Synthesis, Crystal Structure and Luminescent Property of Mg(II) Complex with N-Benzenesulphonyl-L-Leucine and 1,10-Phenanthroline
Abstract
:1. Introduction
2. Results and Discussion
2.1. IR Spectra
2.2. Structure Description
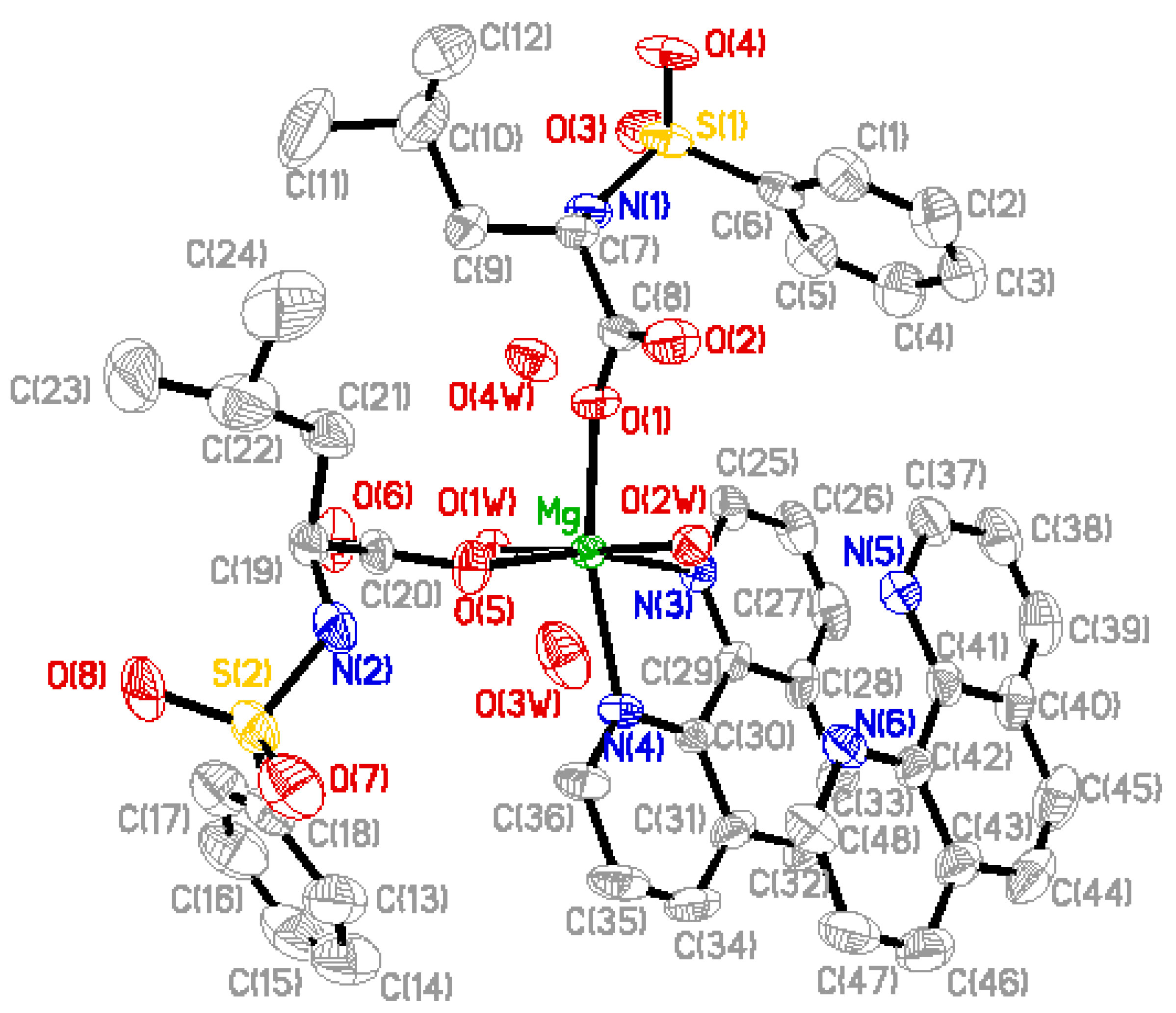

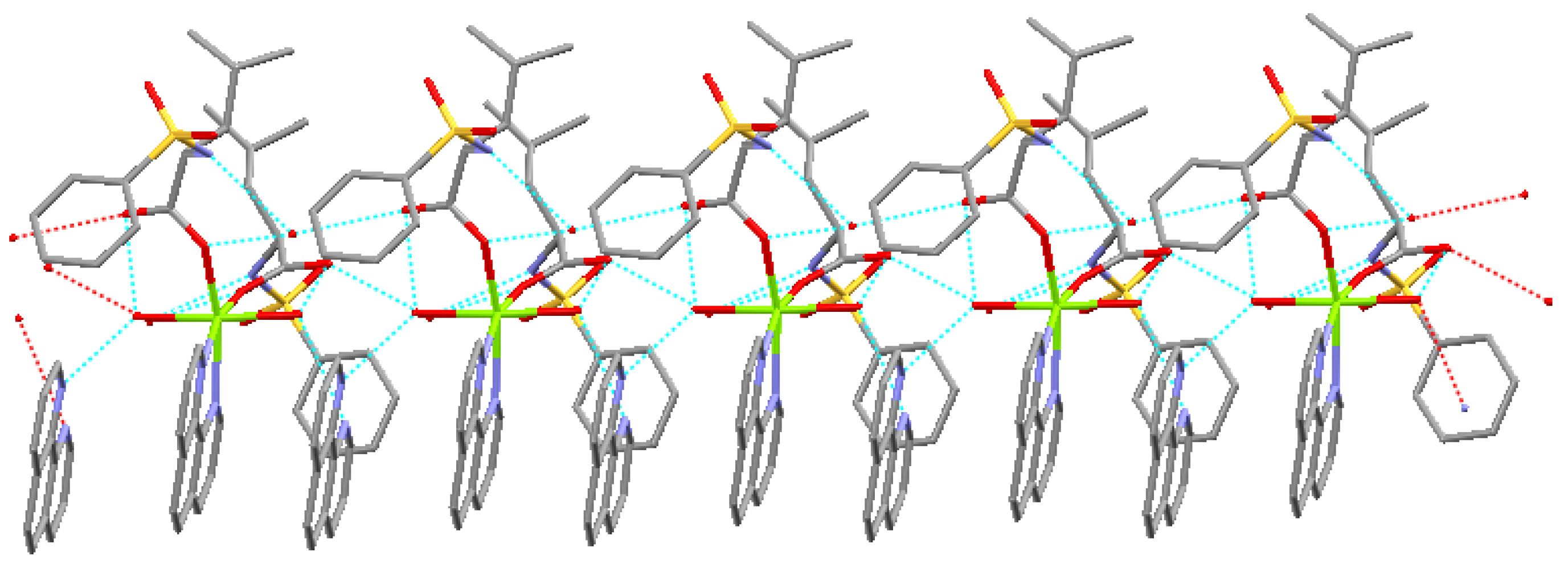
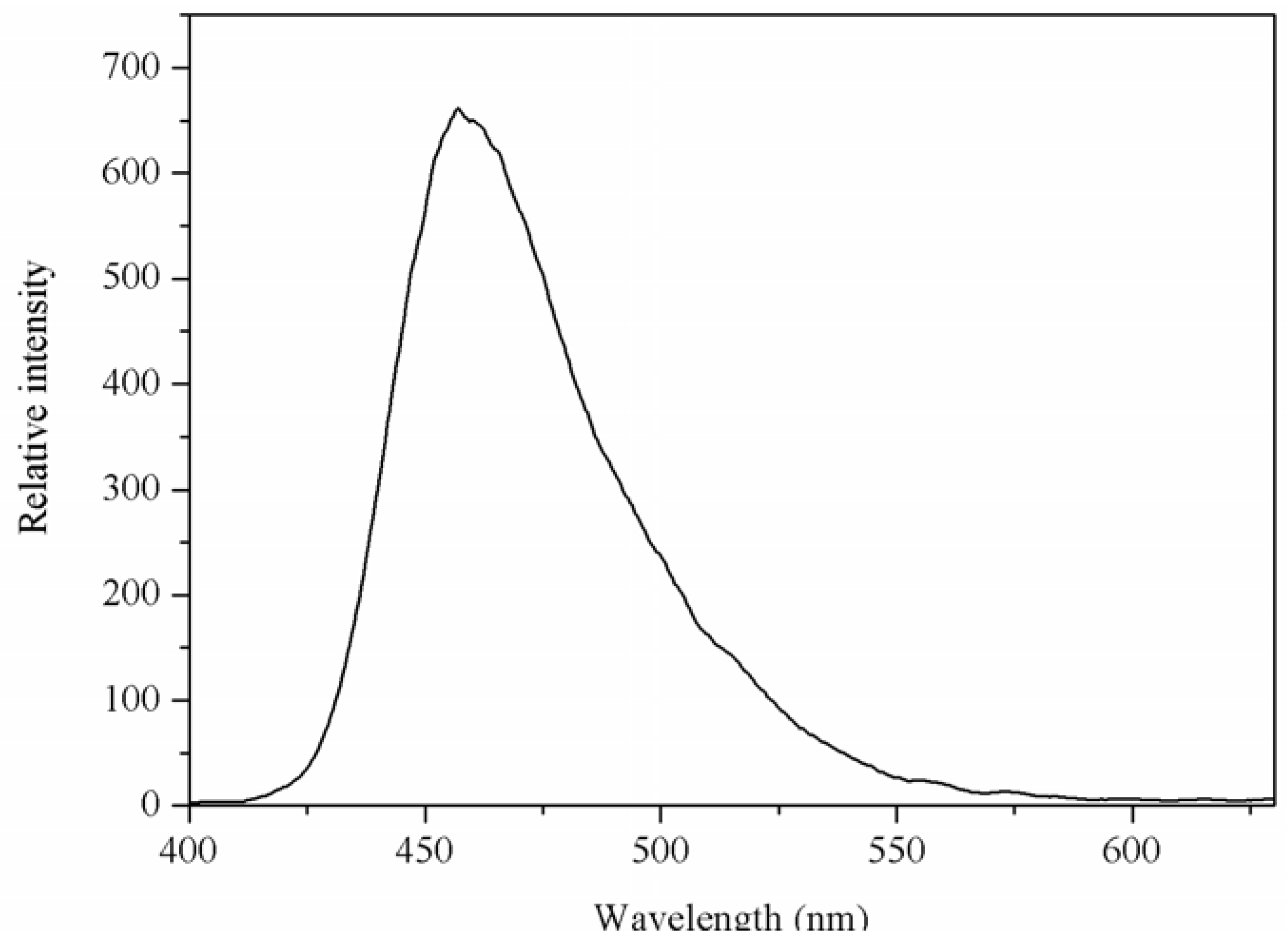
2.3. Luminescent Properties
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of the Ligand
3.3. Synthesis of Mg(II) Complex
3.4. X-ray Crystallography
| Crystallographic parameter | Crystallographic data |
|---|---|
| Formula | C48H56MgN6O12S2 |
| Formula weight | 997.42 |
| Crystal system | Triclinic |
| Space group | P−1 |
| a(Å) | 7.2772(15) |
| b(Å) | 14.279(3) |
| c(Å) | 14.418(3) |
| α(°) | 63.53(3) |
| β(°) | 79.75(3) |
| γ(°) | 81.83(3) |
| Z | 1 |
| F(000) | 526 |
| Temperature (K) | 293(2) |
| V (Å3) | 1,316.3(5) |
| Calculated density (μg·m−3) | 1.258 |
| Crystal size (mm3) | 0.18 × 0.14 × 0.10 |
| μ (mm−1) | 0.177 |
| Limiting indices | −9 ≤ h ≤ 9, −18 ≤ k≤ 16, −18 ≤ l ≤ 18 |
| Reflections collected/unique | 9769/7533 |
| R1, wR2 [all data] | 0.0710, 0.1643 |
| R1, wR2 [I > 2σ(I)] | 0.0506, 0.1328 |
| Largest diff.peak and hole (e·Å−3) | 0.741, −0.286 |
| Bonds | Bond parameter | Bonds | Bond parameter |
|---|---|---|---|
| Mg-O5 | 2.020(2) | S1-O3 | 1.429(3) |
| Mg-O1 | 2.035(2) | S1-O4 | 1.426(3) |
| Mg-O1W | 2.081(2) | S1-N1 | 1.594(3) |
| Mg-O2W | 2.091(2) | S1-C6 | 1.750(5) |
| Mg-N3 | 2.193(3) | N1-C7 | 1.474(5) |
| Mg-N4 | 2.204(3) | ||
| O5-Mg-O1 | 100.07(12) | O1W-Mg-N4 | 98.97(11) |
| O5-Mg-O1W | 89.62(11) | O2W-Mg-N4 | 84.09(11) |
| O1-Mg-O1W | 88.44(10) | N3-Mg-N4 | 75.36(11) |
| O2W-Mg-O5 | 89.76(10) | O3-S1-O4 | 119.1(2) |
| O2W-Mg-O1 | 88.69(10) | O3-S1-N1 | 106.5(2) |
| O1W-Mg-O2W | 176.90(10) | O4-S1-N1 | 108.1(2) |
| O5-Mg-N3 | 165.58(12) | O3-S1-C6 | 107.3(2) |
| O1-Mg-N3 | 93.44(12) | O4-S1-C6 | 106.8(2) |
| O1W-Mg-N3 | 85.72(10) | N1-S1-C6 | 108.67(16) |
| O2W-Mg-N3 | 95.60(11) | O7-S2-O8 | 119.3(2) |
| O5-Mg-N4 | 91.95(12) | O7-S2-N2 | 106.5(2) |
| O1-Mg-N4 | 165.95(12) | N2-S2-O8 | 108.3(2) |
| N1-C7-C9 | 109.9(3) |
4. Conclusions
Acknowledgments
Supplementary Material
References
- Lu, W.G.; Jiang, L.; Feng, X.L.; Lu, T.B. Three 3D coordination polymers constructed by Cd(II) and Zn(II) with imidazole-4,5-dicarboxylate and 4,4’-bipyridyl building blocks. Cryst. Growth Des. 2006, 6, 564–571. [Google Scholar]
- Tian, L.J.; Yang, H.J.; Zheng, X.L.; Ni, Z.H.; Yan, D.M.; Tu, L.L.; Jiang, J.Z. Synthesis, structural characterization and cytotoxic activity of diorganotin(IV) complexes of N-(5-halosalicylidene)tryptophane. Appl. Organomet. Chem. 2009, 23, 24–31. [Google Scholar]
- Wang, L.F.; Hu, Y.X.; Zhang, W.W.; Ren, X.M. Solvothermal synthesis, crystal structure and photoluminescence property of a coordination polymer based on 1,1’-ethynebenzene-3,3’,5,5’-tetracarboxylate. China J. Inorg. Chem. 2011, 27, 542–546. [Google Scholar]
- Gao, S.; Zhang, Z.Y.; Huo, L.H. Synthesis, Crystal structure and thermal behavior of 1D chain coordination polymer [Ca(2-OPA)2(H2O)2]n constructed by 2-oxo-1(4H)-pyridineacetate ligand. China J. Inorg. Chem. 2005, 21, 771–774. [Google Scholar]
- Tai, X.S.; Feng, Y.M.; Wang, L.T.; Tan, M.Y. Synthesis, structure characterization and luminescent properties of ternary zinc acetate complex with 4-aminohippuric acid and 1,10-phenanthroline ligand. Pol. J. Chem. 2009, 83, 1099–1104. [Google Scholar]
- Zhang, A.J.; Ye, C.; Hu, H.M.; Zhu, B.B. A novel Nickel (II) complex adopting a cis-configuration: Solvothermal synthesis and crystal structure of [NiL2(H2O)4](L = 1,4-dihydropyrazine-2,3-dione-5,6-dicarboxylate). Eur. J. Inorg. Chem. 2002, 7, 1595–1598. [Google Scholar]
- Nakamoto, K. Infrared and Ramen Spectra of Inorganic and Coordination Compounds, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1978; Volume 1, pp. 359–368. [Google Scholar]
- Tai, X.S.; Du, L.C.; Zhao, Z.B. Synthesis, crystal structure and antibacterial activity of Magnesium(II) complex with N-benzenesulphonyl-L-phenylalanine and 1,10-phenanthroline. China J. Inorg. Chem. 2011, 27, 575–579. [Google Scholar]
- Guo, D.F.; Ye, Y.; Zeng, Z.Z. Interaction between ternary rare earth complexes of cinnamic acid and phenanthroline with DNA by spectroscopy. J. Rare Earth. 2005, 23, 162–166. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Solution; University of GÖttingen: GÖttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXTL-97, Program for Crystal Structure Refinement; University of GÖttingen: GÖttingen, Germany, 1997. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tai, X.; Wei, N.; Wang, D. Synthesis, Crystal Structure and Luminescent Property of Mg(II) Complex with N-Benzenesulphonyl-L-Leucine and 1,10-Phenanthroline. Materials 2012, 5, 558-565. https://doi.org/10.3390/ma5040558
Tai X, Wei N, Wang D. Synthesis, Crystal Structure and Luminescent Property of Mg(II) Complex with N-Benzenesulphonyl-L-Leucine and 1,10-Phenanthroline. Materials. 2012; 5(4):558-565. https://doi.org/10.3390/ma5040558
Chicago/Turabian StyleTai, Xishi, Na Wei, and Donghao Wang. 2012. "Synthesis, Crystal Structure and Luminescent Property of Mg(II) Complex with N-Benzenesulphonyl-L-Leucine and 1,10-Phenanthroline" Materials 5, no. 4: 558-565. https://doi.org/10.3390/ma5040558





