Manganese-based Materials Inspired by Photosynthesis for Water-Splitting
Abstract
:1. Introduction
2. Photosynthetic Water Splitting
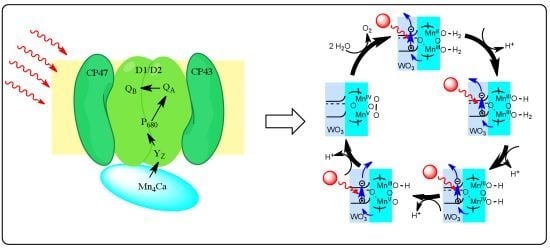
3. Mn-based Catalyst and Their Mechanisms
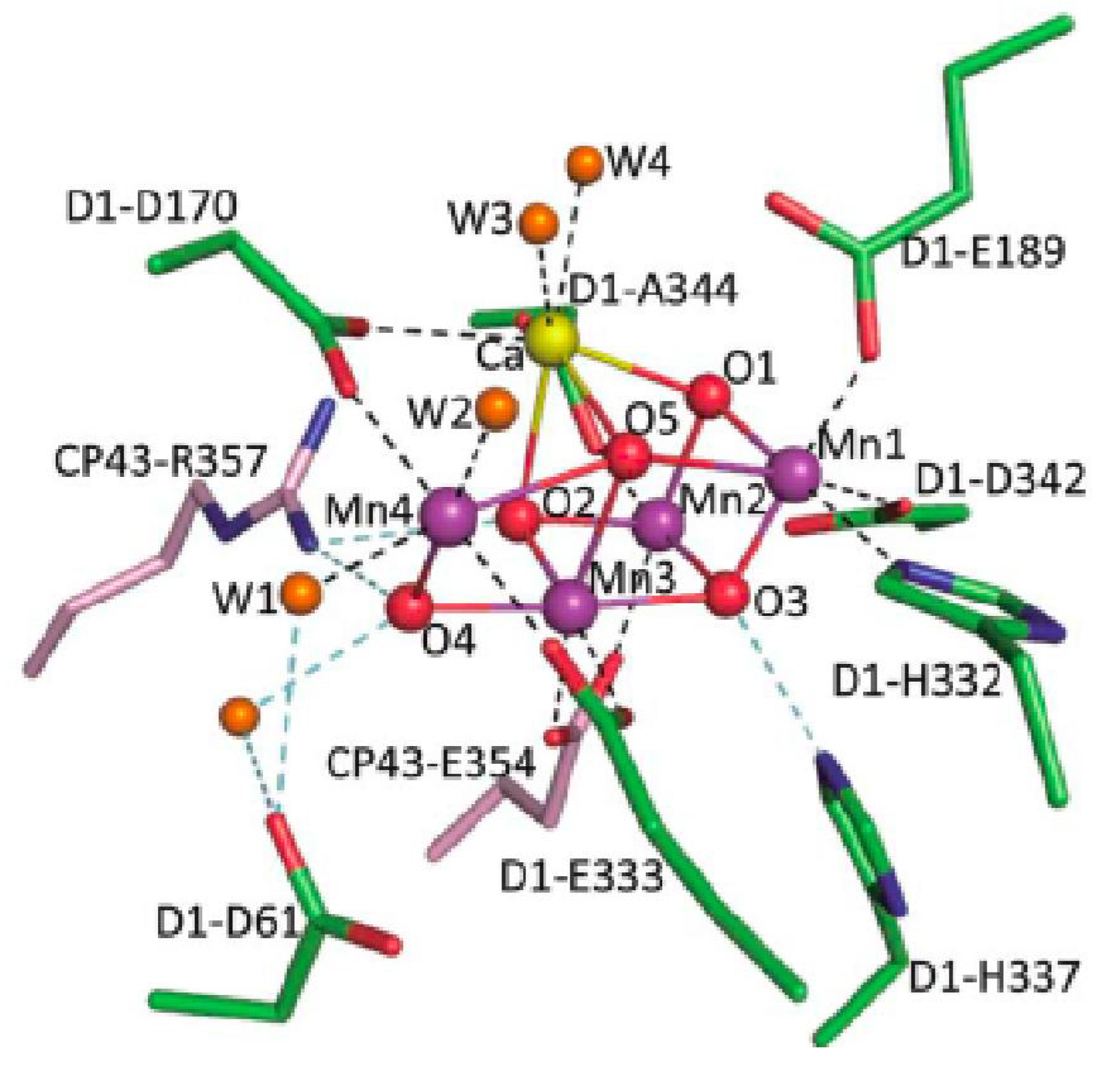
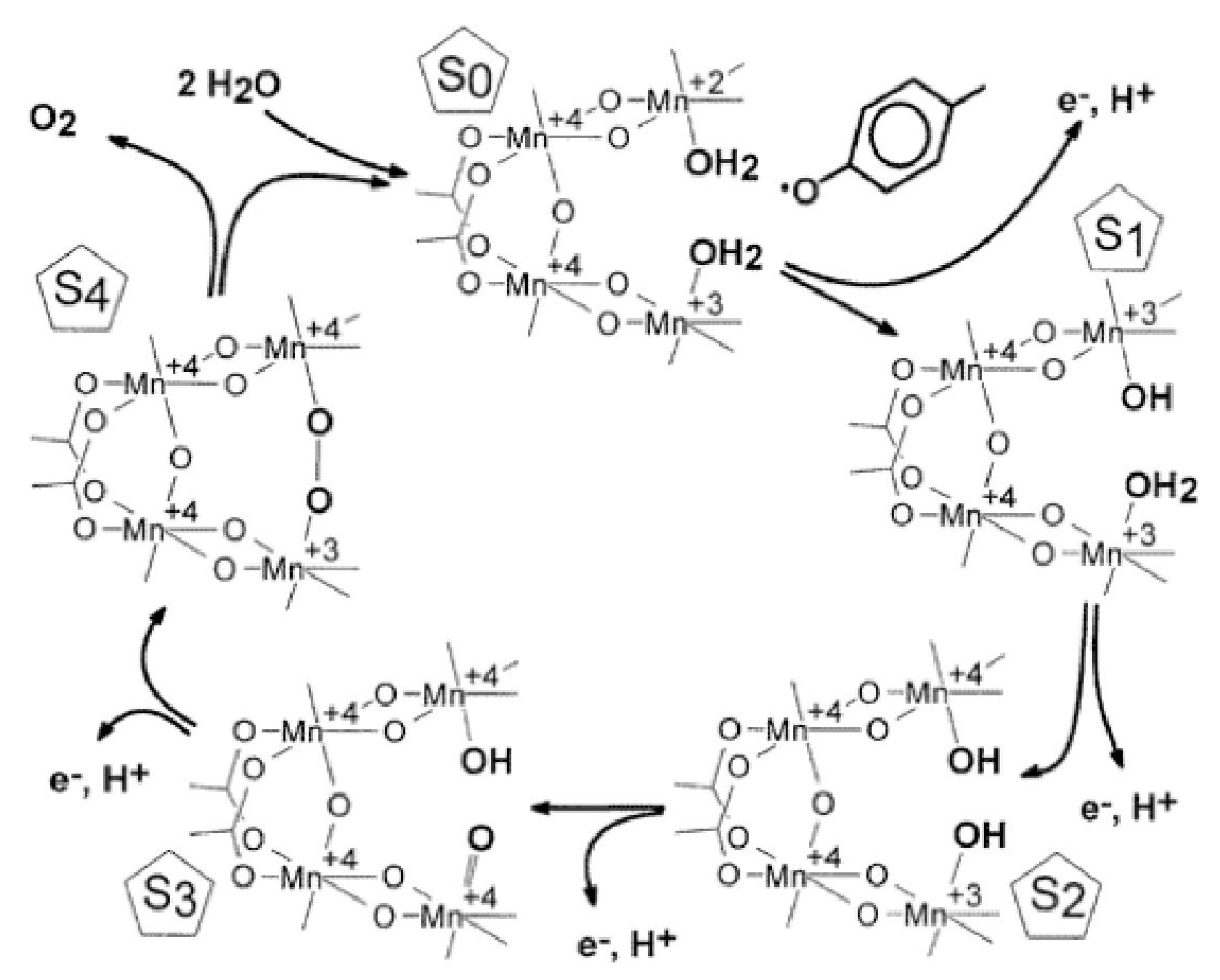
3.1. Mechanism of Photosynthetic Water Splitting
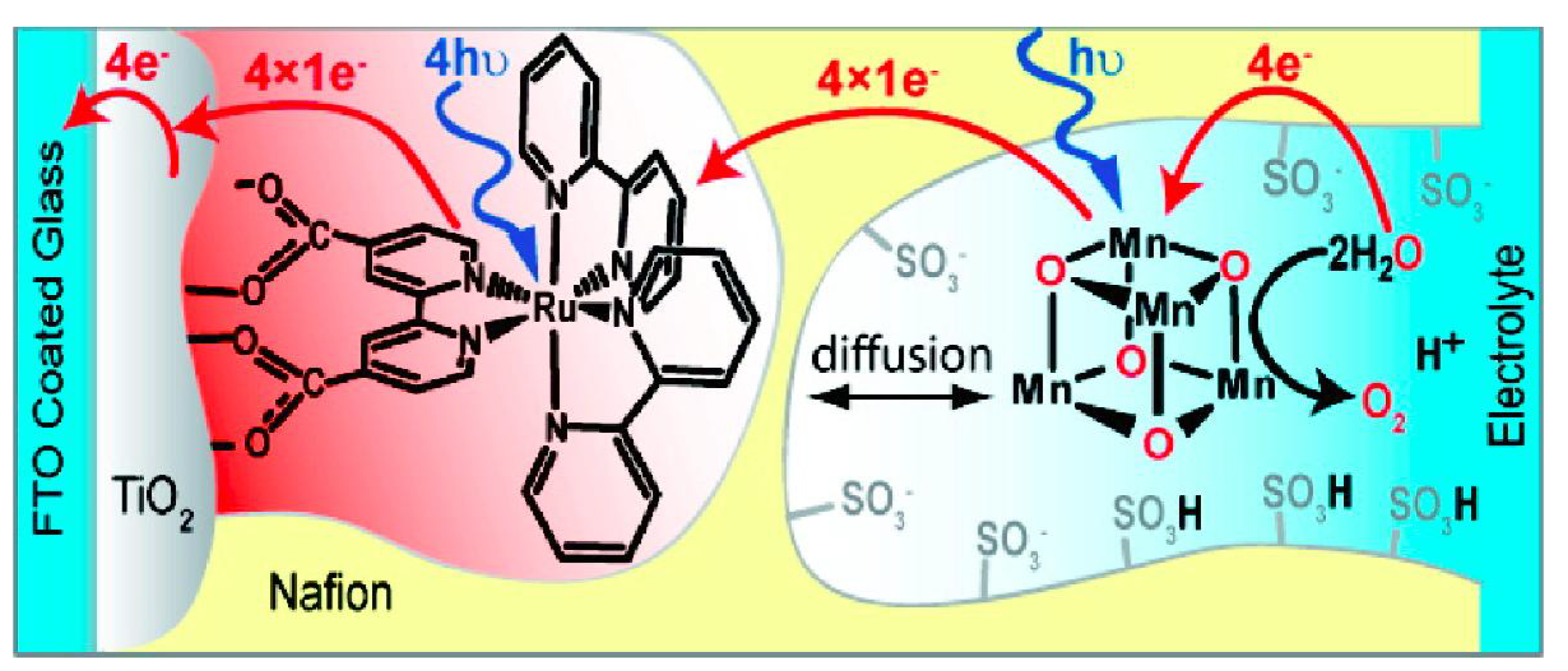
3.2. Mn-oxo tetramer/Nafion and Mn-oxo tetramer/TiO2 System
3.3. Mn-terpy dimer and Mn-terpy dimer/TiO2 System

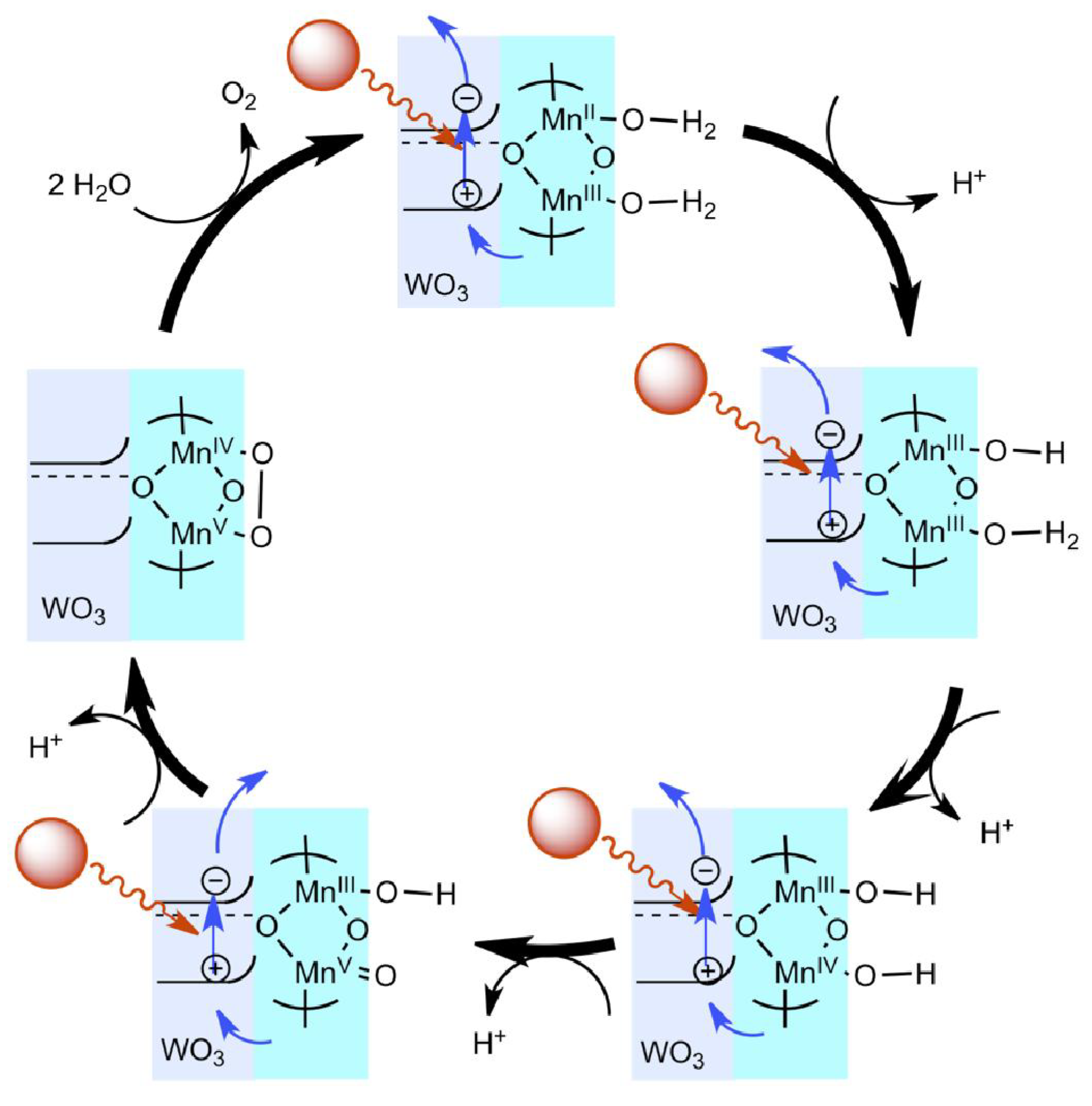
3.4. Mn-oligomer/WO3 System
4. Concluding Remarks
Acknowledgments
References
- Barber, J. Photosynthetic energy conversion: Natural and artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [PubMed]
- Blankenship Robert, E.; Tiede David, M.; Barber, J.; Brudvig Gary, W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer David, M.; Melis, A.; et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 2011, 332, 805–809. [Google Scholar] [PubMed]
- Brudvig, G.W. Water oxidation chemistry of photosystem II. Philos. Trans. R. Soc. B 2008, 363, 1211–1219. [Google Scholar] [CrossRef]
- Barber, J.; Murray, J.W. Revealing the structure of the Mn-cluster of Photosystem II by X-ray crystallography. Coord. Chem. Rev. 2008, 252, 233–243. [Google Scholar] [CrossRef]
- Dismukes, G.C.; Brimblecombe, R.; Felton, G.A.N.; Pryadun, R.S.; Sheats, J.E.; Spiccia, L.; Swiegers, G.F. Development of bioinspired Mn4O4-cubane water oxidation catalysts: Lessons from photosynthesis. Acc. Chem. Res. 2009, 42, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lin, Y.; Chou, L.-Y.; Sheehan, S.W.; He, W.; Zhang, F.; Hou, H.J.M.; Wang, D. Water splitting by tungsten oxide prepared by atomic layer deposition and decoraed with an oxygen-evolving catalyst. Angew. Chem. Int. Ed. 2011, 50, 499–502. [Google Scholar] [CrossRef]
- Blakemore, J.D.; Schley, N.D.; Balcells, D.; Hull, J.F.; Olack, G.W.; Incarvito, C.D.; Eisenstein, O.; Brudvig, G.W.; Crabtree, R.H. Half-sandwich iridium complexes for homogeneous water-oxidation catalysis. J. Am. Chem. Soc. 2010, 132, 16017–16029. [Google Scholar] [CrossRef] [PubMed]
- Kanan, M.; Nocera, D.G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Brimblecombe, R.; Koo, A.; Dismukes, G.C.; Swiegers, G.F.; Spiccia, L. Solar-driven water oxidation by a bio-inspired manganese molecular catalyst. J. Am. Chem. Soc. 2010, 132, 2892–2894. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.K.; Gamelin, D.R. Photoelectrochemical water oxidation by cobalt catalyst (“Co-Pi”)/α-Fe2O3 composite photoanodes: Oxygen evolution and resolution of a kinetic bottleneck. J. Am. Chem. Soc. 2010, 132, 4202–4207. [Google Scholar] [CrossRef] [PubMed]
- Dau, H.; Zaharieva, I. Principles, efficiency, and blueprint character of solar energy conversion in photosynthetic water oxidation. Acc. Chem. Res. 2009, 42, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- McConnell, I.; Li, G.; Brudvig, G.W. Energy conversion in natural and artificial photosynthesis. Chem. Biol. 2010, 17, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Cady, C.W.; Crabtree, R.H.; Brudvig, G.W. Functional models for the oxygen-evolving complex of photosystem II. Coord. Chem. Rev. 2008, 252, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Kanan, M.W.; Surendranath, Y.; Nocera, D.G. Cobalt-phosphate oxygen-evolving compound. Chem. Soc. Rev. 2009, 38, 109–114. [Google Scholar] [CrossRef]
- Hambourger, M.; Moore, G.F.; Kramer, D.M.; Gust, D.; Moore, A.L.; Moore, T.A. Biology and technology for photochemical fuel production. Chem. Soc. Rev. 2009, 38, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2008, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yuan, G.; Liu, R.; Zhou, S.; Sheehan, S.W.; Wang, D. Semiconductor nanostructure-based photoelectrochemical water splitting: A brief review. Chem. Phys. Lett. 2011, 507, 209–215. [Google Scholar] [CrossRef]
- Mishra, A.; Wernsdorfer, W.; Abboud, K.A.; Christou, G. The first high oxidation state manganese-calcium cluster: Relevance to the water oxidizing complex of photosynthesis. Chem. Commun. 2005, 54–56. [Google Scholar] [CrossRef]
- Kanady, J.S.; Tsui, E.Y.; Day, M.W.; Agapie, T. A synthetic model of the Mn3Ca subsite of the oxygen-evolving complex in photosystem II. Science 2011, 333, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.S.; Pecoraro, V.L. Reflections on small molecule manganese models that seek to mimic photosynthetic water oxidation chemistry. Coord. Chem. Rev. 2008, 252, 416–443. [Google Scholar] [CrossRef] [PubMed]
- Joliot, P.; Barbieri, G.; Chabaud, R. Model of the system II photochemical centers. Photochem. Photobiol. 1969, 10, 309–329. [Google Scholar] [CrossRef]
- Kok, B.; Forbush, B.; McGloin, M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem. Photobiol. 1970, 11, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Loll, B.; Kern, J.; Saenger, W.; Zouni, A.; Biesiadka, J. Towards complete cofactor arrangement in the 3.0 A resolution structure of photosystem II. Nature 2005, 438, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Shen, J.R. Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7-A resolution. Proc. Natl. Acad. Sci. USA 2003, 100, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.N.; Iverson, T.M.; Maghlaoui, K.; Barber, J.; Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 2004, 303, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Zouni, A.; Witt, H.T.; Kern, J.; Fromme, P.; Krauss, N.; Saenger, W.; Orth, P. Crystal structure of photosystem II from Synechococcus elongatus at 3.8 A resolution. Nature 2001, 409, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Guskov, A.; Kern, J.; Gabdulkhakov, A.; Broser, M.; Zouni, A.; Saenger, W. Cyanobacterial photosystem II at 2.9 A resolution and the role of quinones, lipid, channels and chloride. Nat. Struct. Mol. Biol. 2009, 16, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Umena, Y.; Kawakami, K.; Shen, J.R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 A. Nature 2011, 473, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Brudvig, G.W.; Crabtree, R.H. Mechanism for photosynthetic O2 evolution. Proc. Natl. Acad. Sci. USA 1986, 83, 4586–4588. [Google Scholar] [CrossRef] [PubMed]
- Christou, G. Manganese carboxylate chemistry and its biological relevance. Acc. Chem. Res. 1989, 22, 328–335. [Google Scholar] [CrossRef]
- Ruettinger, W.; Yagi, M.; Wolf, K.; Bernasek, S.; Dismukes, G.C. O2 evolution from the manganese-oxo cubane core Mn4O46+: A molecular mimic of the photosynthetic water oxidation enzyme? J. Am. Chem. Soc. 2000, 122, 10353–10357. [Google Scholar] [CrossRef]
- Renger, G.; Volker, M.; Eckert, H.J.; Fromme, R.; Hohm-veit, S.; Graber, P. On the mechanism of photosystem II deterioration by UV-B irradiation. Photochem. Photobiol. 1989, 49, 97–105. [Google Scholar] [CrossRef]
- Ohnishi, N.; Allakhverdiev, S.I.; Takahashi, S.; Higashi, S.; Watanabe, M.; Nishiyama, Y.; Murata, N. Two-step mechanism of photodamage to photosystem II: Step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry 2005, 44, 8494–8499. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Cady, C.; Brudvig, G.W.; Hou, H.J.M. Photodamage of a Mn(III/IV)-oxo mix valence compound and photosystem II complexes: Evidence that high-valent manganese species is responsible for UV-induced photodamage of oxygen evolving complex in photosystem II. J. Photochem. Photobiol. B 2011, 104, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Hakala, M.; Tuominen, I.; Keranen, M.; Tyystjarvi, T.; Tyystjarvi, E. Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II. Biochim. Biophys. Acta 2005, 1706, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Vass, I.; Sass, L.; Spetea, C.; Bakou, A.; Ghanotakis, D.F.; Petrouleas, V. UV-B-induced inhibition of photosystem II electron transport studied by EPR and chlorophyll fluorescence. Impairment of donor and acceptor side components. Biochemistry 1996, 35, 8964–8973. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Lo, W.; Armstrong William, H. Illumination with ultraviolet or visible light induces chemical changes in the water soluble manganese complex, [Mn4O6(bpea)4]Br4. Photochem. Photobiol. 2009, 85, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Kern, J.; Irrgang, K.-D.; Latimer, M.J.; Bergmann, U.; Glatzel, P.; Pushkar, Y.; Biesiadka, J.; Loll, B.; Sauer, K.; et al. X-ray damage to the Mn4Ca complex in single crystals of photosystem II: A case study for metalloprotein crystallography. Proc. Natl. Acad. Sci. USA 2005, 102, 12047–12052. [Google Scholar] [CrossRef] [PubMed]
- Brimblecombe, R.; Swiegers, G.F.; Dismukes, G.C.; Spiccia, L. Sustained water oxidation photocatalysis by a bioinspired manganese cluster. Angew. Chem., Int. Ed. 2008, 47, 7335–7338. [Google Scholar] [CrossRef]
- Hocking, R.K.; Brimblecombe, R.; Chang, L.-Y.; Singh, A.; Cheah, M.H.; Clover, C.; Casey, W.H.; Spiccia, L. Water-oxidation catalysis by manganese in a geochemical-like cycle. Nat. Chem. 2011, 3, 461–466. [Google Scholar] [PubMed]
- Limburg, J.; Vrettos, J.S.; Liable-Sands, L.M.; Rheingold, A.L.; Crabtree, R.H.; Brudvig, G.W. A functional model for O-O bond formation by the O2-evolving complex in photosystem II. Science 1999, 283, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Limburg, J.; Vrettos, J.S.; Chen, H.; de Paula, J.C.; Crabtree, R.H.; Brudvig, G.W. Characterization of the O2-evolving reaction catalyzed by [(terpy)(H2O)Mn(III)(O)2Mn(IV)(OH2)(terpy)](NO3)3 (terpy = 2,2':6,2"-terpyridine). J. Am. Chem. Soc. 2001, 123, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Hammarstrom, L.; Styring, S. Coupled electron transfers in artificial photosynthesis. Phil. Trans. R. Soc. B 2008, 363, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- McNamara, W.R.; Milot, R.L.; Song, H.-E.; Snoeberger, R.C., III; Batista, V.S.; Schmuttenmaer, C.A.; Brudvig, G.W.; Crabtree, R.H. Water-stable, hydroxamate anchors for functionalization of TiO2 surfaces with ultrafast interfacial electron transfer. Energy Environ. Sci. 2010, 3, 917–923. [Google Scholar] [CrossRef]
- Li, G.; Sproviero Eduardo, M.; Snoeberger, R.; Iguchi, N.; Black, J.D.; Crabtree George, W.; Brudvig Gary, W.; Batisa, V.S. Deposition of an oxomanganese water oxidation catalyst on TiO2 nanoparticles: Computational modeling, assembly and characterization. Energy Environ. Sci. 2009, 2, 230–238. [Google Scholar] [CrossRef]
- McNamara, W.R.; Snoeberger, R.C.; Li, G.; Schleicher, J.M.; Cady, C.W.; Poyatos, M.; Schmuttenmaer, C.A.; Crabtree, R.H.; Brudvig, G.W.; Batista, V.S. Acetylacetonate anchors for robust functionalization of TiO2 nanoparticles with Mn(II)-terpyridine complexes. J. Am. Chem. Soc. 2008, 130, 14329–14338. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, W.J.; Lee, S.-H.A.; Kobayashi, Y.; Hernandez-Pagan, E.A.; Hoertz, P.G.; Moore, T.A.; Moore, A.L.; Gust, D.; Mallouk, T.E. Photoassisted overall water splitting in a visible light-absorbing dye-sensitized photoelectrochemical cell. J. Am. Chem. Soc. 2009, 131, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, M.; Parkinson, B.A. Combinatorial approaches for the identification and optimization of oxide semiconductors for efficient solar photoelectrolysis. Chem. Soc. Rev. 2008, 38, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.K.; Sun, J.; Inumaru, H.; Gamelin, D.R. Solar water oxidation by composite catalyst/α-Fe2O3 photoanodes. J. Am. Chem. Soc. 2009, 131, 6086–6087. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sproviero, E.M.; McNamara, W.R.; Snoeberger, R.C.; Crabtree, R.H.; Brudvig, G.W.; Batista, V.S. Reversible visible-light photooxidation of an oxomanganese water-oxidation catalyst covalently anchored to TiO2 nanoparticles. J. Phys. Chem. B 2010, 114, 14214–14222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cady, C.W.; Brudvig Gary, W.; Hou, H.J.M. Thermal stability of [Mn(III)(O)2Mn(IV)(H2O)2(Terpy)2](NO3)3 (Terpy = 2,2':6',2"-terpyridine) in aqueous solution. Inorg. Chim. Acta 2011, 366, 128–133. [Google Scholar] [CrossRef]
- Lin, Y.; Yuan, G.; Liu, R.; Zhou, S.; Sheehan, S.W.; Wang, D. Nanonet-based hematite for efficient solar water splitting. J. Am. Chem. Soc. 2011, 133, 2398–2401. [Google Scholar] [CrossRef] [PubMed]
- Dinca, M.; Surendranath, Y.; Nocera Daniel, G. Nickel-borate oxygen-evolving catalyst that functions under benign conditions. Proc. Natl. Acad. Sci. USA 2010, 107, 10337–10341. [Google Scholar] [CrossRef] [PubMed]
- Kanan, M.W.; Yano, J.; Surendranath, Y.; Dinca, M.; Yachandra, V.K.; Nocera, D.G. Structure and valency of a cobalt-phosphate water oxidation catalyst determined by in situ X-ray spectroscopy. J. Am. Chem. Soc. 2010, 132, 13692–13701. [Google Scholar] [CrossRef] [PubMed]
- McAlpin, J.G.; Surendranath, Y.; Dinca, M.; Stich, T.A.; Stoian, S.A.; Casey, W.H.; Nocera, D.G.; Britt, R.D. EPR evidence for Co(IV) species produced during water oxidation at neutral pH. J. Am. Chem. Soc. 2010, 132, 6882–6883. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, J.J.; Jurss, J.W.; Brennaman, M.K.; Hoertz, P.G.; Patrocinio, A.O.T.; Murakami Iha, N.Y.; Templeton, J.L.; Meyer, T.J. Making oxygen with ruthenium complexes. Acc. Chem. Res. 2009, 42, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Sala, X.; Romero, I.; Rodriguez, M.; Escriche, L.; Llobet, A. Molecular catalysts that oxidize water to dioxygen. Angew. Chem. Int. Ed. 2009, 48, 2842–2852. [Google Scholar] [CrossRef]
- Schley, N.D.; Blakemore, J.D.; Subbaiyan, N.K.; Incarvito, C.D.; D’Souza, F.; Crabtree, R.H.; Brudvig, G.W. Distinguishing homogeneous from heterogeneous catalysis in electrode-driven water oxidation with molecular iridium complexes. J. Am. Chem. Soc. 2011, 133, 10473–10481. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Higashi, M.; Lu, D.; Abe, R.; Domen, K. Efficient nonsacrificial water splitting through two-step photoexcitation by visible light using a modified oxynitride as a hydrogen evolution photocatalyst. J. Am. Chem. Soc. 2010, 132, 5858–5868. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Nocera, D.G. Personalized Energy: The home as a solar power station and solar gas station. ChemSusChem 2009, 2, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Hoganson, C.W.; T. Babcock, G.T. A Metalloradical mechanism for the generation of oxygen from water in photosynthesis. Science 1997, 277, 1953–1956. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hou, H.J.M. Manganese-based Materials Inspired by Photosynthesis for Water-Splitting. Materials 2011, 4, 1693-1704. https://doi.org/10.3390/ma4101693
Hou HJM. Manganese-based Materials Inspired by Photosynthesis for Water-Splitting. Materials. 2011; 4(10):1693-1704. https://doi.org/10.3390/ma4101693
Chicago/Turabian StyleHou, Harvey J.M. 2011. "Manganese-based Materials Inspired by Photosynthesis for Water-Splitting" Materials 4, no. 10: 1693-1704. https://doi.org/10.3390/ma4101693