Potentiodynamic Polarization Studies and Surface Chemical Composition of Bismuth Titanate (BixTiyOz) Films Produced through Radiofrequency Magnetron Sputtering
Abstract
:1. Introduction
2. Experimental Procedure


3. Results and Discussion
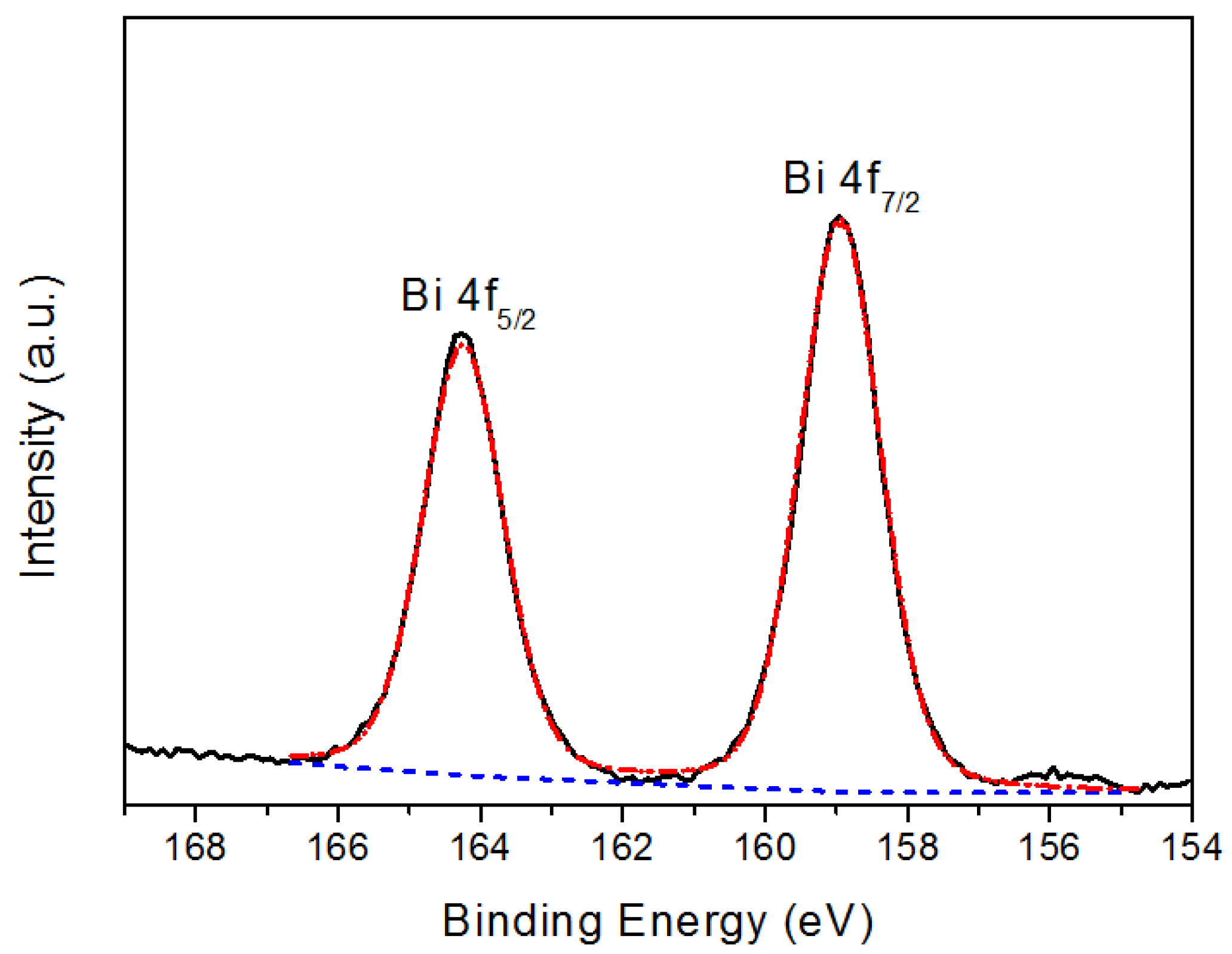
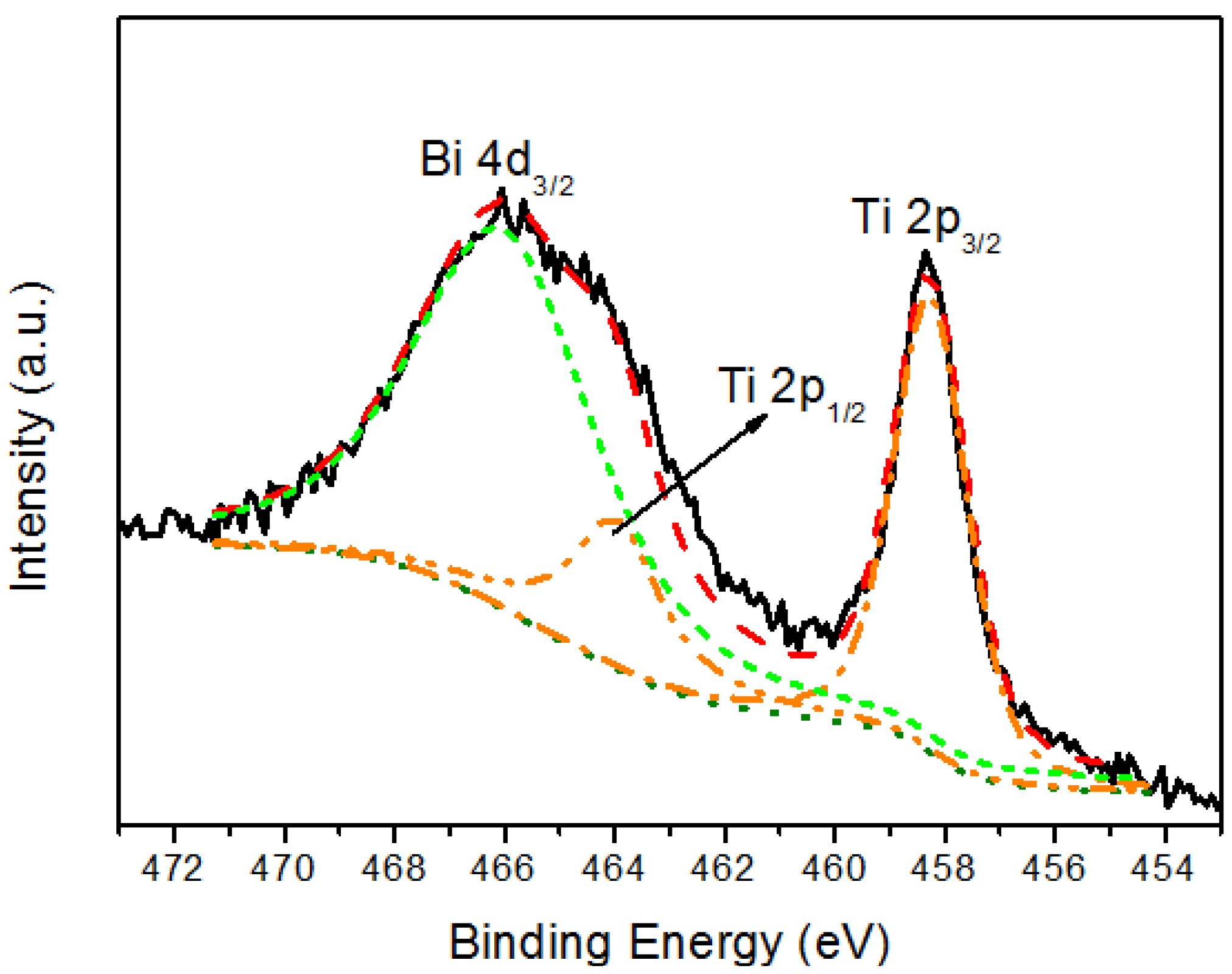
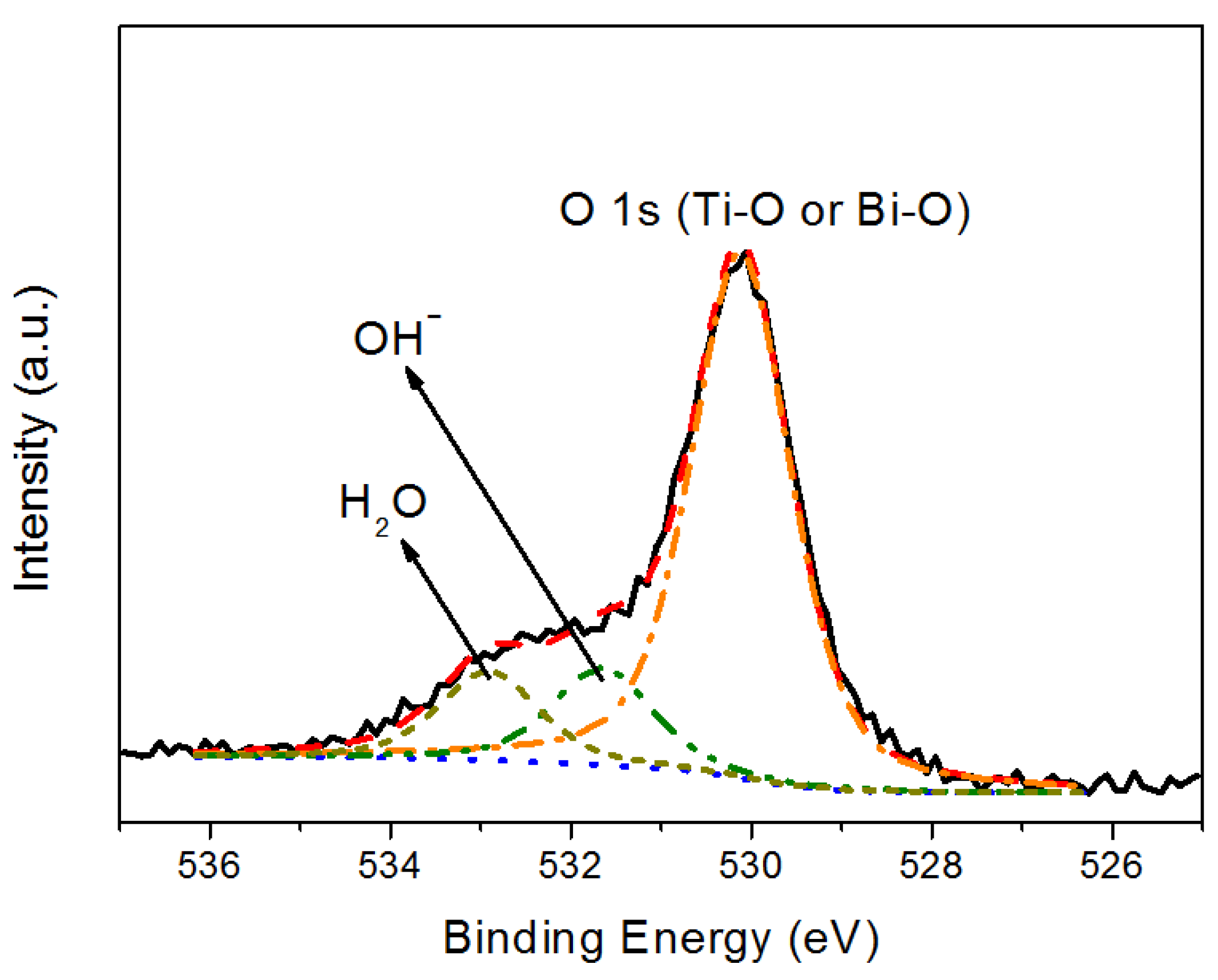


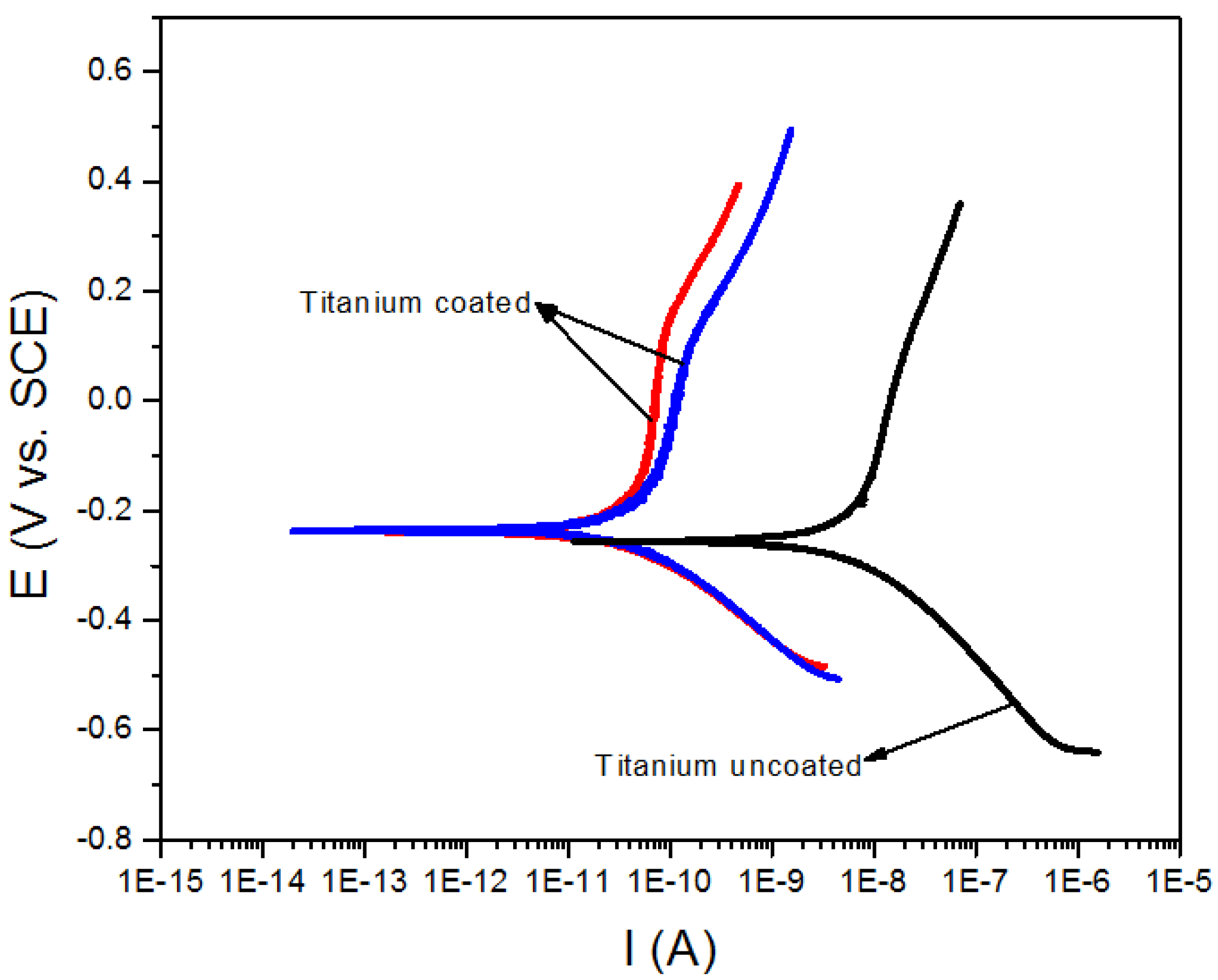
| Sample | Icorr (nA) | Ecorr (mV) | Corrosion rate (mm/y) |
|---|---|---|---|
| Stainless Steel 316L uncoated | 10.60 | −161 | 6.299 × 10−4 |
| Stainless Steel 316L coated | 1.840 | −193 | 1.097 × 10−4 |
| Titanium Alloy uncoated | 6.960 | −247 | 4.166 × 10−4 |
| Titanium Alloy coated | 0.028 | −226 | 1.676 × 10−6 |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bhaskar Kumar, G.; Buddhudu, S. Optical, thermal and dielectric properties of Bi4(TiO4)3 ceramic powders. Ceram. Int. 2010, 36, 1857–1861. [Google Scholar] [CrossRef]
- Hardy, A.; Mondelaers, D.; Vanhoyland, G.; Van Bael, M.K.; Mullens, J.; Van Poucke, L.C. The formation of ferroelectric bismuth titanate (Bi4Ti3O12) from an aqueous metal-chelate GEL. J. Sol-Gel Sci. Technol. 2003, 26, 1103–1107. [Google Scholar] [CrossRef]
- Chakraborty, K.R.; Achary, S.N.; Patwe, S.J.; Krishna, P.S.R.; Shinde, A.B.; Tyagi, A.K. Low temperature neutron diffraction studies on Bi4Ti3O12. Ceram. Int. 2007, 33, 601–604. [Google Scholar] [CrossRef]
- Watcharapasorn, A.; Siriprapa, P.; Jiansirisomboon, S. Grain growth behavior in bismuth titanate-based ceramics. J. Eur. Ceram. Soc. 2010, 30, 87–93. [Google Scholar] [CrossRef]
- Sedlar, M.; Sayer, M. Structural and electrical properties of ferroelectric bismuth titanate thin films prepared by the sol gel method. Ceram. Int. 1996, 22, 241–247. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Nagatomo, T. Preparation and properties of Bi4Ti3O12 thin films grown at low substrate temperatures. Thin Solid Films 1999, 348, 294–298. [Google Scholar] [CrossRef]
- Radosavljevic, I.; Evans, J.S.O.; Sleight, A.W. Synthesis and structure of pyrochlore-type bismuth titanate. J. Solid State Chem. 1998, 136, 63–66. [Google Scholar] [CrossRef]
- Hou, J.; Jiao, S.; Zhu, H; Kumar, R.V. Bismuth titanate pyrochlore microspheres: Directed synthesis and their visible light photocatalytic activity. J. Solid State Chem. 2011, 184, 154–158. [Google Scholar]
- Yao, W.F.; Wang, H.; Xu, X.H.; Zhou, J.T.; Yang, X.N.; Zhang, Y.; Shang, S.X. Photocatalytic property of bismuth titanate Bi2Ti2O7. Appl. Catal. A Gen. 2004, 259, 29–33. [Google Scholar] [CrossRef]
- Wang, S.W.; Lu, W.; Li, N.; Li, Z.F.; Wang, H.; Wang, M.; Shen, X.C. Insulating properties of rapid thermally processed Bi2Ti2O7 thin films by a chemical solution decomposition technique. Mater. Res. Bull. 2002, 37, 1691–1697. [Google Scholar] [CrossRef]
- Xu, S.; Shangguan, W.; Yuan, J.; Shi, J.; Chen, M. Photocatalytic properties of bismuth titanate Bi12TiO20 prepared by co-precipitation processing. Mater. Sci. Eng. B Solid. 2007, 137, 108–111. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, J.; Chen, F. Study on visible light photocatalytic activity and mechanism of spherical Bi12TiO20 nanoparticles prepared by low-power hydrothermal method. Appl. Catal. B Environ. 2011, 102, 316–322. [Google Scholar] [CrossRef]
- Feng, Y.W.; Hong, W.; Xia, S.S.; Hong, X.X.; Na, Y.X.; Dong, W.; Min, W. Preparation and characterization of bismuth titanate Bi12TiO20 nanocrystals. J. Mater. Sci. Lett. 2002, 21, 1803–1805. [Google Scholar] [CrossRef]
- Alfonso, J.E.; Torres, J.; Marco, J.F. Influence of the substrate bias voltage on the crystallographic structure and surface composition of Ti6Al4V thin films deposited by RF magnetron sputtering. Braz. J. Phys. 2006, 36, 994–996. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Gati, D.; Dzhurinskii, B.F.; Sergushin, N.P.; Salyn, Y.V. Simple and coordination compounds. Russ. J. Inorg. Chem. 1975, 20, 2307–2314. [Google Scholar]
- Bender, H.; Chen, W.D.; Portillo, J.; van den Hove, L.; Vandervorst, W. AES and XPS analysis of the interaction of Ti with Si and SiO2 during RTA. Appl. Surf. Sci. 1989, 38, 37–47. [Google Scholar] [CrossRef]
- Debies, T.P.; Rabalais, J.W. X-ray photoelectron spectra and electronic structure of Bi2X3 (X = O, S, Se, Te). Chem Phys. 1977, 20, 277–283. [Google Scholar] [CrossRef]
- Jovalekic, C.; Zdujic, M.; Atanasoska, Lj. Surface analysis of bismuth titanate by Auger and X-ray photoelectron spectroscopy. J. Alloy. Compd. 2009, 469, 441–444. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Zhang, J.B. Bi and O valences in Ba–K–Bi–O, Ba–K–M–Bi–O (M = Rb, La, Eu, In, Tl and Pb) and the related compounds. Solid State Commun. 1994, 90, 709–712. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. X-ray photoelectron spectroscopy studies of solvated metal atom dispersed catalysts. Monometallic iron and bimetallic iron-cobalt particles on alumina. Chem. Mater. 1990, 2, 186–191. [Google Scholar]
- Perron, H.; Vandenborre, J.; Domain, C.; Drot, R.; Roques, J.; Simoni, E.; Ehrhardt, J.J.; Catalette, H. Combined investigation of water sorption on TiO2 rutile (110) single crystal face: XPS vs. periodic DFT. Surf. Sci. 2007, 601, 518–527. [Google Scholar] [CrossRef] [Green Version]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alfonso, J.E.; Olaya, J.J.; Pinzón, M.J.; Marco, J.F. Potentiodynamic Polarization Studies and Surface Chemical Composition of Bismuth Titanate (BixTiyOz) Films Produced through Radiofrequency Magnetron Sputtering. Materials 2013, 6, 4441-4449. https://doi.org/10.3390/ma6104441
Alfonso JE, Olaya JJ, Pinzón MJ, Marco JF. Potentiodynamic Polarization Studies and Surface Chemical Composition of Bismuth Titanate (BixTiyOz) Films Produced through Radiofrequency Magnetron Sputtering. Materials. 2013; 6(10):4441-4449. https://doi.org/10.3390/ma6104441
Chicago/Turabian StyleAlfonso, José E., Jhon J. Olaya, Manuel J. Pinzón, and José F. Marco. 2013. "Potentiodynamic Polarization Studies and Surface Chemical Composition of Bismuth Titanate (BixTiyOz) Films Produced through Radiofrequency Magnetron Sputtering" Materials 6, no. 10: 4441-4449. https://doi.org/10.3390/ma6104441




