mutually soluble dopants. Tribol. Lett. 2007, 26, 19–24. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Liu, W. Characterization and tribological investigation of sol-gel ceramic films on Ti-6Al-4V. Wear 2006, 260, 379–386. [Google Scholar] [CrossRef]
- Zhang, W.G.; Liu, W.M.; Liu, Y.; Wang, C.T. Tribological behaviors of single and dual sol-gel ceramic films on Ti-6Al-4V. Ceram. Int. 2009, 35, 1513–1520. [Google Scholar] [CrossRef]
- Keceli, S.A.; Alanyali, H. A Study on the Evaluation of the Cytotoxicity of Al2O3, Nb2O5, Ta2O5, TiO2 and ZrO2. Turkish J. Eng. Env. Sci. 2004, 28, 49–54. [Google Scholar]
- Wang, Y.; Jin, Y.; Wen, S. The analysis of the friction and wear mechanisms of plasma-sprayed ceramic coatings at 450 °C. Wear 1988, 128, 265–276. [Google Scholar] [CrossRef]
- Dai, W.W.; Ding, C.X.; Li, J.F.; Zhang, Y.F.; Zhang, P.Y. Wear mechanism of plasma-sprayed TiO2 coating against stainless steel. Wear 1996, 196, 238–242. [Google Scholar] [CrossRef]
- Ibrahim, A.; Lima, R.S.; Berndt, C.C.; Marple, B.R. Fatigue and mechanical properties of nanostructured and conventional titania (TiO2) thermal spray coatings. Surf. Coat. Tech. 2007, 201, 7589–7596. [Google Scholar] [CrossRef]
- Fervel, V.; Normand, B.; Coddet, C. Tribological behavior of plasma sprayed Al2O3-based cermet coatings. Wear 1999, 230, 70–77. [Google Scholar] [CrossRef]
- Ahn, H.S.; Kim, J.Y. Lim, D.S. Tribological behaviour of plasma-sprayed zirconia coatings. Wear 1997, 203-204, 77–87. [Google Scholar] [CrossRef]
- Yilmaz, R.; Kurt, A.O.; Demir, A.; TatlI, Z. Effects of TiO2 on the mechanical properties of the Al2O3-TiO2 plasma sprayed coating. J. Eur. Ceram. Soc. 2007, 27, 1319–1323. [Google Scholar] [CrossRef]
- Yang, C.; Bahadur, S. Friction and wear behavior of alumina-based ceramics in dry and lubricated sliding against tool steel. Wear 1992, 157, 263–277. [Google Scholar] [CrossRef]
- Zum Gahr, K.H.; Bundschuh, W.; Zimmerlin, B. Effect of grain size on friction and sliding wear of oxide ceramics. Wear 1993, 162-164, 269–279. [Google Scholar] [CrossRef]
- Fauchais, P.; Etchart-Salas, R.; Rat, V.; Coudert, J.F.; Caron, N.; Wittmann-Ténèze, K. Parameters Controlling Liquid Plasma Spraying: Solutions, Sols, or Suspensions. J. Therm. Spray Technol. 2008, 17, 31–59. [Google Scholar] [CrossRef]
- He, C.; Wang, Y.S.; Wallace, J.S.; Hsu, S.M. Effect of microstructure on the wear transition of zirconia-toughened alumina. Wear 1993, 162-164, 314–321. [Google Scholar] [CrossRef]
- Chen, H.; Ding, C.; Zhang, P.; La, P.; Lee, S.W. Wear of plasma-sprayed nanostructured zirconia coatings against stainless steel under distilled-water conditions. Surf. Coat. Tech. 2003, 173, 144–149. [Google Scholar] [CrossRef]
- Kerkwijk, B.; Winnubst, A.J.A.; Verweij, H.; Mulder, E.J.; Metselaar, H.S.C.; Schipper, D.J. Tribological properties of nanoscale alumina-zirconia composites. Wear 1999, 225-229, 1293–1302. [Google Scholar] [CrossRef]
- Lin, X.; Zeng, Y.; Ding, C.; Zhang, P. Effects of temperature on tribological properties of nanostructured and conventional Al2O3-3 wt.% TiO2 coatings. Wear 2004, 256, 1018–1025. [Google Scholar] [CrossRef]
- Fu, Y.; Batchelor, A.W.; Xing, H.; Gu, Y. Wear behaviour of laser-treated plasma-sprayed ZrO2 coatings. Wear 1997, 210, 157–164. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Y.; Liu, Z.; Chuang, Y. Laser remelting of plasma sprayed Al2O3 ceramic coatings and subsequent wear resistance. Mater. Sci. Eng. A 2000, 291, 168–172. [Google Scholar] [CrossRef]
- Xin, Y.C.; Liu, C.L.; Huo, K.F.; Tang, G.Y.; Tian, X.B.; Chu, P.K. Corrosion behavior of ZrN/Zr coated biomedical AZ91 magnesium alloy. Surf. Coat. Tech. 2009, 203, 2554–2557. [Google Scholar] [CrossRef]
- Yan, C.; Zheng, Y.F. A study of ZrN/Zr coatings deposited on NiTi alloy by PIIID technique. IEEE Trans. Plasma Sci. 2006, 34, 1105–1108. [Google Scholar] [CrossRef]
- Hübler, R.; Cozza, A.; Marcondes, T.L.; Souza, R.B.; Fiori, F.F. Wear and corrosion protection of 316-L femoral implants by deposition of thin films. Surf. Coat. Tech. 2001, 142-144, 1078–1083. [Google Scholar] [CrossRef]
- Hendry, J.A.; Pilliar, R.M. The fretting corrosion resistance of PVD surface-modified orthopedic implant alloys. J. Biomed. Mater. Res. 2001, 58, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.J.; Yu, G.P.; Huang, J.H. Corrosion resistance of ZrN films on AISI 304 stainless steel substrate. Surf. Coat. Tech. 2003, 167, 59–67. [Google Scholar] [CrossRef]
- Kertzman, Z.; Marchal, J.; Suarez, M.; Staia, M.H.; Filip, P.; Kohli, P.; Aouadi, S.M. Mechanical, tribological, and biocompatibility properties of ZrN-Ag nanocomposite films. J. Biomed. Mater. Res. A 2008, 84, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Kelesoglu, E.; Mitterer, C.; Ürgen, M. Corrosion characteristics of plain carbon steel coated with TiN and ZrN under high-flux ion bombardment. Surf. Coat. Tech. 2002, 160, 82–86. [Google Scholar] [CrossRef]
- Gispert, M.P.; Serro, A.P.; Colaco, R.; do Rego, A.M.B.; Alves, E.; da Silva, R.C.; Brogueira, P.; Pires, E.; Saramago, B. Tribological behaviour of Cl-implanted TiN coatings for biomedical applications. Wear 2007, 262, 1337–1345. [Google Scholar] [CrossRef]
- Kao, C.T.; Ding, S.J.; Chen, Y.C.; Huang, T.H. The anticorrosion ability of titanium nitride (TiN) plating on an orthodontic metal bracket and its biocompatibility. J. Biomed. Mater. Res. 2002, 63, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zheng, Y.F. Formation of TiN films on biomedical NiTi shape memory alloy by PIIID. Mater. Sci. Eng. A Struct. Mater. 2006, 434, 99–104. [Google Scholar] [CrossRef]
- Li, Y.; Qu, L.; Wang, F. The electrochemical corrosion behavior of TiN and (Ti,Al)N coatings in acid and salt solution. Corr. Sci. 2003, 45, 1367–1381. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Matthews, A. Tribological and electrochemical performance of PVD TiN coatings on the femoral head of Ti-6Al-4V artificial hip joints. Surf. Coat. Tech. 2003, 163-164, 597–604. [Google Scholar] [CrossRef]
- Monticelli, C.; Zucchi, F.; Tampieri, A. Triboelectrochemical behaviour of a Si3N4-TiN ceramic composite and a titanium alloy commonly used in biomedical applications. Wear 2009, 266, 327–336. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, D. Nanoindentation and corrosion studies of TiN/NiTi thin films for biomedical applications. Surf. Coat. Tech. 2009, 204, 1132–1136. [Google Scholar] [CrossRef]
- Liu, C.L.; Lin, G.Q.; Yang, D.Z.; Qi, M. In vitro corrosion behavior of multilayered Ti/TiN coating on biomedical AISI 316L stainless steel. Surf. Coat. Tech. 2006, 200, 4011–4016. [Google Scholar] [CrossRef]
- Cheng, Y.; Zheng, Y.F. A study of ZrN/Zr coatings deposited on NiTi alloy by PIIID technique. IEEE Trans. Plasma Sci. 2006, 34, 1105–1108. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Du, H.J.; Zhang, S. Deposition of TiN layer on TiNi thin films to improve surface properties. Surf. Coat. Tech. 2003, 167, 129–136. [Google Scholar] [CrossRef]
- da Silva, L.L.G.; Ueda, M.; Silva, M.M.; Codaro, E.N. Corrosion behavior of Ti-6Al-4V alloy treated by plasma immersion ion implantation process. Surf. Coat. Tech. 2007, 201, 8136–8139. [Google Scholar] [CrossRef]
- Pelletier, J.; Anders, A. Plasma-blased ion implantation and deposition: A review of physics, technology, and applications. IEEE Trans. Plasma Sci. 2005, 33, 1944–1959. [Google Scholar] [CrossRef]
- Oztarhan, A.; Brown, I.; Bakkaloglu, C.; Watt, G.; Evans, P.; Oks, E.; Nikolaev, A.; Tek, Z. Metal vapour vacuum arc ion implantation facility in Turkey. Surf. Coat. Tech. 2005, 196, 327–332. [Google Scholar] [CrossRef]
- Ueda, M.; Gomes, G.F.; Kostov, K.G.; Reuther, H.; Lepienski, C.M.; Soares, P.C.; Takai, O.; Silva, M.M. Results from experiments on hybrid plasma immersion ion implantation/nitriding processing of materials. Braz. J. Phys. 2004, 34, 1632–1637. [Google Scholar] [CrossRef]
- Lei, M.K.; Zhang, Z.L.; Ma, T.C. Plasma-based low-energy ion implantation for low-temperature surface engineering. Surf. Coat. Tech. 2000, 131, 317–325. [Google Scholar] [CrossRef]
- Abadias, G. Stress and preferred orientation in nitride-based PVD coatings. Surf. Coat. Tech. 2008, 202, 2223–2235. [Google Scholar] [CrossRef]
- Vadiraj, A.; Kamaraj, M.; Gnanomoorthy, R. Fretting wear studies on PVD TiN coated, ion implanted and thermally oxidised biomedical titanium alloys. Surf. Eng. 2007, 23, 209–215. [Google Scholar] [CrossRef]
- Rodríguez, R.J.; García, J.A.; Medrano, A.; Rico, M.; Sánchez, R.; Martínez, R.; Labrugère, C.; Lahaye, M.; Guette, A. Tribological behaviour of hard coatings deposited by arc-evaporation PVD. Vacuum 2002, 67, 559–566. [Google Scholar] [CrossRef]
- Ochoa, E.A.; Figueroa, C.A. Influence of the microstructure on steel hardening in pulsed plasma nitriding. J. Vac. Sci. Technol. A 2008, 26, 328–332. [Google Scholar] [CrossRef]
- Clem, W.C.; Konovalov, V.V.; Chowdhury, S.; Vohra, Y.K.; Catledge, S.A.; Bellis, S.L. Mesenchymal stem cell adhesion and spreading on microwave plasma-nitrided titanium alloy. J. Biomed. Mater. Res. A 2006, 76A, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.K.Y.; Kwok, S.C.H.; Chen, P.; Yang, P.; Ngai, R.H.C.; Tian, X.B.; Chu, P.K. Surface modification of cemented carbide using plasma nitriding and metal ion implantation. Surf. Coat. Tech. 2005, 196, 150–154. [Google Scholar] [CrossRef]
- Sun, F.J.; Liu, J.Y.; Yang, Y.L.; Yu, H.J. Nitridation of iron by CW-CO2 laser nitriding technologies. Mater. Sci. Eng. B 2005, 122, 29–33. [Google Scholar] [CrossRef]
- Schaaf, P.; Han, M.; Lieb, K.P.; Carpene, E. Laser nitriding of iron with laser pulses from femtosecond to nanosecond pulse duration. Appl. Phys. Lett. 2002, 80, 1091–1093. [Google Scholar] [CrossRef]
- Schaaf, P. Laser nitriding of metals. Prog. Mater. Sci. 2002, 47, 1–161. [Google Scholar] [CrossRef]
- Li, D.J.; Cui, F.Z.; Gu, H.Q. Studies of diamond-like carbon films coated on PMMA by ion beam assisted deposition. Appl. Surf. Sci. 1999, 137, 30–37. [Google Scholar] [CrossRef]
- Ma, W.J.; Ruys, A.J.; Mason, R.S.; Martin, P.J.; Bendavid, A.; Liu, Z.W.; Ionescu, M.; Zreiqat, H. DLC coatings: Effects of physical and chemical properties on biological response. Biomaterials 2007, 28, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Seung, L.C.; Lee, K.R.; Yong, E.K.; Hyun, Y.K.; Hee, H.J. Structure and properties of Si incorporated tetrahedral amorphous carbon films prepared by hybrid filtered vacuum arc process. Diam. Relat. Mater. 2002, 11, 198–203. [Google Scholar] [CrossRef]
- Zou, Y.S.; Wang, W.; Song, G.H.; Du, H.; Gong, J.; Huang, R.F.; Wen, L.S. Influence of the gas atmosphere on the microstructure and mechanical properties of diamond-like carbon films by arc ion plating. Mater. Lett. 2004, 58, 3271–3275. [Google Scholar] [CrossRef]
- Thorwarth, G.; Hammerl, C.; Kuhn, M.; Assmann, W.; Schey, B.; Stritzker, B. Investigation of DLC synthesized by plasma immersion ion implantation and deposition. Surf. Coat. Tech. 2005, 193, 206–212. [Google Scholar] [CrossRef]
- Sánchez, N.A.; Rincón, C.; Zambrano, G.; Galindo, H.; Prieto, P. Characterization of diamond-like carbon (DLC) thin films prepared by r.f. magnetron sputtering. Thin Solid Films 2000, 373, 247–250. [Google Scholar] [CrossRef]
- He, X.M.; Li, W.Z.; Li, H.D. Structure and properties of carbon nitride films synthesized by low energy ion bombardment. J. Mater. Res. 1997, 12, 1595–1602. [Google Scholar] [CrossRef]
- Shim, K.S.; Kim, S.M.; Bae, S.H.; Lee, S.Y.; Jung, H.S.; Park, H.H. Fabrication and characterization of diamond-like carbon thin films by pulsed laser deposition. Appl. Surf. Sci. 2000, 154-155, 482–484. [Google Scholar] [CrossRef]
- Kalish, R.; Lifshitz, Y.; Nugent, K.; Prawer, S. Thermal stability and relaxation in diamond-like-carbon. A Raman study of films with different sp3 fractions (ta-C to a-C). Appl. Phys. Lett. 1999, 74, 2936–2938. [Google Scholar] [CrossRef]
- Kulik, J.; Lifshitz, Y.; Lempert, G.D.; Rabalais, J.W.; Marton, D. Erectron-energy-loss spectroscopy of mass-selected ion-beam-deposited diamond-like carbon. J. Appl. Phys. 1994, 76, 5063–5069. [Google Scholar] [CrossRef]
- Erdemir, A.; Donnet, C. Tribology of diamond-like carbon films: recent progress and future prospects. J. Phy D Appl. Phys. 2006, 39, R311–R327. [Google Scholar] [CrossRef]
- Grill, A. Diamond-like carbon coatings as biocompatible materials—An overview. Diam. Relat. Mater. 2003, 12, 166–170. [Google Scholar] [CrossRef]
- Bhushan, B. Chemical, mechanical and tribological characterization of ultra-thin and hard amorphous carbon coatings as thin as 3.5 nm: recent developments. Diam. Relat. Mater. 1999, 8, 1985–2015. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Dearnaley, G.; Arps, J.H. Biomedical applications of diamond-like carbon (DLC) coatings: A review. Surf. Coat. Tech. 2005, 200, 2518–2524. [Google Scholar] [CrossRef]
- Hauert, R. A review of modified DLC coatings for biological applications. Diam. Relat. Mater. 2003, 12, 583–589. [Google Scholar] [CrossRef]
- Bhushan, B.; Kellock, A.J.; Cho, N.H.; Ager, J.W. Characterization of chemical bonding and physical characteristics of diamond-like amorphous carbon and diamond films. J. Mater. Res. 1992, 7, 404–410. [Google Scholar] [CrossRef]
- Ronkainen, H.; Varjus, S.; Holmberg, K. Tribological performance of different DLC coatings in water-lubricated conditions. Wear 2001, 249, 267–271. [Google Scholar] [CrossRef]
- Fisher, J.; Firkins, P.; Reeves, E.A.; Hailey, J.L.; Isaac, G.H. The influence of scratches to metallic counterfaces on the wear of ultra‐high molecular weight polyethylene. ARCHIVE: Proc. Inst. Mech. Eng. H J. Eng. Med. 1995, 209, 263–264. [Google Scholar] [CrossRef]
- Jiang, J.; Arnell, R.D. The effect of substrate surface roughness on the wear of DLC coatings. Wear 2000, 239, 1–9. [Google Scholar] [CrossRef]
- Ikeyama, M.; Nakao, S.; Miyagawa, Y.; Miyagawa, S. Effects of Si content in DLC films on their friction and wear properties. Surf. Coat. Tech. 2005, 191, 38–42. [Google Scholar] [CrossRef]
- Meneve, J.; Jacobs, R.; Eersels, L.; Smeets, J.; Dekempeneer, E. Friction and wear behaviour of amorphous hydrogenated Si1-x Cx films. Surf. Coat. Tech. 1993, 62, 577–582. [Google Scholar] [CrossRef]
- Neerinck, D.; Persoone, P.; Sercu, M.; Goel, A.; Venkatraman, C.; Kester, D.; Halter, C.; Swab, P.; Bray, D. Diamond-like nanocomposite coatings for low-wear and low-friction applications in humid environments. Thin Solid Films 1998, 317, 402–404. [Google Scholar] [CrossRef]
- Sheeja, D.; Tay, B.K.; Lau, S.P.; Shi, X.; Ding, X. Structural and tribological characterization of multilayer ta-C films prepared by filtered cathodic vacuum arc with substrate pulse biasing. Surf. Coat. Tech. 2000, 132, 228–232. [Google Scholar] [CrossRef]
- Roy, R.K.; Lee, K.R. Biomedical applications of diamond-like carbon coatings: A review. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 83B, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lakes, R.S. Biomaterials, 3rd ed.; Springer Science + Business Media: New York, NY, USA, 2007. [Google Scholar]
- Kokubo, T. Bioactive glass ceramics: properties and applications. Biomaterials 1991, 12, 155–63. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Puleo, D.A.; Nanci, A. Understanding and controlling the bone-implant interface. Biomaterials 1999, 20, 2311–21. [Google Scholar] [CrossRef] [PubMed]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. R Rep. 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. Engl. 2002, 41, 3130–3146. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cements. Injury 2000, 31, D37–D47. [Google Scholar] [CrossRef]
- Jaffe, W.L.; Scott, D.F. Current concepts review—Total hip Arthroplasty with hydroxyapatite-coated prostheses. J. Bone Joint Surg. Am. 1996, 78, 1918–1934. [Google Scholar] [PubMed]
- Choi, J.; Bogdanski, D.; Köller, M.; Esenwein, S.A.; Müller, D.; Muhr, G.; Epple, M. Calcium phosphate coating of nickel-titanium shape-memory alloys. Coating procedure and adherence of leukocytes and platelets. Biomaterials 2003, 24, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Grandfield, K.; Sun, F.; FitzPatrick, M.; Cheong, M.; Zhitomirsky, I. Electrophoretic deposition of polymer-carbon nanotube-hydroxyapatite composites. Surf. Coat. Tech. 2009, 203, 1481–1487. [Google Scholar] [CrossRef]
- Herø, H.; Wie, H.; Jørgensen, R.B.; Ruyter, I.E. Hydroxyapatite coatings on Ti produced by hot isostatic pressing. J. Biomed. Mater. Res. 1994, 28, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Nelea, V.; Ristoscu, C.; Chiritescu, C.; Ghica, C.; Mihailescu, I.N.; Pelletier, H.; Mille, P.; Cornet, A. Pulsed laser deposition of hydroxyapatite thin films on Ti-5Al-2.5Fe substrates with and without buffer layers. Appl. Surf. Sci. 2000, 168, 127–131. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.S.; Khor, K.A.; Li, H.; Cheang, P.; Marple, B.R. HVOF spraying of nanostructured hydroxyapatite for biomedical applications. Mater. Sci. Eng. A 2005, 396, 181–187. [Google Scholar] [CrossRef]
- Maitz, M.F.; Pham, M.T.; Matz, W.; Reuther, H.; Steiner, G.; Richter, E. Ion beam treatment of titanium surfaces for enhancing deposition of hydroxyapatite from solution. Biomol. Eng. 2002, 19, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, E.; Velten, D.; Breme, J. Biomimetic implant coatings. Biomol. Eng. 2007, 24, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ye, X.; Tang, G.; Zhao, N.; Gong, Y.; Zhao, Y.; Zhao, J.; Zhang, X. Biomimetic coating of compound titania and hydroxyapatite on titanium. J. Biomed. Mater. Res. A 2007, 83, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Duran, A.; Conde, A.; Coedo, A.G.; Dorado, T.; Garcia, C.; Cere, S. Sol-gel coatings for protection and bioactivation of metals used in orthopaedic devices. J. Mater. Chem. 2004, 14, 2282–2290. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, A. Bioactive materials for biomedical applications using sol-gel technology. Biomed. Mater. 2008, 3. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process-an alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Groot, K.; Geesink, R.; Klein, C.; Serekian, P. Plasma sprayed coatings of hydroxyapatite. J. Biomed. Mater. Res. 1987, 21, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Furlong, R.J.; Osborn, J.F. Fixation of hip prostheses by hydroxyapatite ceramic coatings. J. Bone Joint Surg. Br. 1991, 73, 741–745. [Google Scholar] [PubMed]
- Yang, C.Y.; Wang, B.C.; Chang, E.; Wu, J.D. The influences of plasma spraying parameters on the characteristics of hydroxyapatite coatings: a quantitative study. J. Mater. Sci.: Mater. Med. 1995, 6, 249–257. [Google Scholar] [CrossRef]
- Montanaro, L.; Arciola, C.R.; Campoccia, D.; Cervellati, M. In vitro effects on MG63 osteoblast-like cells following contact with two roughness-differing fluorohydroxyapatite-coated titanium alloys. Biomaterials 2002, 23, 3651–3659. [Google Scholar] [CrossRef] [PubMed]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001, 22, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Liu, Q.; Wolke, J.; Zhang, D.; De Groot, K. The role of amorphous phase in nucleating bone-like apatite on plasma-sprayed hydroxyapatite coatings in simulated body fluid. J. Mater. Sci. Lett. 1997, 16, 335–337. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, M.; Ding, C. Bond strength of plasma-sprayed hydroxyapatite/Ti composite coatings. Biomaterials 2000, 21, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Leyland, A.; Matthews, A. Deposition of layered bioceramic hydroxyapatite/TiO2 coatings on titanium alloys using a hybrid technique of micro-arc oxidation and electrophoresis. Surf. Coat. Tech. 2000, 125, 407–414. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.W.; Lee, E.J.; Li, L.H.; Kim, H.E. Hydroxyapatite-TiO2 Hybrid Coating on Ti Implants. J. Biomater. Appl. 2006, 20, 195–208. [Google Scholar] [CrossRef] [PubMed]
- He, L.P.; Mai, Y.W.; Chen, Z.Z. Fabrication and characterization of nanometer CaP(aggregate)/Al2O3 composite coating on titanium. Mater. Sci. Eng. A 2004, 367, 51–56. [Google Scholar] [CrossRef]
- Gautier, S.; Champion, E.; Bernache-Assollant, D. Processing, microstructure and toughness of Al2O3 platelet-reinforced hydroxyapatite. J. Eur. Ceram. Soc. 1997, 17, 1361–1369. [Google Scholar] [CrossRef]
- Singh, D.; de la Cinta Lorenzo-Martin, M.; Gutiérrez-Mora, F.; Routbort, J.L.; Case, E.D. Self-joining of zirconia/hydroxyapatite composites using plastic deformation process. Acta Biomater. 2006, 2, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Inuzuka, M.; Nakamura, S.; Kishi, S.; Yoshida, K.; Hashimoto, K.; Toda, Y.; Yamashita, K. Hydroxyapatite-doped zirconia for preparation of biomedical composites ceramics. Solid State Ionics 2004, 172, 509–513. [Google Scholar] [CrossRef]
- Tercero, J.E.; Namin, S.; Lahiri, D.; Balani, K.; Tsoukias, N.; Agarwal, A. Effect of carbon nanotube and aluminum oxide addition on plasma-sprayed hydroxyapatite coating's mechanical properties and biocompatibility. Mater. Sci. Eng. C 2009, 29, 2195–2202. [Google Scholar] [CrossRef]
- Shokuhfar, T.; Makradi, A.; Titus, E.; Cabral, G.; Ahzi, S.; Sousa, A.C.M.; Belouettar, S.; Gracio, J. Prediction of the mechanical properties of hydroxyapatite/polymethyl methacrylate/carbon nanotubes nanocomposite. J. Nanosci. Nanotechnol. 2008, 8, 4279–4284. [Google Scholar] [CrossRef] [PubMed]
- Balani, K.; Anderson, R.; Laha, T.; Andara, M.; Tercero, J.; Crumpler, E.; Agarwal, A. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts In vitro. Biomaterials 2007, 28, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.; Jansen, J.A. Thin Calcium Phosphate Coatings for Biomedical Implants; Springer: Pennsville, NJ, USA, 2009. [Google Scholar]
- Bao, Q.H.; Chen, C.Z.; Wang, D.G.; Li, Q.M.; Lei, T.Q. Pulsed laser deposition and its current research status in preparing hydroxyapatite thin films. Appl. Surf. Sci. 2005, 252, 1538–1544. [Google Scholar] [CrossRef]
- Koch, C.F.; Johnson, S.; Kumar, D.; Jelinek, M.; Chrisey, D.B.; Doraiswamy, A.; Jin, C.; Narayan, R.J.; Mihailescu, I.N. Pulsed laser deposition of hydroxyapatite thin films. Mater. Sci. Eng. C 2007, 27, 484–494. [Google Scholar] [CrossRef]
- Torrisi, L. Structural investigations on laser deposited hydroxyapatite films. Thin Solid Films 1994, 237, 12–15. [Google Scholar] [CrossRef]
- Torrisi, L.; Steola, R. Thermally assisted hydroxyapatite obtained by pulsed-laser deposition on titanium substrates. Thin Solid Films 1993, 227, 32–36. [Google Scholar] [CrossRef]
- Singh, R.K.; Qian, F.; Nagabushnam, V.; Damodaran, R.; Moudgil, B.M. Excimer laser deposition of hydroxypaptite thin films. Biomaterials 1994, 15, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pradas, J.M.; Sardin, G.; Clèries, L.; Serra, P.; Ferrater, C.; Morenza, J.L. Deposition of hydroxyapatite thin films by excimer laser ablation. Thin Solid Films 1998, 317, 393–396. [Google Scholar] [CrossRef]
- Lo, W.J.; Grant, D.M.; Ball, M.D.; Welsh, B.S.; Howdle, S.M.; Antonov, E.N.; Bagratashvili, V.N.; Popov, V.K. Physical, chemical , and biological characterization of pulsed laser deposited and plasma sputtered hydroxyapatite thin films on titanium alloy. J. Biomed. Mater. Res. 2000, 50, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.D.; Downes, S.; Scotchford, C.A.; Antonov, E.N.; Bagratashvili, V.N.; Popov, V.K.; Lo, W.J.; Grant, D.M.; Howdle, S.M. Osteoblast growth on titanium foils coated with hydroxyapatite by pulsed laser ablation. Biomaterials 2001, 22, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Parada, J.M.; Sardin, G.; Clèries, L.; Serra, P.; Ferrater, C.; Morenza, J.L. Depostion of hydroxyapatite thin films by excimer laser ablation. Thin Solid Films 1998, 317, 393–396. [Google Scholar] [CrossRef]
- Feddes, B.; Vredenberg, A.M.; Wehner, M.; Wolke, J.C.G.; Jansen, J.A. Laser-induced crystallization of calcium phosphate coatings on polyethylene (PE). Biomaterials 2005, 26, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Serra, P.; Morenza, J.L. Evidence of chemical reactions in the hydroxyapatite laser ablation plume with a water atmosphere. J. Appl. Phys. 1999, 85, 3289–3293. [Google Scholar] [CrossRef]
- Serra, P.; Clèries, L.; Morenza, J.L. Analysis of the expansion of hydroxyapatite laser ablation plumes. Appl. Surf. Sci. 1996, 96-98, 216–221. [Google Scholar]
- Ong, J.L.; Lucas, L.C.; Lacefield, W.R.; Rigney, E.D. Strength of thin calcium phosphate coatings produced by ion beam sputter deposition. Biomaterials 1992, 13, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.T.; Chittur, K.K.; Lacefield, W.R. Dissolution/reprecipitation of calcium phosphate thin films produced by ion beam sputter deposition technique. Biomaterials 1999, 20, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.Z.; Luo, Z.S.; Feng, Q.L. Highly adhesive hydroxyapatite coatings on titanium alloy formed by ion beam assisted deposition. J. Mater. Sci.: Mater. Med. 1997, 8, 403–405. [Google Scholar] [CrossRef]
- Ong, J.L.; Lucas, L.C. Post-deposition heat treatments for ion beam sputter deposited calcium phosphate coatings. Biomaterials 1994, 15, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Ide-Ektessabi, A. Preparation of hydroxyapatite layer by ion beam assisted simultaneous vapor deposition. Surf. Coat. Technol. 2003, 163-164, 362–367. [Google Scholar]
- Lee, I.-S.; Whang, C.N.; Lee, G.H.; Cui, F.Z.; Ito, A. Effects of ion beam assist on the formation of calcium phosphate films. Nucl. Instrum. Meth. Phys. Res. B 2003, 206, 522–526. [Google Scholar] [CrossRef]
- Yoshinari, M.; Ohtsuka, Y.; Dérand, T. Thin hydroxyapatite coating produced by the ion beam dynamic mixing method. Biomaterials 1994, 15, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.Z.; Luo, Z.S. Biomaterials modification by ion-beam processing. Surf. Coat. Technol. 1999, 112, 278–285. [Google Scholar] [CrossRef]
- Yoshinari, M.; Klinge, B.; Dérand, T. The biocompatibility (cell culture and histological study) of hydroxy-apatite-coated implants created by ion beam dynamic mixing. Clin. Oral Impl. Res. 1996, 7, 96–100. [Google Scholar] [CrossRef]
- Yoshinari, M.; Ohtsuka, Y.; Dérand, T. Thin hydroxyapatite coatings produced by ion beam dynamic mixing method. Biomaterials 1994, 15, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, Y.; Matsuura, M.; Chida, N. Formation of hydroxyapatite coatings on pure titanium substrates by ion beam dynamic mixing. Surf. Coat. Technol. 1994, 65, 224–230. [Google Scholar] [CrossRef]
- Lee, I.S.; Kim, D.H.; Kim, H.E.; Jung, Y.C.; Han, C.H. Biological performance of calcium phosphate films formed on commercially pure Ti by electron-beam evaporation. Biomaterials 2002, 23, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, K.; Yuhta, T.; Fukui, Y. A functionally graded titanium/hydroxyapatite film obtained by sputtering. J. Mater. Sci.: Mater. Med. 2002, 13, 253–258. [Google Scholar] [CrossRef]
- Nelea, V.; Morosanu, C.; Iliescu, M. Mihailescu. Microstructure and mechnical properties of hydroxyapatite thin films grown by RF magnetron sputtering. Surf. Coat. Technol. 2003, 173, 315–322. [Google Scholar] [CrossRef]
- Ding, S.J.; Ju, C.P.; Chern Lin, J.H. Immersion behavior of RF magnetron-assisted sputtered hydroxyapatite/titanium coatings in simulated body fluid. J. Biomed. Mater. Res. 1999, 47, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Nelea, V.; Morosanu, C.; Iliescu, M.; Mihailescu, I.N. Hydroxyapatite thin films grown by pulsed laser depostion and radio-frequency magnetron sputtering: comparative study. Appl. Surf. Sci. 2004, 228, 346–356. [Google Scholar] [CrossRef]
- Jansen, J.A.; Wolke, J.G.C.; Swann, S.; van der Waerden, J.P.C.M.; de Groot, K. Application of magnetron sputtering for producing ceramic coatings on implant materials. Clin. Oral. Impl. Res. 1993, 4, 28–34. [Google Scholar] [CrossRef]
- Wie, H.; Hero, H.; Solheim, T. Hot isostatic pressing-processed hydroxyapatite-coated titanium implants: light microscopic and scanning electron microscopy investigations. Int. J. Oral. Maxillofac. Implants 1998, 13, 837–844. [Google Scholar] [PubMed]
- Habibovic, P.; Barrere, F.; Blitterswijk van, C.A.; Groot de, K. Biomimetic hydroxyapatite coating on metal implants. J. Am. Ceram. Soc. 2002, 85, 517–522. [Google Scholar] [CrossRef]
- Forsgren, J.; Svahn, F.; Jarmar, T.; Engqvist, H. Formation and adhesion of biomimetic hydroxyapatite deposited on titanium substrates. Acta Biomater. 2007, 3, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Yang, Q.Z.; Troczynski, T. Sol-gel hydroxyapatite coatings on stainless steel substrates. Biomaterials 2002, 23, 691–698. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, H.E.; Knowles, J.C. Fluor-hydroxyapatite sol-gel coatings on titanium substrate for hard tissue implants. Biomaterials 2004, 25, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Balani, K.; Chen, Y.; Harimkar, S.P.; Dahotre, N.B.; Agarwal, A. Tribological behavior of plasma-sprayed carbon nanotube-reinforced hydroxyapatite coating in physiological solution. Acta Biomater. 2007, 3, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.; Anderson, O. Bioactive glass. In An Introduction to Bioceramics; Hench, L., Wilson, L., Eds.; World Scientific: Pennsville, NJ, USA, 1993; p. 41. [Google Scholar]
- Bolelli, G.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Rauch, J. Microstructural and In vitro characterisation of high-velocity suspension flame sprayed (HVSFS) bioactive glass coatings. J. Eur. Ceram. Soc. 2009, 29, 2249–2257. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass® 45S5 dissolution. J. Biomed. Mater. Res. 2001, 55, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, I.; Buttery, L.D.K.; Tai, G.; Hench, L.L.; Polak, J.M. Characterization of human fetal osteoblasts by microarray analysis following stimulation with 58S bioactive gel-glass ionic dissolution products. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Salerno, A.; Locardi, B.; Riccio, V.; Della Ragione, F.; Iardino, P.; Zappia, V. Behaviour of human osteoblasts cultured on bioactive glass coatings. Biomaterials 1998, 19, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.H.; Doost Mohammadi, A. Preparation and characterization of sol-gel bioactive glass coating for improvement of biocompatibility of human body implant. Mater. Sci. Eng. A 2008, 474, 128–133. [Google Scholar] [CrossRef]
- Foppiano, S.; Marshall, S.J.; Marshall, G.W.; Saiz, E.; Tomsia, A.P. Bioactive glass coatings affect the behavior of osteoblast-like cells. Acta Biomater. 2007, 3, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Zhitomirsky, D.; Roether, J.A.; Boccaccini, A.R.; Zhitomirsky, I. Electrophoretic deposition of bioactive glass/polymer composite coatings with and without HA nanoparticle inclusions for biomedical applications. J. Mater. Process. Technol. 2009, 209, 1853–1860. [Google Scholar] [CrossRef]
- Wang, C.X.; Chen, Z.Q.; Wang, M. Fabrication and characterization of bioactive glass coatings produced by the ion beam sputter deposition technique. J. Mater. Sci. Mater. Med. 2002, 13, 247–251. [Google Scholar] [CrossRef]
- Comesaña, R.; Quintero, F.; Lusquiños, F.; Pascual, M.J.; Boutinguiza, M.; Durán, A.; Pou, J. Laser cladding of bioactive glass coatings. Acta Biomater. 2010, 6, 953–961. [Google Scholar] [CrossRef] [PubMed]
- D'Alessio, L.; Teghil, R.; Zaccagnino, M.; Zaccardo, I.; Ferro, D.; Marotta, V. Pulsed laser ablation and deposition of bioactive glass as coating material for biomedical applications. Appl. Surf. Sci. 1999, 138, 527–532. [Google Scholar] [CrossRef]
- Chern Lin, J.H.; Liu, M.L.; Ju, C.P. Structure and properties of hydroxyapatite-bioactive glass composites plasma sprayed on Ti6Al4V. J. Mater. Sci. Mater. Med. 1994, 5, 279–283. [Google Scholar] [CrossRef]
- Gomez-Vega, J.M.; Saiz, E.; Tomsia, A.P. Glass-based coatings for titanium implant alloys. J. Biomed. Mater. Res. 1999, 46, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, J.; Galliano, P.; Durán, A. Bioactive and protective sol-gel coatings on metals for orthopaedic prostheses. J. Sol-Gel Sci. Technol. 2001, 21, 65–74. [Google Scholar] [CrossRef]
- Liu, X.; Morra, M.; Carpi, A.; Li, B. Bioactive calcium silicate ceramics and coatings. Biomed. Pharmacother. 2008, 62, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Ding, C.X.; Wang, Z.Y. Apatite formed on the surface of plasma-sprayed wollastonite coating immersed in simulated body fluid. Biomaterials 2001, 22, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Ding, C.X. Phase compositions and microstructure of plasma sprayed wollastonite coating. Surf. Coat. Tech. 2001, 141, 269–274. [Google Scholar] [CrossRef]
- Liu, X.Y.; Xie, Y.T.; Ding, C.X.; Chu, P.K. Early apatite deposition and osteoblast growth on plasma-sprayed dicalcium silicate coating. J. Biomed. Mater. Res. A 2005, 74A, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.C.; Liu, X.Y.; Zheng, X.B.; Ding, C.X. Plasma-sprayed diopside coatings for biomedical applications. Surf. Coat. Tech. 2004, 185, 340–345. [Google Scholar] [CrossRef]
- Liu, X.Y.; Tao, S.Y.; Ding, C.X. Bioactivity of plasma sprayed dicalcium silicate coatings. Biomaterials 2002, 23, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Ding, C.X.; Chu, P.K. Mechanism of apatite formation on wollastonite coatings in simulated body fluids. Biomaterials 2004, 25, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.T.; Liu, X.Y.; Ding, C.X.; Chu, P.K. Bioconductivity and mechanical properties of plasma-sprayed dicalcium silicate/zirconia composite coating. Mater. Sci. Eng. C 2005, 25, 509–515. [Google Scholar] [CrossRef]
- Liu, X.Y.; Ding, C.X. Plasma-sprayed wollastonite 2M/ZrO2 composite coating. Surf. Coat. Tech. 2003, 172, 270–278. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.; Liu, X.; Wei, L.; Wang, G.; Meng, F. Proliferation and gene expression of osteoblasts cultured in DMEM containing the ionic products of dicalcium silicate coating. Biomed. Pharmacother. 2009, 63, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Ramaswamy, Y.; Chang, J.; Woods, J.; Chen, Y.Q.; Zreiqat, H. The effect of Zn contents on phase composition, chemical stability and cellular bioactivity in Zn-Ca-Si system ceramics. J. Biomed. Mater. Res.: Appl. Biomater. 2008, 87B, 346–353. [Google Scholar] [CrossRef]
- Wu, C.T.; Ramaswamy, Y.; Kwik, D.; Zreiqat, H. The effect of strontium incorporation into CaSiO3 ceramics on their physical and biological properties. Biomaterials 2007, 28, 3171–3181. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, Y.; Wu, C.T.; Hummel, A.V.; Combes, V.; George, G.; Zreiqat, H. The responses of osteoblast, osteoclast and endothelial cells to zirconium modified calcium-silicate-based ceramic. Biomaterials 2008, 29, 4392–4402. [Google Scholar] [CrossRef] [PubMed]
- Kuromoto, N.K.; Simão, R.A.; Soares, G.A. Titanium oxide films produced on commercially pure titanium by anodic oxidation with different voltages. Mater. Chara. 2007, 58, 114–121. [Google Scholar] [CrossRef]
- Yang, B.; Uchida, M.; Kim, H.M.; Zhang, X.; Kokubo, T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Advincula, M.C.; Rahemtulla, F.G.; Advincula, R.C.; Ada, E.T.; Lemons, J.E.; Bellis, S.L. Osteoblast adhesion and matrix mineralization on sol-gel-derived titanium oxide. Biomaterials 2006, 27, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Meng, F.; Ding, C.; Chu, P.K.; Liu, X. Microstructure, bioactivity and osteoblast behavior of monoclinic zirconia coating with nanostructured surface. Acta Biomater. 2010, 6, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Sollazzo, V.; Palmieri, A.; Pezzetti, F.; Bignozzi, C.A.; Argazzi, R.; Massari, L.; Brunelli, G.; Carinci, F. Genetic effect of zirconium oxide coating on osteoblast-like cells. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, D.; Sun, J.; Zhang, Y.; Xu, K. UV-enhanced bioactivity and cell response of micro-arc oxidized titania coatings. Acta Biomater. 2008, 4, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; Groot, K.d. The role of hydrated silica, titania, and alumina in inducing apatite on implants. J. Biomed. Mater. Res. 1994, 28, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Kim, H.M.; Kokubo, T.; Fujibayashi, S.; Nakamura, T. Structural dependence of apatite formation on titania gels in a simulated body fluid. J. Biomed. Mater. Res. A 2003, 64, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, X.; Ding, C. Phase composition and in vitro bioactivity of plasma sprayed calcia stabilized zirconia coatings. Surf. Coat. Tech. 2008, 202, 5824–5831. [Google Scholar] [CrossRef]
- Yan, Y.; Han, Y.; Lu, C. The effect of chemical treatment on apatite-forming ability of the macroporous zirconia films formed by micro-arc oxidation. Appl. Surf. Sci. 2008, 254, 4833–4839. [Google Scholar] [CrossRef]
- Han, Y.; Yan, Y.; Lu, C. Ultraviolet-enhanced bioactivity of ZrO2 films prepared by micro-arc oxidation. Thin Solid Films 2009, 517, 1577–81. [Google Scholar] [CrossRef]
- Yan, Y.; Han, Y. Structure and bioactivity of micro-arc oxidized zirconia films. Surf. Coat. Tech. 2007, 201, 5692–5695. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Li, B.; Cao, C.; Dong, Y.; Ding, C.; Chu, P.K. UV-irradiation-induced bioactivity on TiO2 coatings with nanostructural surface. Acta Biomater. 2008, 4, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.; Balázsi, C.; Yang, J.H.C.; Gouma, P.I. Biopolymer-hydroxyapatite composite coatings prepared by electrospinning. Polym. Adv. Technol. 2006, 17, 902–906. [Google Scholar] [CrossRef]
- Bigi, A.; Panzavolta, S.; Roveri, N. Hydroxyapatite-gelatin films: a structural and mechanical characterization. Biomaterials 1998, 19, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Kino, R.; Ikoma, T.; Yunoki, S.; Nagai, N.; Tanaka, J.; Asakura, T.; Munekata, M. Preparation and characterization of multilayered hydroxyapatite/silk fibroin film. J. Biosci. Bioeng. 2007, 103, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.A.; Czernuszka, C.J. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cell Mater. 2006, 11, 43–56. [Google Scholar] [PubMed]
- Sato, M.; Slamovich, E.B.; Webster, T.J. Enhanced osteoblast adhesion on hydrothermally treated hydroxyapatite/titania/poly(lactide-co-glycolide) sol-gel titanium coatings. Biomaterials 2005, 26, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jordan, E.H.; Gell, M.; Wei, M. Apatite formation on alkaline-treated dense TiO2 coatings deposited using the solution precursor plasma spray process. Acta Biomater. 2008, 4, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, M.; Pätsi, M.; Rahiala, H.; Peltola, T.; Ritala, M.; Rosenholm, J.B. Influence of sol and surface properties on In vitro bioactivity of sol-gel-derived TiO2 and TiO2-SiO2 films deposited by dip-coating method. J. Biomed. Mater. Res. 1998, 42, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, X.; Fu, R.K.Y.; Ho, J.P.Y.; Ding, C.; Chu, P.K. Plasma-treated nanostructured TiO2 surface supporting biomimetic growth of apatite. Biomaterials 2005, 26, 6143–6150. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Yen, S.K. Biomimetic growth of apatite on electrolytic TiO2 coatings in simulated body fluid. Mater. Sci. Eng. C 2006, 26, 54–64. [Google Scholar] [CrossRef]
- Cui, X.; Kim, H.M.; Kawashita, M.; Wang, L.; Xiong, T.; Kokubo, T.; Nakamura, T. Preparation of bioactive titania films on titanium metal via anodic oxidation. Dental Mater. 2009, 25, 80–86. [Google Scholar] [CrossRef]
- Liu, X.; Huang, A.; Ding, C.; Chu, P.K. Bioactivity and cytocompatibility of zirconia (ZrO2) films fabricated by cathodic arc deposition. Biomaterials 2006, 27, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kim, K.H.; Lee, J.Y.; Ku, Y.; Lee, S.J.; Min, B.M.; Chung, C.P. Immobilization of bone morphogenetic protein-2 on a nanofibrous chitosan membrane for enhanced guided bone regeneration. Biotechnol. Appl. Biochem. 2006, 43, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Schliephak, H.; Scharnweber, D. Chemical and biological functionalization of titanium for dental implants. J. Mater. Chem. 2008, 18, 2404–2414. [Google Scholar] [CrossRef]
- Adden, N.; Gamble, L.J.; Castner, D.G.; Hoffman, A.; Gross, G.; Menzel, H. Phosphonic Acid monolayers for binding of bioactive molecules to titanium surfaces. Langmuir 2006, 22, 8197–8204. [Google Scholar] [CrossRef] [PubMed]
- Tebbe, D.; Thull, R.; Gbureck, U. Influence of spacer length on heparin coupling efficiency and fibrinogen adsorption of modified titanium surfaces. Biomed. Eng. Online 2007, 6. [Google Scholar] [CrossRef]
- Nanci, A.; Wuest, J.D.; Peru, L.; Brunet, P.; Sharma, V.; Zalzal, S.; McKee, M.D. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J. Biomed. Mater. Res. 1998, 40, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma Methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization—A review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Chatelier, R.C.; Xie, X.; Gengenbach, T.R.; Griesser, H.J. Effects of plasma modification conditions on surface restructuring. Langmuir 1995, 11, 2585–2591. [Google Scholar] [CrossRef]
- Meyer-Plath, A.A.; Finke, B.; Schröder, K.; Ohl, A. Pulsed and cw microwave plasma excitation for surface functionalization in nitrogen-containing gases. Surf. Coat. Tech. 174-175, 877–881.
- Gancarz, I.; Bryjak, J.; Bryjak, M.; Pozniak, G.; Tylus, W. Plasma modified polymers as a support for enzyme immobilization 1.: Allyl alcohol plasma. Eur. Polym. J. 2003, 39, 1615–1622. [Google Scholar] [CrossRef]
- Swaraj, S.; Oran, U.; Lippitz, A.; Friedrich, J.F.; Unger, W.E.S. Study of influence of external plasma parameters on plasma polymerised films prepared from organic molecules (acrylic acid, allyl alcohol, allyl amine) using XPS and NEXAFS. Surf. Coat. Tech. 2005, 200, 494–497. [Google Scholar] [CrossRef]
- Puleo, D.A.; Kissling, R.A.; Sheu, M.S. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4), on titanium alloy. Biomaterials 2002, 23, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Herr, G.; Hartwig, C.H.; Boll, C.; Küsswetter, W. Ectopic bone formation by composites of BMP and metal implants in rats. Acta Orthop. 1996, 67, 606–610. [Google Scholar] [CrossRef]
- Seol, Y.J.; Park, Y.J.; Lee, S.C.; Kim, K.H.; Lee, J.Y.; Kim, T.I.; Lee, Y.M.; Ku, Y.; Rhyu, I.C.; Han, S.B.; Chung, C.P. Enhanced osteogenic promotion around dental implants with synthetic binding motif mimicking bone morphogenetic protein (BMP)-2. J. Biomed. Mater. Res. A 2006, 77A, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Länge, K.; Herold, M.; Scheideler, L.; Geis-Gerstorfer, J.; Wendel, H.P.; Gauglitz, G. Investigation of initial pellicle formation on modified titanium dioxide (TiO2) surfaces by reflectometric interference spectroscopy (RIfS) in a model system. Dent. Mater. 2004, 20, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, M.; Oda, Y.; Kato, T.; Okuda, K. Influence of surface modifications to titanium on antibacterial activity In vitro. Biomaterials 2001, 22, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Wikesjö, U.M.E.; Qahash, M.; Thomson, R.C.; Cook, A.D.; Rohrer, M.D.; Wozney, J.M.; Hardwick, W.R. rhBMP-2 significantly enhances guided bone regeneration. Clinl. Oral Implants Res. 2004, 15, 194–204. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Cromme, F.; Kandziora, F.; Haas, N.P.; Raschke, M. Bone morphogenetic protein-2 coating of titanium implants increases biomechanical strength and accelerates bone remodeling in fracture treatment: a biomechanical and histological study in rats. Bone 2002, 30, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Schmidmaier, G.; Wildemann, B.; Bail, H.; Lucke, M.; Fuchs, T.; Stemberger, A.; Flyvbjerg, A.; Haas, N.P.; Raschke, M. Local application of growth factors (insulin-like growth factor-1 and transforming growth factor-[beta, #2799]1) from a biodegradable poly(-lactide) coating of osteosynthetic implants accelerates fracture healing in rats. Bone 2001, 28, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Puleo, D.A. Release and Retention of Biomolecules in Collagen Deposited on Orthopedic. Biomaterials 1999, 27, 65–75. [Google Scholar]
- Agrawal, C.M.; Pennick, A.; Wang, X.; Schenck, R.C. Porous-coated titanium implant impregnated with a biodegradable protein delivery system. J. Biomed. Mater. Res. 1997, 36, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Welsh, W.R.; Kim, H.D.; Jong, Y.S.; Valentini, R.F. Controlled release of platelet-derived growth factor using ethylene vinyl acetate copolymer (EVAc) coated on stainless-steel wires. Biomaterials 1995, 16, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A. Bioactive biomaterials. Curr. Opin. Biotechnol. 1999, 10, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Castner, D.G.; Ratner, B.D. Biomedical surface science: Foundations to frontiers. Surf. Sci. 2002, 500, 28–60. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of Infections Associated with Surgical Implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Tunney, M.M.; Patrick, S.; Curran, M.D.; Ramage, G.; Hanna, D.; Nixon, J.R.; Gorman, S.P.; Davis, R.I.; Anderson, N. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and pcr amplification of the bacterial 16S rRNA gene. J. Clin. Microbiol. 1999, 37, 3281–3290. [Google Scholar] [PubMed]
- Hetrick, E.M.; Prichard, H.L.; Klitzman, B.; Schoenfisch, M.H. Reduced foreign body response at nitric oxide-releasing subcutaneous implants. Biomaterials 2007, 28, 4571–4580. [Google Scholar] [CrossRef] [PubMed]
- Grainger, D.W. Controlled-release and local delivery of therapeutic antibodies. Expert Opin. Biol. Ther. 2004, 4, 1029–1044. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Cohen, R.E.; Rubner, M.F. Antibacterial properties of ag nanoparticle loaded multilayers and formation of magnetically directed antibacterial microparticles. Langmuir 2005, 21, 9651–9659. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, W.J.; Rosenberg, H. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J. Bacteriol. 1982, 152, 7–13. [Google Scholar] [PubMed]
- Holt, K.B.; Bard, A.J. Interaction of Silver(I) Ions with the Respiratory Chain of Escherichia coli: An Electrochemical and Scanning Electrochemical Microscopy Study of the Antimicrobial Mechanism of Micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Ghandour, W.; Hubbard, J.A.; Deistung, J.; Hughes, M.N.; Poole, R.K. The uptake of silver ions by Escherichia coli K12: toxic effects and interaction with copper ions. Appl. Microbiol. Biotechnol. 1988, 28, 559–565. [Google Scholar]
- Zeiri, L.; Bronk, B.V.; Shabtai, Y.; Eichler, J.; Efrima, S. Surface-Enhanced raman spectroscopy as a tool for probing specific biochemical components in bacteria. Appl. Spectrosc. 2004, 58, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Yi, S.C.; Oh, S.G. Preparation and antibacterial effects of Ag-SiO2 thin films by sol-gel method. Biomaterials 2003, 24, 4921–4928. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.X.; Shen, L.R.; Ren, L.; Xu, Z.J.; Zhao, A.S.; Leng, Y.X.; Huang, N. The biomedical properties of polyethylene terephthalate surface modified by silver ion implantation. Nucl. Instrum. Methods Phys. Res. B 2007, 257, 141–145. [Google Scholar] [CrossRef]
- Zhao, J.; Cai, X.M.; Tang, H.Q.; Liu, T.; Gu, H.Q.; Cui, R.Z. Bactericidal and biocompatible properties of TiN/Ag multilayered films by ion beam assisted deposition. J. Mater. Sci.: Mater. Med. 2009, 20, 101–105. [Google Scholar] [CrossRef]
- Necula, B.S.; Fratila-Apachitei, L.E.; Zaat, S.A.J.; Apachitei, I.; Duszczyk, J. In vitro antibacterial activity of porous TiO2-Ag composite layers against methicillin-resistant Staphylococcus aureus. Acta Biomater. 2009, 5, 3573–3580. [Google Scholar] [CrossRef] [PubMed]
- Galya, T.; Sedlarik, V.; Kuritka, I.; Novotny, R.; Sedlarikova, J.; Saha, P. Antibacterial poly(vinyl alcohol) film containing silver nanoparticles: preparation and characterization. J. Appl. Polym. Sci. 2008, 110, 3178–3185. [Google Scholar] [CrossRef]
- Evans, P.; Sheel, D.W. Photoactive and antibacterial TiO2 thin films on stainless steel. Surf. Coat. Tech. 2007, 201, 9319–9324. [Google Scholar] [CrossRef]
- Stobie, N.; Duffy, B.; Hinder, S.J.; McHale, P.; McCormack, D.E. Silver doped perfluoropolyether-urethane coatings: antibacterial activity and surface analysis. Colloids. Surf. B Biointerfaces 2009, 72, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.; Tam, P.; Chiu, J.F.; Che, C.M. Silver nanoparticles: partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? a study of the gram-negative bacterium escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Ren, L.; Zhao, A.; Li, P.; Leng, Y.; Sun, H.; Huang, N. Antibacterial activity of silver surface modified polyethylene terephthalate by filtered cathodic vacuum arc method. Surf. Coat. Tech. 2007, 201, 6893–6896. [Google Scholar] [CrossRef]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, L.; Ogino, A.; Nagatsu, M. Investigation into the antibacterial property of carbon films. Diam. Relat. Mater. 17, 1416–1419.
- Suketa, N.; Sawase, T.; Kitaura, H.; Naito, M.; Baba, K.; Nakayama, K.; Wennerberg, A.; Atsuta, M. An antibacterial surface on dental implants, based on the photocatalytic bactericidal effect. Clin. Implant Dent. Relat. Res. 2005, 7, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Marciano, F.R.; Lima-Oliveira, D.A.; Da-Silva, N.S.; Diniz, A.V.; Corat, E.J.; Trava-Airoldi, V.J. Antibacterial activity of DLC films containing TiO2 nanoparticles. J. Colloid. Interface Sci. 2009, 340, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Applerot, G.; Perkas, N.; Amirian, G.; Girshevitz, O.; Gedanken, A. Coating of glass with ZnO via ultrasonic irradiation and a study of its antibacterial properties. Appl. Surf. Sci. 2009, 256S, S3–S8. [Google Scholar] [CrossRef]
- Dowling, D.P.; Betts, A.J.; Pope, C.; McConnell, M.L.; Eloy, R.; Arnaud, M.N. Anti-bacterial silver coatings exhibiting enhanced activity through the addition of platinum. Surf. Coat. Tech. 2003, 163-164, 637–640. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Maness, P.C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal Activity of Photocatalytic TiO2 Reaction: toward an Understanding of Its Killing Mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [PubMed]
- Kikuchi, Y.; Sunada, K.; Iyoda, T.; Hashimoto, K.; Fujishima, A. Photocatalytic bactericidal effect of TiO2 thin films: dynamic view of the active oxygen species responsible for the effect. J. Photochem. Photobiol. A Chem. 1997, 106, 51–56. [Google Scholar] [CrossRef]
- Marciano, F.R.; Bonetti, L.F.; Santos, L.V.; Da-Silva, N.S.; Corat, E.J.; Trava-Airoldi, V.J. Antibacterial activity of DLC and Ag-DLC films produced by PECVD technique. Diam. Relat. Mater. 2009, 18, 1010–1014. [Google Scholar] [CrossRef]
- Boeckh, C.; Schumacher, E.; Podbielski, A.; Haller, B. Antibacterial activity of restorative dental biomaterials in vitro. Caries Res. 2002, 36, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Tobias, R.S.; Browne, R.M.; Wilson, C.A. Antibacterial activity of dental restorative materials. Int. Endod. J. 1985, 18, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Sugimoto, M.; Kato, M.; Tsukagoshi, K.; Tanigawa, T.; Sugimoto, H. Preparation of zinc oxide ceramics with a sustainable antibacterial activity under dark conditions. Ceram. Int. 2010, 36, 497–506. [Google Scholar] [CrossRef]
- Yamamoto, O.; Komatsu, M.; Sawai, J.; Nakagawa, Z. Effect of lattice constant of zinc oxide on antibacterial characteristics. J. Mater. Sci.: Mater. Med. 2004, 15, 847–851. [Google Scholar] [CrossRef]
- Ohira, T.; Yamamoto, O.; Iida, Y.; Nakagawa, Z. Antibacterial activity of ZnO powder with crystallographic orientation. J. Mater. Sci.: Mater. Med. 2008, 19, 1407–1412. [Google Scholar] [CrossRef]
- Sawai, J.; Kawada, E.; Kanou, F.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M. Detection of active oxygen generated from ceramic powders having antibacterial activity. J. Chem. Eng. Jpn. 1996, 29, 627–633. [Google Scholar] [CrossRef]
- Sawai, J.; Shoji, S.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M.; Kojima, H. Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J. Ferment. Bioeng. 1998, 86, 521–522. [Google Scholar] [CrossRef]
- Wu, P.; Grainger, D.W. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials 2006, 27, 2450–2467. [Google Scholar] [CrossRef] [PubMed]
- Stigter, M.; Bezemer, J.; de Groot, K.; Layrolle, P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J. Control. Release 2004, 99, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Shintani, H. Modification of medical device surface to attain anti-infection. Trends Biomater. Artif. Organs 2004, 18, 1–8. [Google Scholar]
- Basak, P.; Adhikari, B.; Banerjee, I.; Maiti, T.K. Sustained release of antibiotic from polyurethane coated implant materials. J. Mater. Sci. Mater. Med. 2009, 20, 213–221. [Google Scholar] [CrossRef]
- Schierholz, J.M.; Steinhauser, H.; Rump, A.F.E.; Berkels, R.; Pulverer, G. Controlled release of antibiotics from biomedical polyurethanes: morphological and structural features. Biomaterials 1997, 18, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Rojas, I.A.; Slunt, J.B.; Grainger, D.W. Polyurethane coatings release bioactive antibodies to reduce bacterial adhesion. J. Control. Release 2000, 63, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Poelstra, K.A.; Barekzi, N.A.; Rediske, A.M.; Felts, A.G.; Slunt, J.B. Prophylactic treatment of gram-positive and gram-negative abdominal implant infections using locally delivered polyclonal antibodies. J. Biomed. Mater. Res. 2002, 60, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Stigter, M.; de Groot, K.; Layrolle, P. Incorporation of tobramycin into biomimetic hydroxyapatite coating on titanium. Biomaterials 2002, 23, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Radin, S.; Campbell, J.T.; Ducheyne, P.; Cuckler, J.M. Calcium phosphate ceramic coatings as carriers of vancomycin. Biomaterials 1997, 18, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Bitschnau, A.; Österling, J.; Sewing, A.; Meyer, C.; Kraus, R.; Meissner, S.A.; Wenisch, S.; Domann, E.; Schnettler, R. The effects of combined gentamicin-hydroxyapatite coating for cementless joint prostheses on the reduction of infection rates in a rabbit infection prophylaxis model. Biomaterials 2006, 27, 4627–4634. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension 1990, 16, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Nablo, B.J.; Prichard, H.L.; Butler, R.D.; Klitzman, B.; Schoenfisch, M.H. Inhibition of implant-associated infections via nitric oxide release. Biomaterials 2005, 26, 6984–6990. [Google Scholar] [CrossRef] [PubMed]
- Ischiropoulos, H.; Zhu, L.; Beckman, J.S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 1992, 298, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Cho, H.; Calaycay, J.; Mumford, R.; Swiderek, K.; Lee, T.; Ding, A.; Troso, T.; Nathan, C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 1992, 256, 225–228. [Google Scholar] [CrossRef] [PubMed]
- O'Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Riccio, D.A.; Dobmeier, K.P.; Hetrick, E.M.; Privett, B.J.; Paul, H.S.; Schoenfisch, M.H. Nitric oxide-releasing S-nitrosothiol-modified xerogels. Biomaterials 2009, 30, 4494–4502. [Google Scholar] [CrossRef] [PubMed]
- Marxer, S.M.; Rothrock, A.R.; Nablo, B.J.; Robbins, M.E.; Schoenfisch, M.H. Preparation of nitric oxide (no)-releasing sol−gels for biomaterial applications. Chem. Mater. 2003, 15, 4193–4199. [Google Scholar] [CrossRef]
- Nablo, B.J.; Schoenfisch, M.H. Antibacterial properties of nitric oxide-releasing sol-gels. J. Biomed. Mater. Res. Part A 2003, 67A, 1276–1283. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Antibacterial nitric oxide-releasing xerogels: Cell viability and parallel plate flow cell adhesion studies. Biomaterials 2007, 28, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Nablo, B.J.; Chen, T.Y.; Schoenfisch, M.H. Sol−Gel derived nitric-oxide releasing materials that reduce bacterial adhesion. J. Am. Chem. Soc. 2001, 123, 9712–9713. [Google Scholar] [CrossRef] [PubMed]
- Nablo, B.J.; Rothrock, A.R.; Schoenfisch, M.H. Nitric oxide-releasing sol-gels as antibacterial coatings for orthopedic implants. Biomaterials 2005, 26, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Hogg, N. Biological chemistry and clinical potential of S-nitrosothiols. Free Radic. Biol. Med. 2000, 28, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Guyomard, A.; Dé, E.; Jouenne, T.; Malandain, J.J.; Muller, G.; Glinel, K. Incorporation of a hydrophobic antibacterial peptide into amphiphilic polyelectrolyte multilayers: a bioinspired approach to prepare biocidal thin coatings. Adv. Funct. Mate.l 2008, 18, 758–765. [Google Scholar] [CrossRef]
- Shukla, A.; Fleming, K.E.; Chuang, H.F.; Chau, T.M.; Loose, C.R.; Stephanopoulos, G.N.; Hammond, P.T. Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials 2010, 31, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Diamond, G. The role of cationic antimicrobial peptides in innate host defences. Trends in Microbiology 2000, 8, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. The Role of antimicrobial peptides in innate immunity. Integr Comp Biol 2003, 43, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; McCarthy, T.J. Layer-by-Layer deposition: A tool for polymer surface modification. Macromolecules 1997, 30, 78–86. [Google Scholar]
- Etienne, O.; Picart, C.; Taddei, C.; Haikel, Y.; Dimarcq, J.L.; Schaaf, P.; Voegel, J.C.; Ogier, J.A.; Egles, C. Multilayer Polyelectrolyte Films Functionalized by Insertion of Defensin: A New Approach to Protection of Implants from Bacterial Colonization. Antimicrob. Agents Chemother. 2004, 48, 3662–3669. [Google Scholar] [CrossRef] [PubMed]
- Etienne, O.; Gasnier, C.; Taddei, C.; Voegel, J.C.; Aunis, D.; Schaaf, P.; Metz-Boutigue, M.H.; Bolcato-Bellemin, A.L.; Egles, C. Antifungal coating by biofunctionalized polyelectrolyte multilayered films. Biomaterials 2005, 26, 6704–6712. [Google Scholar] [CrossRef] [PubMed]
- Humblot, V.; Yala, J.F.; Thebault, P.; Boukerma, K.; Héquet, A.; Berjeaud, J.M.; Pradier, C.M. The antibacterial activity of Magainin I immobilized onto mixed thiols Self-Assembled Monolayers. Biomaterials 2009, 30, 3503–3512. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Vallet-Regí, M.; Kupferschmidt, N.; Terasaki, O.; Schmidtchen, A.; Malmsten, M. Incorporation of antimicrobial compounds in mesoporous silica film monolith. Biomaterials 2009, 30, 5729–5736. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors. Licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, G.; Zreiqat, H. Functional Coatings or Films for Hard-Tissue Applications. Materials 2010, 3, 3994-4050. https://doi.org/10.3390/ma3073994
Wang G, Zreiqat H. Functional Coatings or Films for Hard-Tissue Applications. Materials. 2010; 3(7):3994-4050. https://doi.org/10.3390/ma3073994
Chicago/Turabian StyleWang, Guocheng, and Hala Zreiqat. 2010. "Functional Coatings or Films for Hard-Tissue Applications" Materials 3, no. 7: 3994-4050. https://doi.org/10.3390/ma3073994