Alkylation of Benzene with Propylene in a Flow-Through Membrane Reactor and Fixed-Bed Reactor: Preliminary Results
Abstract
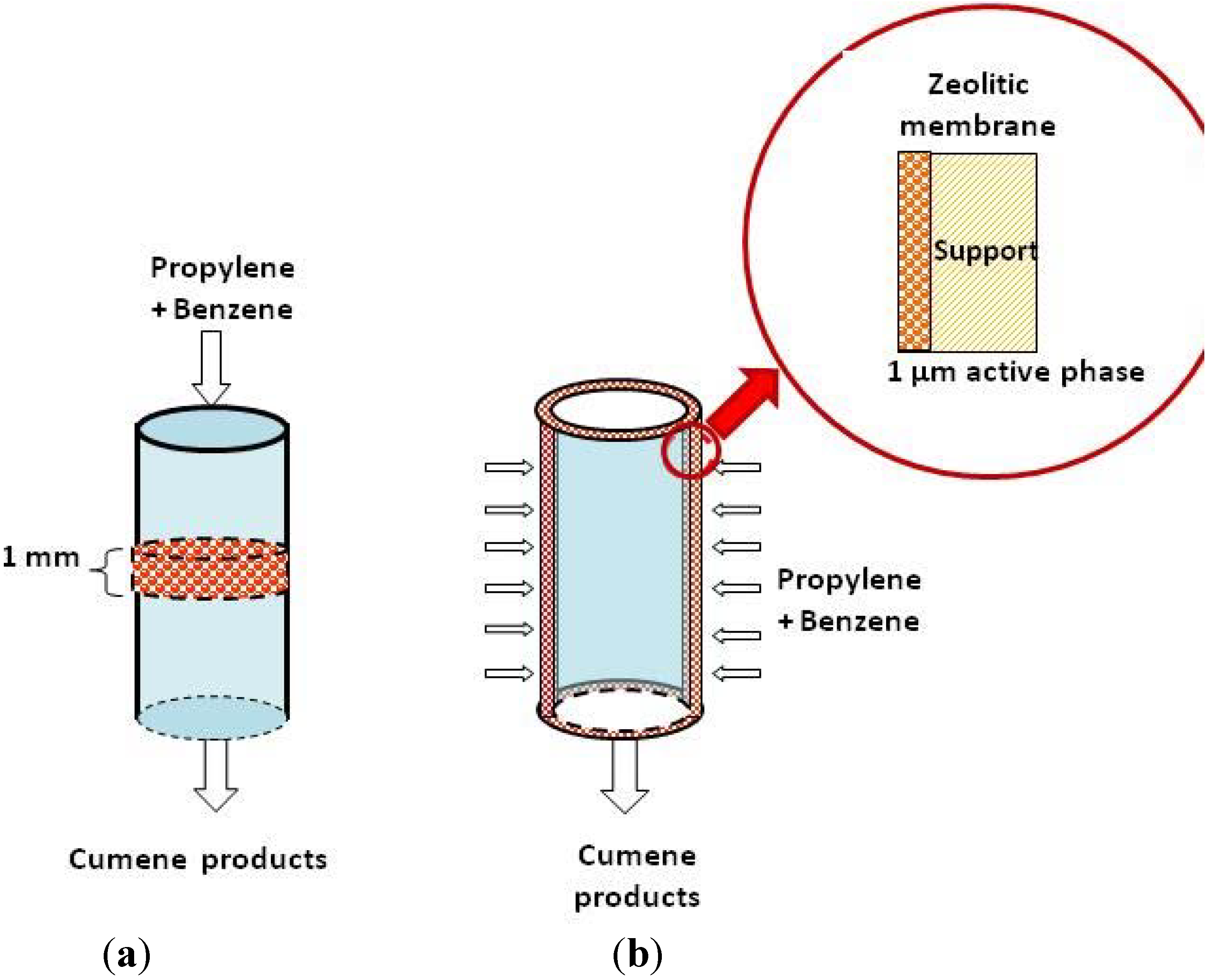
:1. Introduction
2. Experimental Section
2.1. Zeolite Synthesis
2.2. Membrane Synthesis
2.3. Characterization
3. Catalytic Activity Tests
3.1. Fixed-bed Differential Reactor (FBDR)
3.2. Flow-Through Membrane Reactor (FTMR)
4. Results and Discussion
4.1. Characterization


| Specific surface area m2/g | Pore volume cm3/g | Average pore diameter Å | Acidity mmol NH3/g |
|---|---|---|---|
| 396.76 | 0.2087 | 21.79 | 3.4 |
4.2. Catalytic Activity


5. Conclusions
References
- Picciotti, M. Petrochemicals—Today and tomorrow in developing countries. Hydrocarbon Process. 2006, April, 88–89. [Google Scholar]
- Degnan, T.F.; Smith, C.M.; Venkat, C.R. Alkylation of aromatics with ethylene and propylene: Recent developments in commercial processes. Appl. Catal. A Gen. 2001, 221, 283–294. [Google Scholar] [CrossRef]
- Bellussi, G.; Pazzuconi, G.; Perego, C.; Girotti, G.; Terzoni, G. Liquid-phase alkylation of benzene with light olefins catalyzed by β-zeolites. J. Catal. 1995, 157, 227–231. [Google Scholar] [CrossRef]
- Tanabe, K.; Hölderich, W.F. Industrial application of solid acid-base catalysts. Appl. Catal. A Gen. 1999, 181, 399–434. [Google Scholar] [CrossRef]
- Van Laak, A.N.C.; Sagala, S.L.; Zecĕvic, J.; Friedrich, H.; de Jongh, P.E.; de Jong, K.P. Mesoporous mordenites obtained by sequential acid and alkaline treatments—Catalysts for cumene production with enhanced accessibility. J. Catal. 2010, 276, 170–180. [Google Scholar] [CrossRef]
- Joni, J.; Haumann, M.; Wasserscheid, P. Continuous gas-phase isopropylation of toluene and cumene using highly acidic supported ionic liquid phase (SILP) catalysts. Appl. Catal. A Gen. 2010, 372, 8–15. [Google Scholar] [CrossRef]
- Popova, M.M.; Mihályi, R.M.; Pá-Borbély, G.; Minchev, Ch. Transalkylation of toluene with cumene over zeolites Y dealuminated in solid-state: Part II. Effect of the introduced Lewis acid sites. Appl. Catal. A Gen. 2003, 248, 197–209. [Google Scholar] [CrossRef]
- Q-MaxTM Process; UOP: Des Plaines, IL, USA, 2006. Available online: http://www.dequi.eel.usp.br/~barcza/CumenoUOP.pdf (accessed on 25 April 2012).
- Bokade, V.V.; Kharul, U.K. Selective synthesis of cumene by isopropylation of benzene using catalytic membrane reactor. Chem. Eng. J. 2009, 147, 97–101. [Google Scholar] [CrossRef]
- Casanave, D.; Moueddeb, H.; Ciavarella, P.; Fiaty, K.; Dalmon, J.A. Optimisation of a fixed-bed membrane reactor for isobutane dehydrogenation. Comparison of modeling and experimental results. In Proceedings of the 4th Workshop on Optimisation of Catalytic Membrane Reactors Systems, Oslo, Norway, 30–31 May 1997.
- Matsukata, M.; Sawamura, K.; Shirai, T.; Takada, M.; Sekine, Y.; Kikuchi, E. Controlled growth for synthesizing a compact mordenite membrane. J. Memb. Sci. 2008, 316, 18–27. [Google Scholar] [CrossRef]
- O’Brien-Abraham, J.; Kanezashi, M.; Lin, Y.S. A comparative study on permeation and mechanical properties of random and oriented MFI-type zeolite membranes. Micropor. Mesopor. Mater. 2007, 105, 140–148. [Google Scholar] [CrossRef]
- Kusakabe, K.; Kuroda, T.; Murata, A.; Morooka, S. Formation of a Y-type zeolite membrane on a porous α-alumina tube for gas separation. Ind. Eng. Chem. Res. 1997, 36, 649–655. [Google Scholar] [CrossRef]
- Torres, M.; Domínguez, J.M.; Maubert, M.; Gutiérrez, M.; Mantilla, A.; Ferrat, G. Preparation of beta zeolite supported on a ceramic membrane for the isopaffins/olefins alkylation reaction. In Proceedings of International Conference on Catalysis in Membrane Reactors, Copenhagen, Denmark, 11–14 September 1998; p. 31.
- Coronas, J.; Santamaría, J. Catalytic reactors based on porous ceramic membranes. Catal. Today 1999, 51, 377–389. [Google Scholar] [CrossRef]
- Arruebo, M.; Mallada, R.; Pina, P. Handbook of Membrane Separations; Pabby, A., Rizvi, S., Sastre, A., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 269–324. [Google Scholar]
- Westermann, T.; Melin, T. Flow-through catalytic membrane reactors—Principles and applications. Chem. Eng. Process 2009, 48, 17–28. [Google Scholar] [CrossRef]
- Torres, M.; Lopez, L.; Dominguez, J.M.; Mantilla, A.; Ferrat, G.; Gutiérrez, M.; Maubert, M. Olefins catalytic oligomerization on new composites of beta-zeolite films supported on α-Al2O3 membranes. Chem. Eng. J. 2003, 92, 1–6. [Google Scholar] [CrossRef]
- Gong, Y.; Dou, T.; Kang, S.; Li, Q.; Hu, Y. Deep desulfurization of gasoline using ion-exchange zeolites: Cu(I)- and Ag(I)-beta. Fuel Process. Technol. 2009, 90, 122–129. [Google Scholar] [CrossRef]
- Fritsch, D.; Randjelovic, I.; Keil, F. Application of a forced-flow catalytic membrane reactor for the dimerisation of isobutene. Catal. Today 2004, 98, 295–308. [Google Scholar] [CrossRef]
- Taborda, F.; Wang, Z.; Willhammar, T.; Montes, C.; Zou, X. Synthesis of Al-Si-beta and Ti-Si-beta by the aging-drying method. Micropor. Mesopor. Mater. 2012, 150, 38–46. [Google Scholar] [CrossRef]
- Moreau, P.; Finiels, A.; Meric, P.; Fajula, F. Acetylation of 2-methoxynaphthalene in the presence of beta zeolites: Influence of reaction conditions and textural properties of the catalysts. Catal. Lett. 2003, 85, 199–203. [Google Scholar] [CrossRef]
- Čejka, J.; Wichterlova, B. Acid-catalyzed synthesis of mono- and dialkyl-benzenes over zeolites: Active sites, zeolite topology, and reaction mechanisms. Catal. Rev. 2002, 44, 375–421. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Torres-Rodríguez, M.; Gutiérrez-Arzaluz, M.; Mugica-Álvarez, V.; Aguilar-Pliego, J.; Pergher, S. Alkylation of Benzene with Propylene in a Flow-Through Membrane Reactor and Fixed-Bed Reactor: Preliminary Results. Materials 2012, 5, 872-881. https://doi.org/10.3390/ma5050872
Torres-Rodríguez M, Gutiérrez-Arzaluz M, Mugica-Álvarez V, Aguilar-Pliego J, Pergher S. Alkylation of Benzene with Propylene in a Flow-Through Membrane Reactor and Fixed-Bed Reactor: Preliminary Results. Materials. 2012; 5(5):872-881. https://doi.org/10.3390/ma5050872
Chicago/Turabian StyleTorres-Rodríguez, Miguel, Mirella Gutiérrez-Arzaluz, Violeta Mugica-Álvarez, Julia Aguilar-Pliego, and Sibele Pergher. 2012. "Alkylation of Benzene with Propylene in a Flow-Through Membrane Reactor and Fixed-Bed Reactor: Preliminary Results" Materials 5, no. 5: 872-881. https://doi.org/10.3390/ma5050872






