Synthesis and Characterization of Self-Assembled Nanogels Made of Pullulan
Abstract
:1. Introduction
2. Results and Discussion
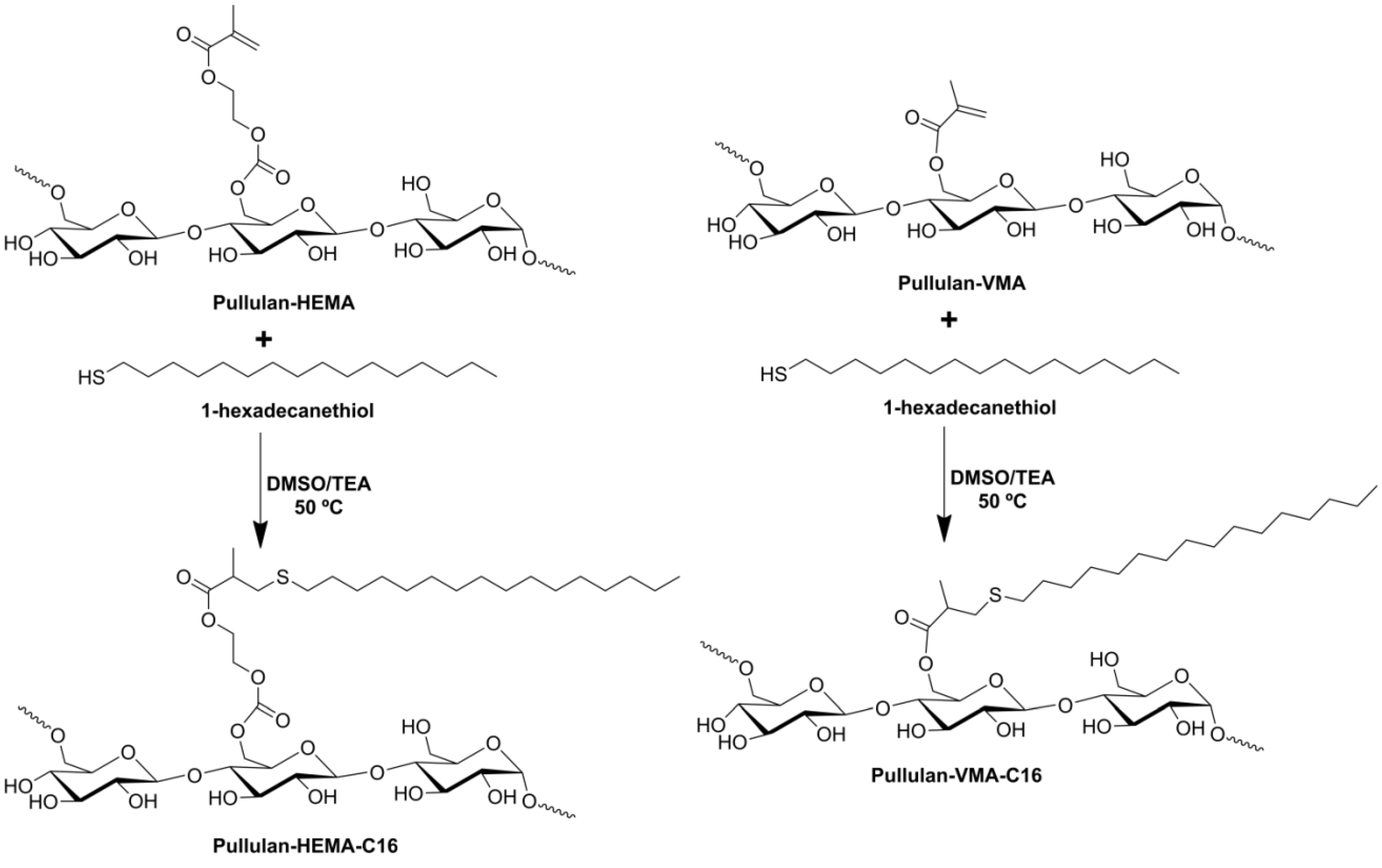
2.1. Synthesis of Pullulan-C16
| tDSHEMA or tDSVMAa | DSHEMA or DSVMA b | tDSC16 c | DSC16d | DSC16/DSHEMA or DSC16/DSVMA e | Pullulan-C16 f |
|---|---|---|---|---|---|
| Pullulan-HEMA-C16 | |||||
| 20 | 5.6 | 120 | 1.3 | 23.2 | PHC16-5.6-1.3 |
| 25 | 8 | 80 | 4.6 | 57.5 | PHC16-8-4.6 |
| 200 | 4.3 | 53.8 | PHC16-8-4.3 | ||
| 40 | 10 | 80 | 1.2 | 12 | PHC16-10-1.2 |
| 200 | 5.9 | 59 | PHC16-10-5.9 | ||
| Pullulan-VMA-C16 | |||||
| 25 | 8.8 | 200 | 6 | 68.2 | PVC16-8.8-6 |
| 50 | 10 | 200 | 7 | 70 | PVC16-10-7 |
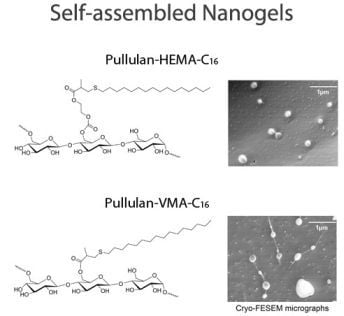
2.2. Self-assembly of Pullulan-C16
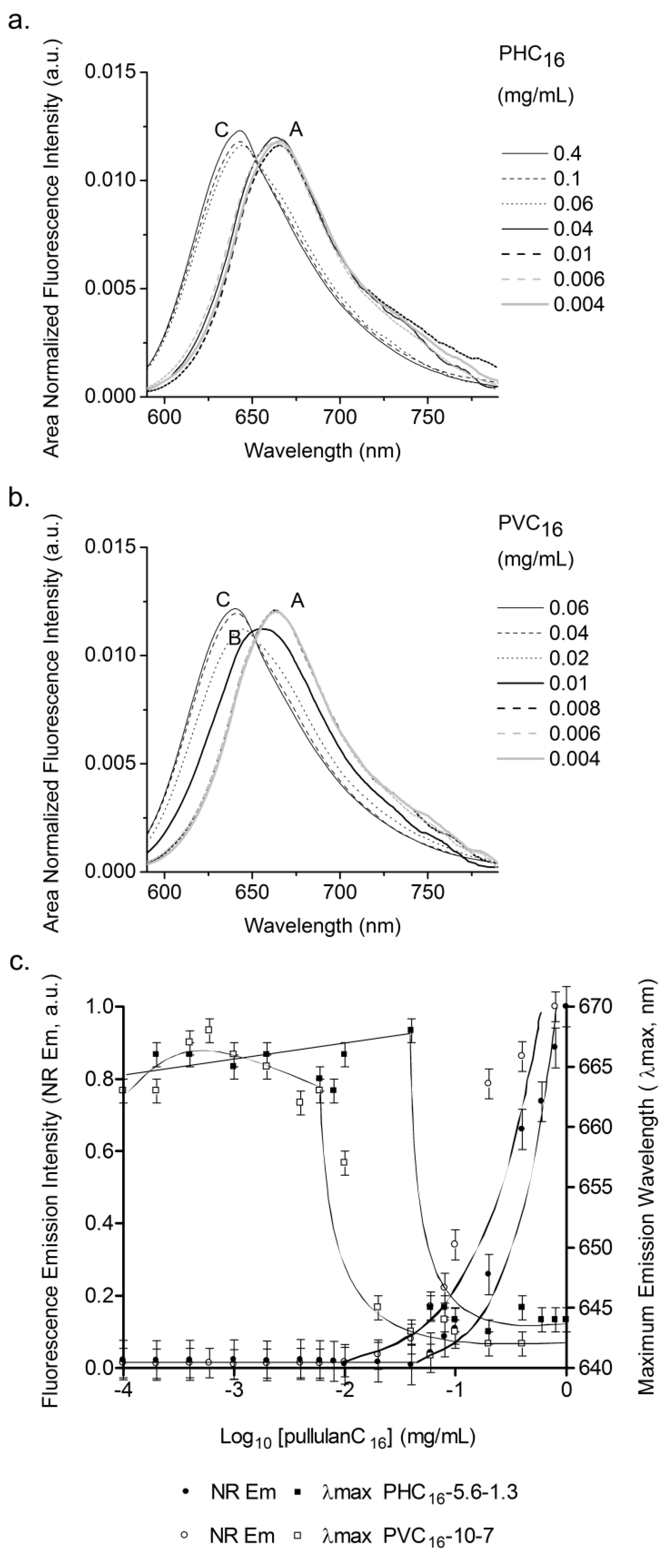
2.3. Characterization of Pullulan-C16 Nanogels
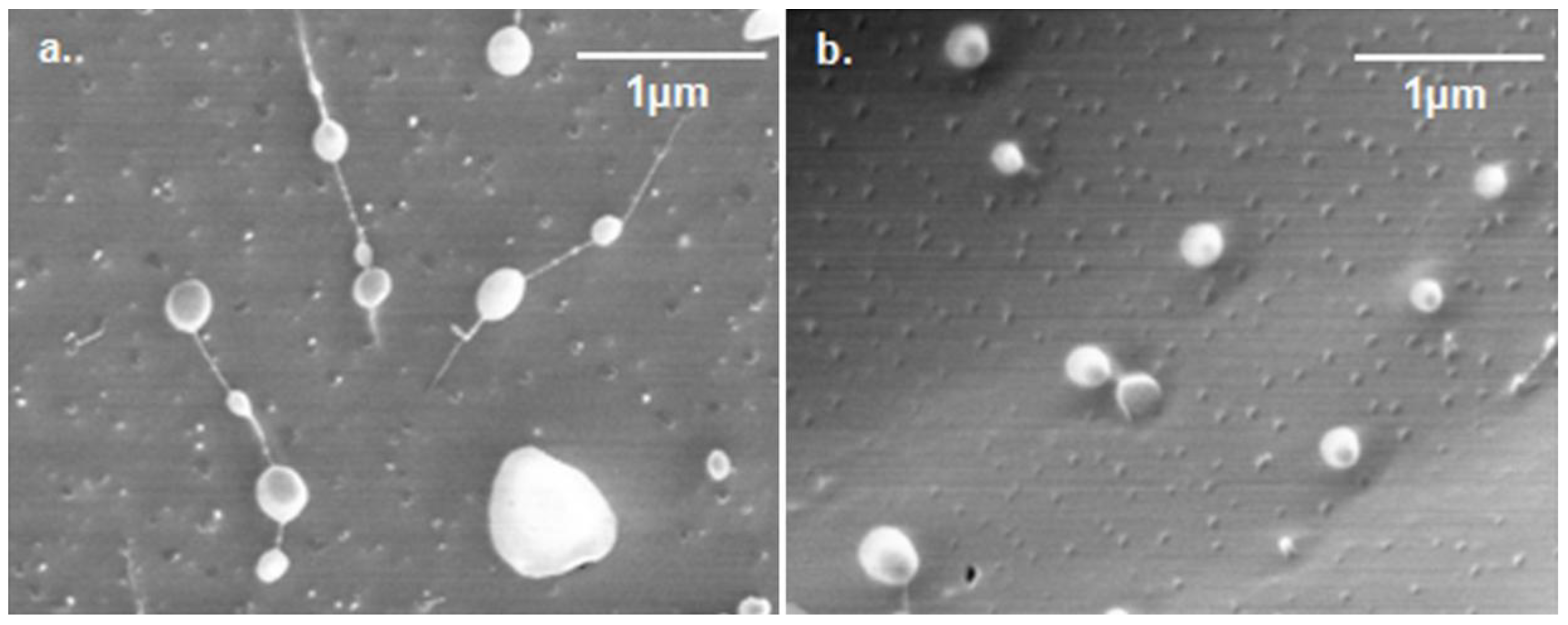
2.3.1. Size and shape
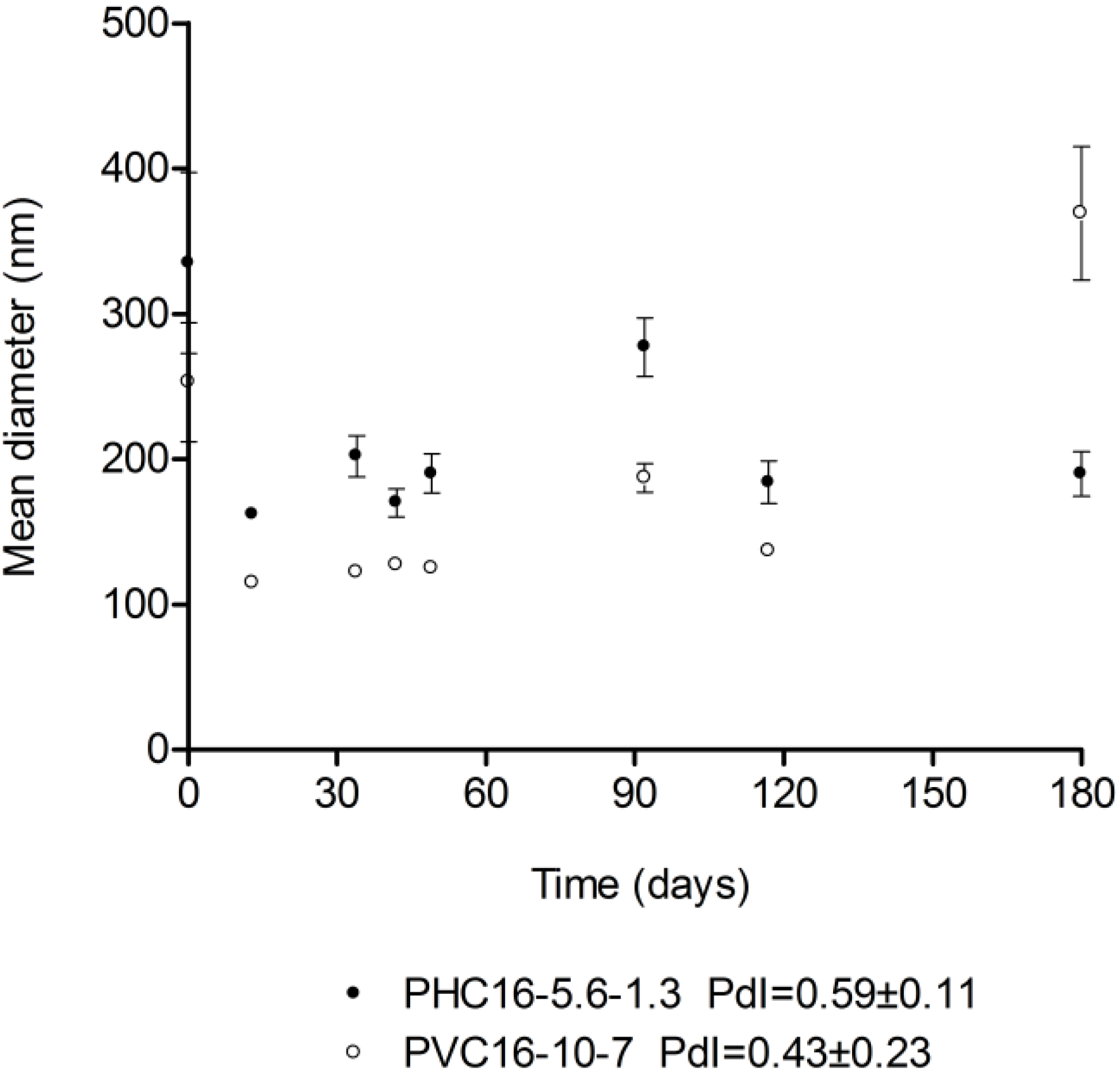
2.3.2. Storage
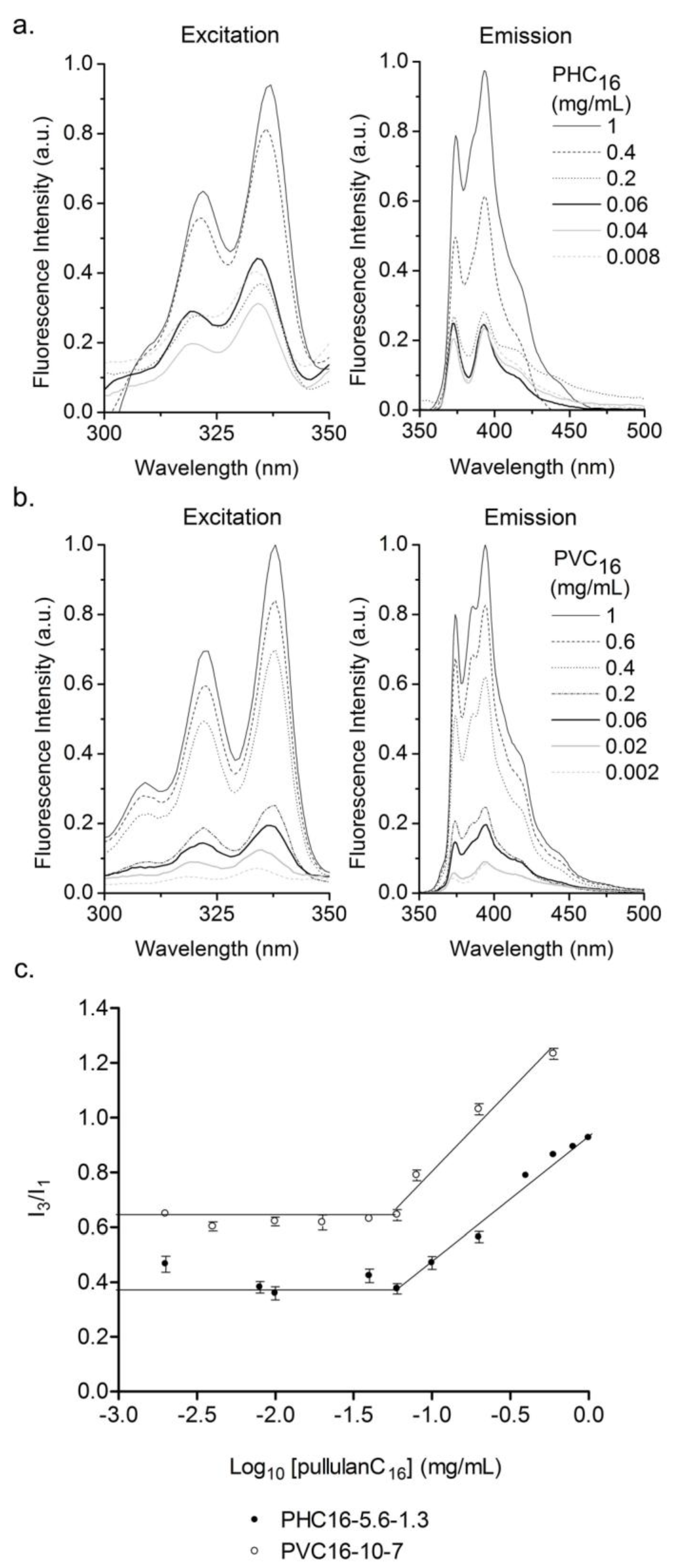
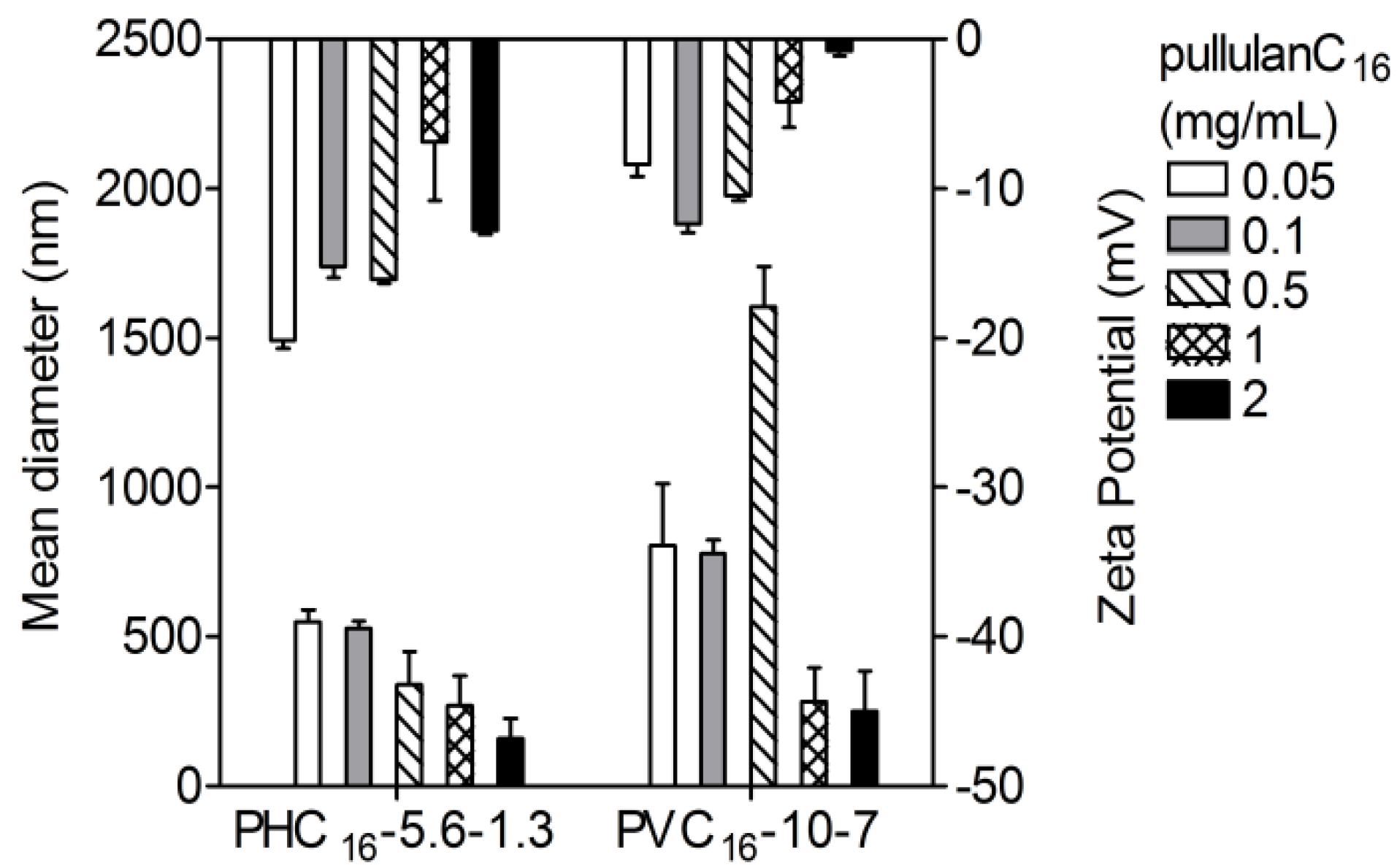
2.3.3. Effect of the Concentration of Pullulan-C16
2.3.4. Effect of Urea
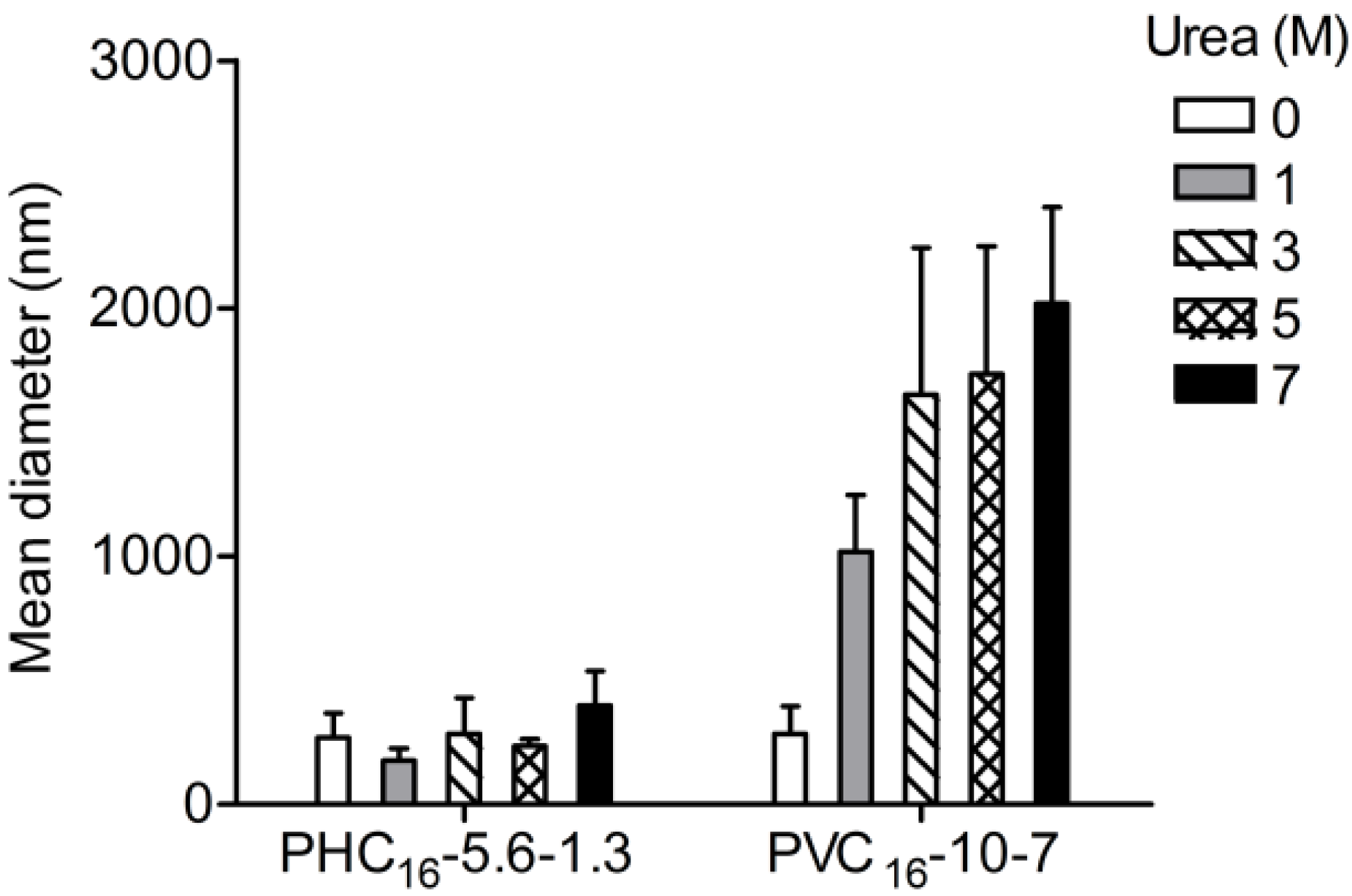
2.3.5. Effect of Ionic Strength
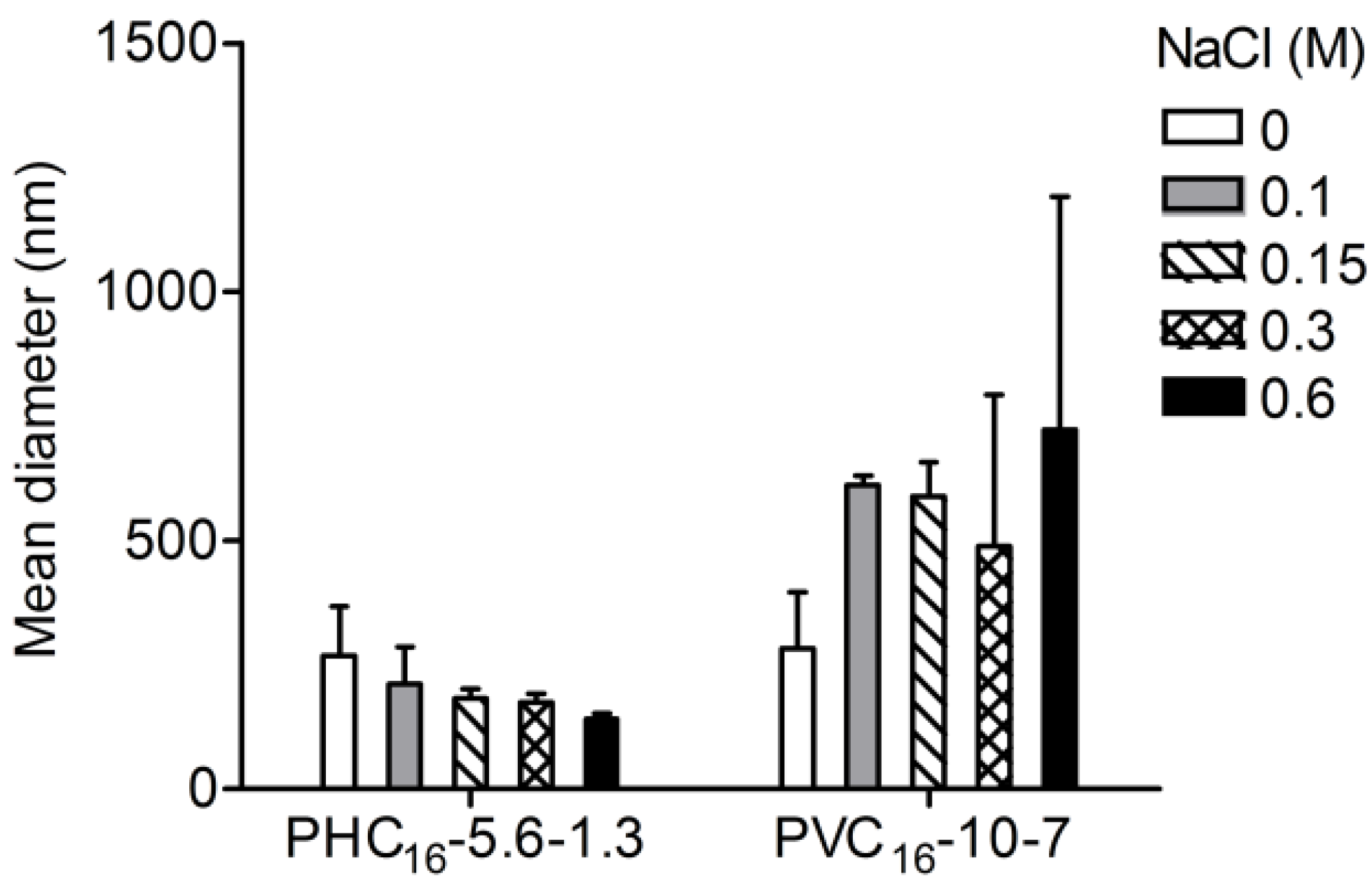
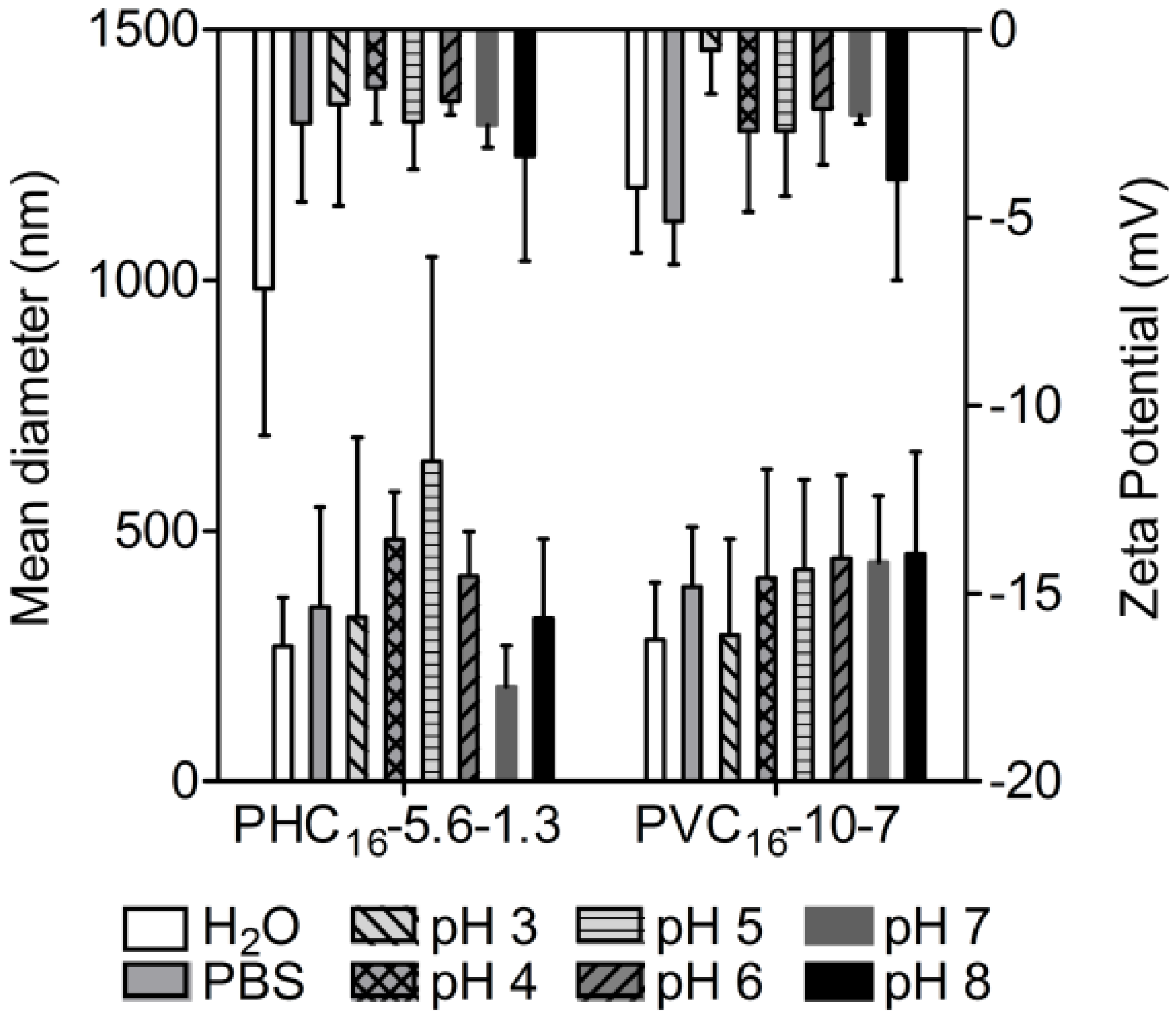
2.3.6. Effect of pH
3. Experimental Section
3.1. Materials
3.2. Synthesis of Amphiphilic Pullulan-C16
3.3. Characterization of pullulan-C16 nanogels
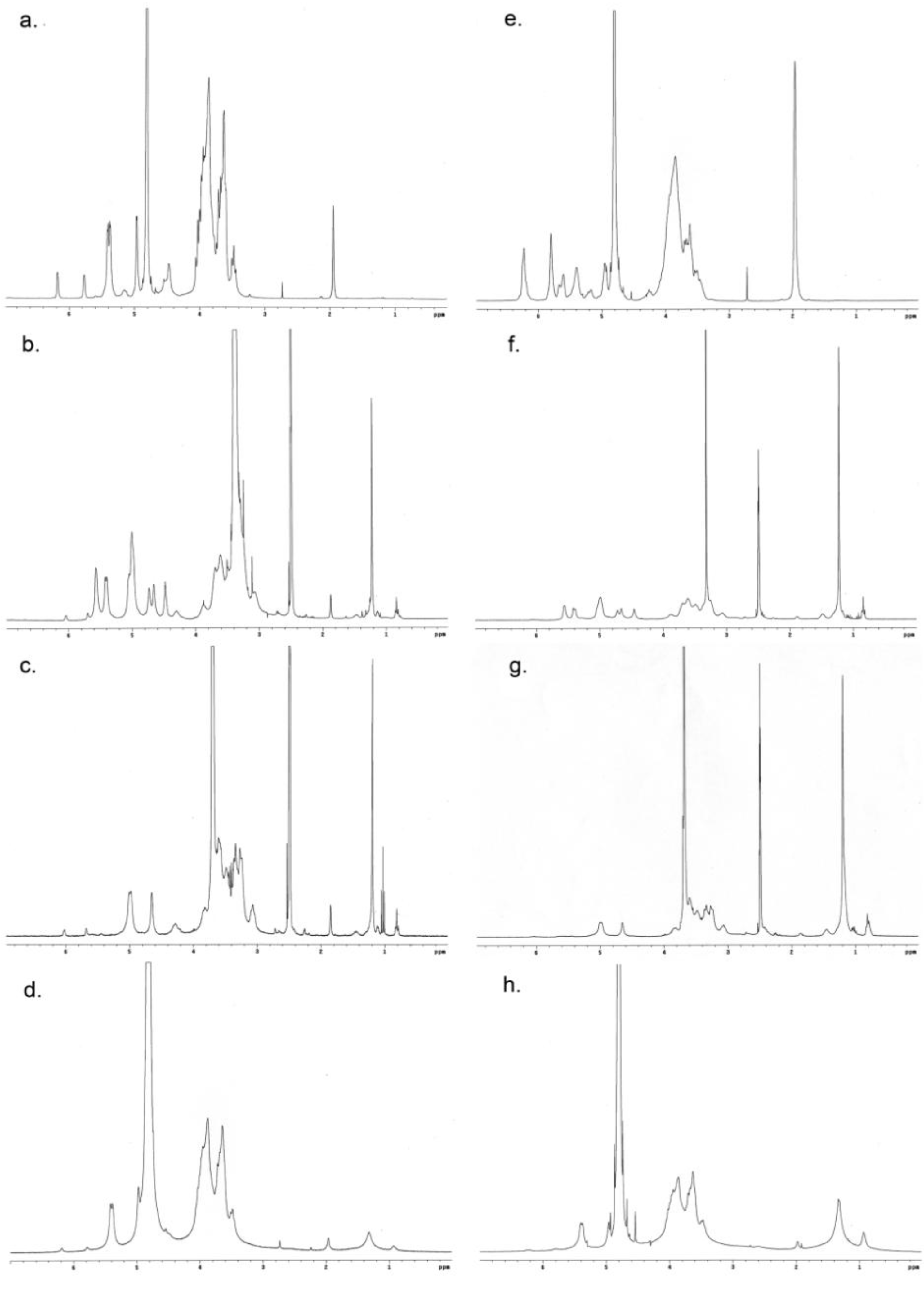
3.3.1. 1H NMR Spectroscopy
3.3.2. Fluorescence Spectroscopy
3.3.3. Cryo-FESEM
3.3.4. DLS
4. Conclusions
Acknowledgements
References
- Catley, B.J.; Whelan, W.J. Observations on the structure of pullulan. Arch. Biochem. Biophys. 1971, 143, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Jennings, H.J.; Smith, I.C. Composition, sequence, and conformation of polymers and oligomers of glucose as revealed by carbon-13 nuclear magnetic resonance. J. Am. Chem. Soc. 1974, 96, 8081–8087. [Google Scholar] [CrossRef]
- Kasaai, M.R. Intrinsic viscosity-molecular weight relationship and hydrodynamic volume for pullulan. J. Appl. Polym. Sci. 2006, 100, 4325–4332. [Google Scholar] [CrossRef]
- Jiao, Y.H.; Fu, Y.; Jiang, Z.H. Synthesis and characterization of poly(ethylene glycol) grafted on pullulan. Abstr. Paper Am. Chem. Soc. 2003, 225, U576–U576. [Google Scholar]
- Masuda, K.; Sakagami, M.; Horie, K.; Nogusa, H.; Hamana, H.; Hirano, K. Evaluation of carboxymethylpullulan as a novel carrier for targeting immune tissues. Pharm. Res. 2001, 18, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, T.; Shibuya, T.; Shiobara, S. Safety studies of a novel starch, pullulan: Chronic toxicity in rats and bacterial mutagenicity. Food Chem. Toxicol. 1997, 35, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Shingel, K.I. Current knowledge on biosynthesis, biological activity, and chemical modification of the exopolysaccharide, pullulan. Carbohydr. Res. 2004, 339, 447–460. [Google Scholar] [CrossRef]
- Nomura, Y.; Ikeda, M.; Yamaguchi, N.; Aoyama, Y.; Akiyoshi, K. Protein refolding assisted by self-assembled nanogels as novel artificial molecular chaperone. FEBS Lett. 2003, 553, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Shingel, K.I.; Petrov, P.T. Behavior of gamma-ray-irradiated pullulan in aqueous solutions of cationic (cetyltrimethylammonium hydroxide) and anionic (sodium dodecyl sulfate) surfactants. Colloid Polym. Sci. 2002, 280, 176–182. [Google Scholar] [CrossRef]
- Leathers, T.D. Biotechnological production and applications of pullulan. Appl. Microbiol. Biotechnol. 2003, 62, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Hasuda, H.; Kwon, O.H.; Kang, I.K.; Ito, Y. Synthesis of photoreactive pullulan for surface modification. Biomaterials 2005, 26, 2401–2406. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Nogawa, M. Preparation of a protein micro-array using a photo-reactive polymer for a cell-adhesion assay. Biomaterials 2003, 24, 3021–3026. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, H.; Aoyama, T.; Ogawa, O.; Tabata, Y. Liver targeting of plasmid DNA by pullulan conjugation based on metal coordination. J. Contr. Release 2002, 83, 287–302. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, A.K. Hydrogel pullulan nanoparticles encapsulating pBUDLacZ plasmid as an efficient gene delivery carrier. J. Contr. Release 2004, 99, 157–166. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Kobayashi, S.; Shichibe, S.; Mix, D.; Baudys, M.; Kim, S.W.; Sunamoto, J. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: Complexation and stabilization of insulin. J. Contr. Release 1998, 54, 313–320. [Google Scholar] [CrossRef]
- Suginoshita, Y.; Tabata, Y.; Matsumura, T.; Toda, Y.; Nabeshima, M.; Moriyasu, F.; Kada, Y.; Chiba, T. Liver targeting of human interferon-beta with pullulan based on metal coordination. J. Contr. Release 2002, 83, 75–88. [Google Scholar] [CrossRef]
- Nogusa, H.; Yamamoto, K.; Yano, T.; Kajiki, M.; Hamana, H.; Okuno, S. Distribution characteristics of carboxymethylpullulan-peptide-doxorubicin conjugates in tumor-bearing rats: Different sequence of peptide spacers and doxorubicin contents. Biol. Pharm. Bull. 2000, 23, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Bum Lee, T.; Park, K.H.; Shin, E.K.; Lee, Y.B.; Choi, H.K. Self-assembled nanoparticles of hydrophobically-modified polysaccharide bearing vitamin H as a targeted anti-cancer drug delivery system. Eur. J. Pharm. Sci. 2003, 18, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Seong Lee, E.; Bae, Y.H. Adriamycin loaded pullulan acetate/sulfonamide conjugate nanoparticles responding to tumor pH: pH-dependent cell interaction, internalization and cytotoxicity in vitro. J. Contr. Release 2003, 87, 3–13. [Google Scholar] [CrossRef]
- Hasegawa, U.; Nomura, S.M.; Kaul, S.C.; Hirano, T.; Akiyoshi, K. Nanogel-quantum dot hybrid nanoparticles for live cell imaging. Biochem. Biophys. Res. Commun. 2005, 331, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Shin, D.; Yun, K.; Park, K.H.; Lee, K.C. Conjugation of heparin into carboxylated pullulan derivatives as an extracellular matrix for endothelial cell culture. Biotechnol. Lett. 2003, 25, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Tabata, Y.; Ikada, Y. Body distribution profile of polysaccharides after intravenous administration. Drug Deliv. 1993, 1, 75–82. [Google Scholar] [CrossRef]
- Kaneo, Y.; Tanaka, T.; Nakano, T.; Yamaguchi, Y. Evidence for receptor-mediated hepatic uptake of pullulan in rats. J. Contr. Release 2001, 70, 365–373. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Deguchi, S.; Moriguchi, N.; Yamaguchi, S.; Sunamoto, J. Self-Aggregates of Hydrophobized Polysaccharides in Water––Formation and Characteristics of Nanoparticles. Macromolecules 1993, 26, 3062–3068. [Google Scholar] [CrossRef]
- Nishikawa, T.; Akiyoshi, K.; Sunamoto, J. Macromolecular complexation between bovine serum albumin and the self-assembled hydrogel nanoparticle of hydrophobized polysaccharides. J. Am. Chem. Soc. 1996, 118, 6110–6115. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Kobayashi, S.; Shichibe, S.; Mix, D.; Baudys, M.; Kim, S.W.; Sunamoto, J. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: Complexation and stabilization of insulin. J. Contr. Release 1998, 54, 313–320. [Google Scholar] [CrossRef]
- Sawada, S.I.; Akiyoshi, K. Nano-Encapsulation of Lipase by Self-Assembled Nanogels: Induction of High Enzyme Activity and Thermal Stabilization. Macromol. Biosci. 2010, 10, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kitano, S.; Kageyama, S.; Nagata, Y.; Miyahara, Y.; Hiasa, A.; Naota, H.; Okumura, S.; Imai, H.; Shiraishi, T.; Masuya, M.; Nishikawa, M.; Sunamoto, J.; Akiyoshi, K.; Kanematsu, T.; Scott, A. M.; Murphy, R.; Hoffman, E.W.; Old, L.J.; Shiku, H. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin. Cancer Res. 2006, 12, 7397–7405. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.G.; Schmitt, M.; Hiasa, A.; Nagata, Y.; Ikeda, H.; Sasaki, Y.; Akiyoshi, K.; Sunamoto, J.; Nakamura, H.; Kuribayashi, K.; Shiku, H. A novel hydrophobized polysaccharide/oncoprotein complex vaccine induces in vitro and in vivo cellular and humoral immune responses against HER2-expressing murine sarcomas. Cancer Res. 1998, 58, 3385–3390. [Google Scholar] [PubMed]
- Shiku, H.; Wang, L.; Ikuta, Y.; Okugawa, T.; Schmitt, M.; Gu, X.; Akiyoshi, K.; Sunamoto, J.; Nakamura, H. Development of a cancer vaccine: Peptides, proteins, and DNA. Cancer Chemother. Pharmacol. 2000, 46, S77–82. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kishida, T.; Hasegawa, U.; Ueda, Y.; Imanishi, J.; Yamagishi, H.; Akiyoshi, K.; Otsuji, E.; Mazda, O. Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochem. Biophys. Res. Commun. 2008, 367, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, U.; Sawada, S.; Shimizu, T.; Kishida, T.; Otsuji, E.; Mazda, O.; Akiyoshi, K. Raspberry-like assembly of cross-linked nanogels for protein delivery. J. Contr. Release 2009, 140, 312–317. [Google Scholar] [CrossRef]
- Ayame, H.; Morimoto, N.; Akiyoshi, K. Self-assembled cationic nanogels for intracellular protein delivery. Bioconjugate Chem. 2008, 19, 882–890. [Google Scholar] [CrossRef]
- Morimoto, N.; Endo, T.; Iwasaki, Y.; Akiyoshi, K. Design of hybrid hydrogels with self-assembled nanogels as cross-linkers: interaction with proteins and chaperone-like activity. Biomacromolecules 2005, 6, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Hirakura, T.; Yasugi, K.; Nemoto, T.; Sato, M.; Shimoboji, T.; Aso, Y.; Morimoto, N.; Akiyoshi, K. Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier: New system for sustained delivery of protein with a chaperone-like function. J. Contr. Release 2010, 142, 483–489. [Google Scholar] [CrossRef]
- Gonçalves, C.; Pereira, P.; Gama, M. Self-Assembled Hydrogel Nanoparticles for Drug Delivery Applications. Materials 2010, 3, 1420–1460. [Google Scholar] [CrossRef] [Green Version]
- Masci, G.; Bontempo, D.; Crescenzi, V. Synthesis and characterization of thermoresponsive N-isopropylacrylamide/methacrylated pullulan hydrogels. Polymer 2002, 43, 5587–5593. [Google Scholar] [CrossRef]
- vanDijkWolthuis, W.N.E.; Tsang, S.K.Y.; KettenesvandenBosch, J.J.; Hennink, W.E. A new class of polymerizable dextrans with hydrolyzable groups: Hydroxyethyl methacrylated dextran with and without oligolactate spacer. Polymer 1997, 38, 6235–6242. [Google Scholar] [CrossRef]
- Ferreira, L.; Gil, M.H.; Dordick, J.S. Enzymatic synthesis of dextran-containing hydrogels. Biomaterials 2002, 23, 3957–3967. [Google Scholar] [CrossRef] [PubMed]
- Glinel, K.; Sauvage, J.P.; Oulyadi, H.; Huguet, J. Determination of substituents distribution in carboxymethylpullulans by NMR spectroscopy. Carbohydr. Res. 2000, 328, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, C.; Martins, J.A.; Gama, F.M. Self-assembled nanoparticles of dextrin substituted with hexadecanethiol. Biomacromolecules 2007, 8, 392–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrkach, J.S.; Peracchia, M.T.; Domb, A.; Lotan, N.; Langer, R. Nanotechnology for biomaterials engineering: Structural characterization of amphiphilic polymeric nanoparticles by H-1 NMR spectroscopy. Biomaterials 1997, 18, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, K.; Thomas, J.K. Environmental Effects on Vibronic Band Intensities in Pyrene Monomer Fluorescence and Their Application in Studies of Micellar Systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Dong, D.C.; Winnik, M.A. The Py Scale of Solvent Polarities. Solvent effects on the vibrionic fine structure of pyrene fluorescence and empirical correlations with ET and Y values. Can. J. Chem. 1984, 62, 2560–2565. [Google Scholar] [CrossRef]
- Coutinho, P.J.G.; Castanheira, E.M.S.; Rei, M.C.; Oliveira, M.E.C.D.R. Nile red and DCM fluorescence Anisotropy studies in C12E7/DPPC mixed systems. J. Phys. Chem. B 2002, 106, 12841–12846. [Google Scholar] [CrossRef]
- Jones, M.C.; Leroux, J.C. Polymeric micelles––A new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999, 48, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, P.; Ray, A. Effect of Urea on Micelle Formation and Hydrophobic Bonding. J. Phys. Chem. 1963, 67, 190–192. [Google Scholar] [CrossRef]
- Moore, D.R.; Mathias, L.J. Molecular Composites Via Insitu Polymerization - Poly(Phenylene Terephthalamide)-Nylon 3. J. Appl. Polym. Sci. 1986, 32, 6299–6315. [Google Scholar] [CrossRef]
- Hierrezuelo, J.M.; Molina-Bolívar, J.A.; Carnero Ruiz, C. On the Urea Action Mechanism: A Comparative Study on the Self-Assembly of Two Sugar-Based Surfactants. J. Phys. Chem. B 2009, 113, 7178–7187. [Google Scholar] [CrossRef] [PubMed]
- Dijk-Wolthuis, W.N.E.v.; Steenbergen, M.J.v.; Underberg, W.J.M.; Hennink, W.E. Degradation kinetics of methacrylated dextrans in aqueous solution. J. Pharm. Sci. 1997, 86, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Smedsrod, B. Clearance function of scavenger endothelial cells. Comp. Hepatol. 2004, 3, S22. [Google Scholar] [PubMed]
- Carvalho, V.; Castanheira, P.; Faria, T.Q.; Goncalves, C.; Madureira, P.; Faro, C.; Domingues, L.; Brito, R.M.; Vilanova, M.; Gama, M. Biological activity of heterologous murine interleukin-10 and preliminary studies on the use of a dextrin nanogel as a delivery system. Int. J. Pharm. 2010, 400, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.; Goncalves, C.; Gil, A.M.; Gama, F.M. Production and characterization of a new dextrin based hydrogel. Eur. Polym. J. 2007, 43, 3050–3059. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ferreira, S.A.; Coutinho, P.J.G.; Gama, F.M. Synthesis and Characterization of Self-Assembled Nanogels Made of Pullulan. Materials 2011, 4, 601-620. https://doi.org/10.3390/ma4040601
Ferreira SA, Coutinho PJG, Gama FM. Synthesis and Characterization of Self-Assembled Nanogels Made of Pullulan. Materials. 2011; 4(4):601-620. https://doi.org/10.3390/ma4040601
Chicago/Turabian StyleFerreira, Sílvia A., Paulo J. G. Coutinho, and Francisco M. Gama. 2011. "Synthesis and Characterization of Self-Assembled Nanogels Made of Pullulan" Materials 4, no. 4: 601-620. https://doi.org/10.3390/ma4040601