Coal Combustion Wastes Reuse in Low Energy Artificial Aggregates Manufacturing
Abstract
:1. Introduction
1.1. Research Significance
- Energy saving in production processes;
- Decrease of natural raw materials consumption;
- Recycling of wastes/by-products of other industrial processes.

1.2. Coal Combustion Residues Environmental Issues and Recycling Strategy
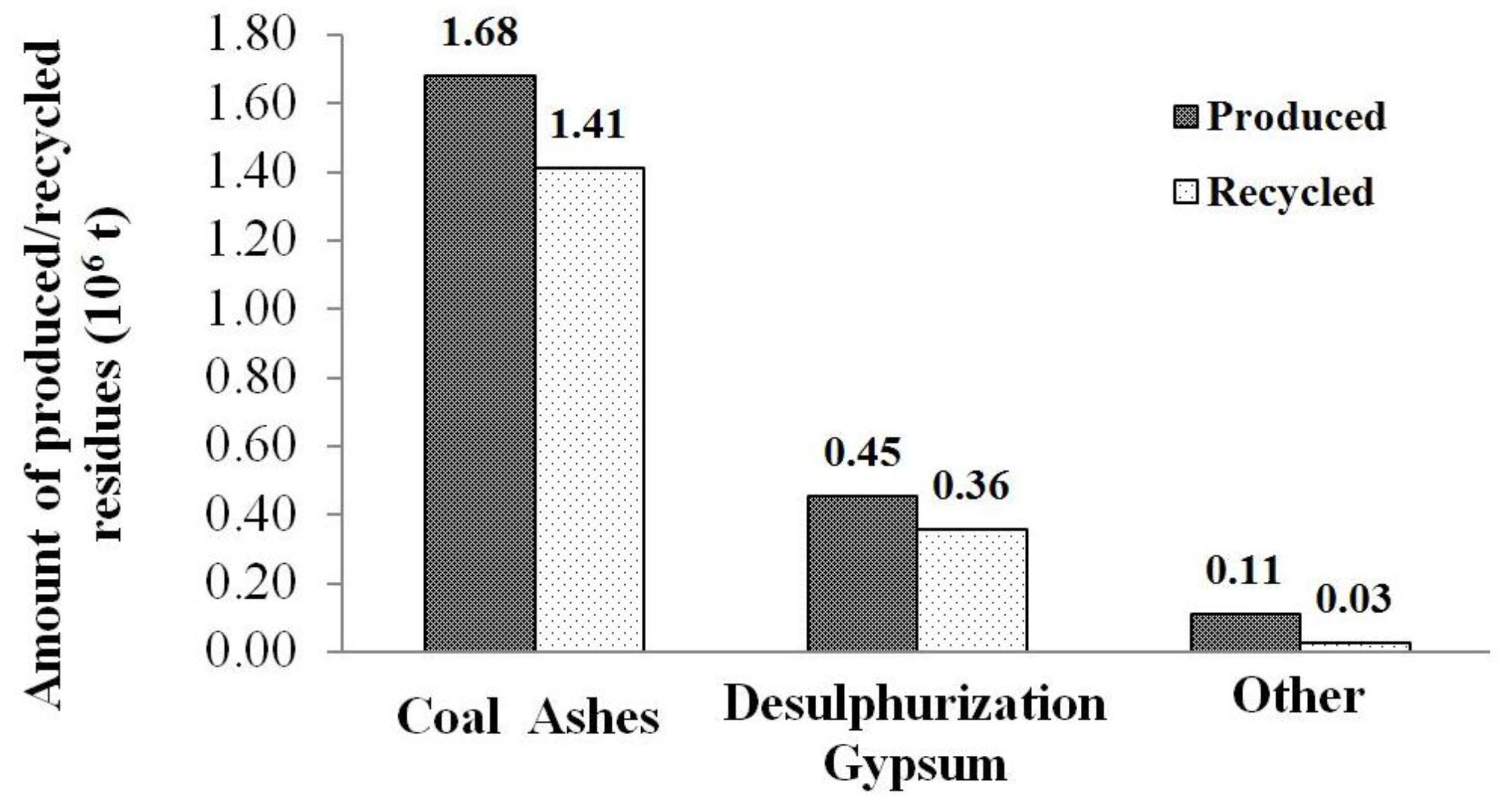
2. Experimental Program
2.1. Coal Combustion Residues Physical and Chemical Characterization
2.2. Artificial Aggregates Production and Testing
| Mixtures | WFA | L | DDS | WTS | W/S ratio |
|---|---|---|---|---|---|
| C90 | 90 | 10 | – | – | 0.22 |
| C80 | 80 | 20 | – | – | 0.25 |
| C60 | 60 | 40 | – | – | 0.29 |
| C30W50 | 30 | 20 | – | 50 | 0.25 |
| C24W60 | 24 | 16 | – | 60 | 0.30 |
| C40D30 | 40 | 30 | 30 | – | 0.25 |
| C25D10 | 25 | 15 | 10 | 50 | 0.28 |
| C20D15 | 20 | 15 | 15 | 50 | 0.31 |
3. Experimental Results and Discussion
3.1. Physico-Chemical Characterization of Residues
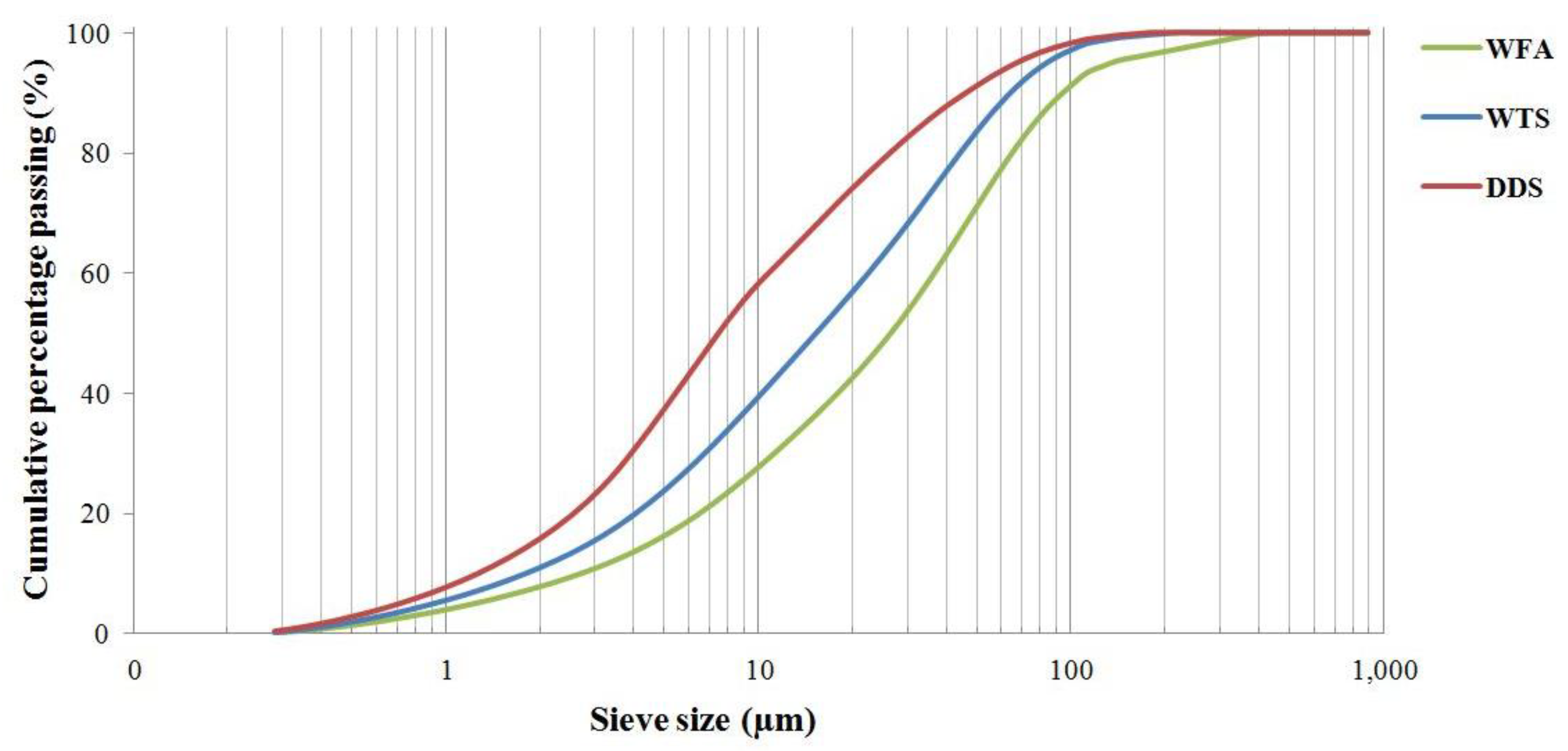
| Analyte | Al2O3 | Na2O | K2O | SO3 | CaO | Fe2O3 | MgO | MnO | P2O5 | TiO2 | SiO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WFA | 28.10 | 0.87 | 1.89 | 0.08 | 4.32 | 6.99 | 1.59 | 0.06 | 1.12 | 0.04 | 53.71 | 6.0 |
| WTS | 6.43 | 0.52 | 0.17 | 2.74 | 32.70 | 19.70 | 7.01 | 0.10 | 0.29 | 0.31 | 7.50 | 34.5 |
| DDS | 4.60 | 1.33 | 0.26 | 38.80 | 22.30 | 1.00 | 1.97 | 0.03 | 0.28 | 0.20 | 12.05 | 11.1 |
| Analyte | Cd | Cr | Cu | Ni | Pb | Se | V | Zn |
|---|---|---|---|---|---|---|---|---|
| WFA | 10 | 40 | 30 | 50 | 20 | 10 | 110 | 50 |
| WTS | 10 | 60 | 100 | 80 | 10 | 10 | 160 | 1390 |
| DDS | 10 | 20 | 10 | 20 | 10 | 10 | 50 | 10 |
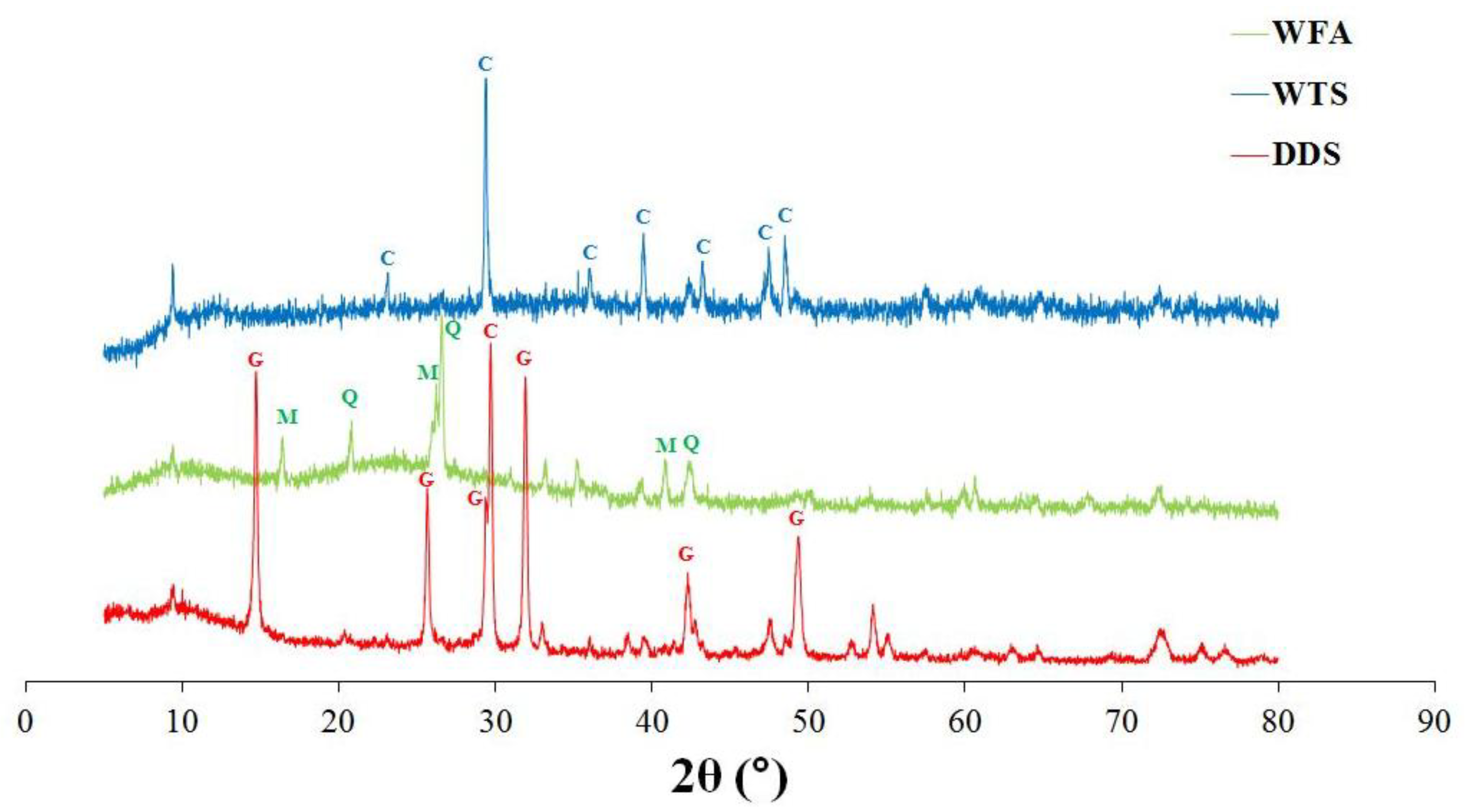
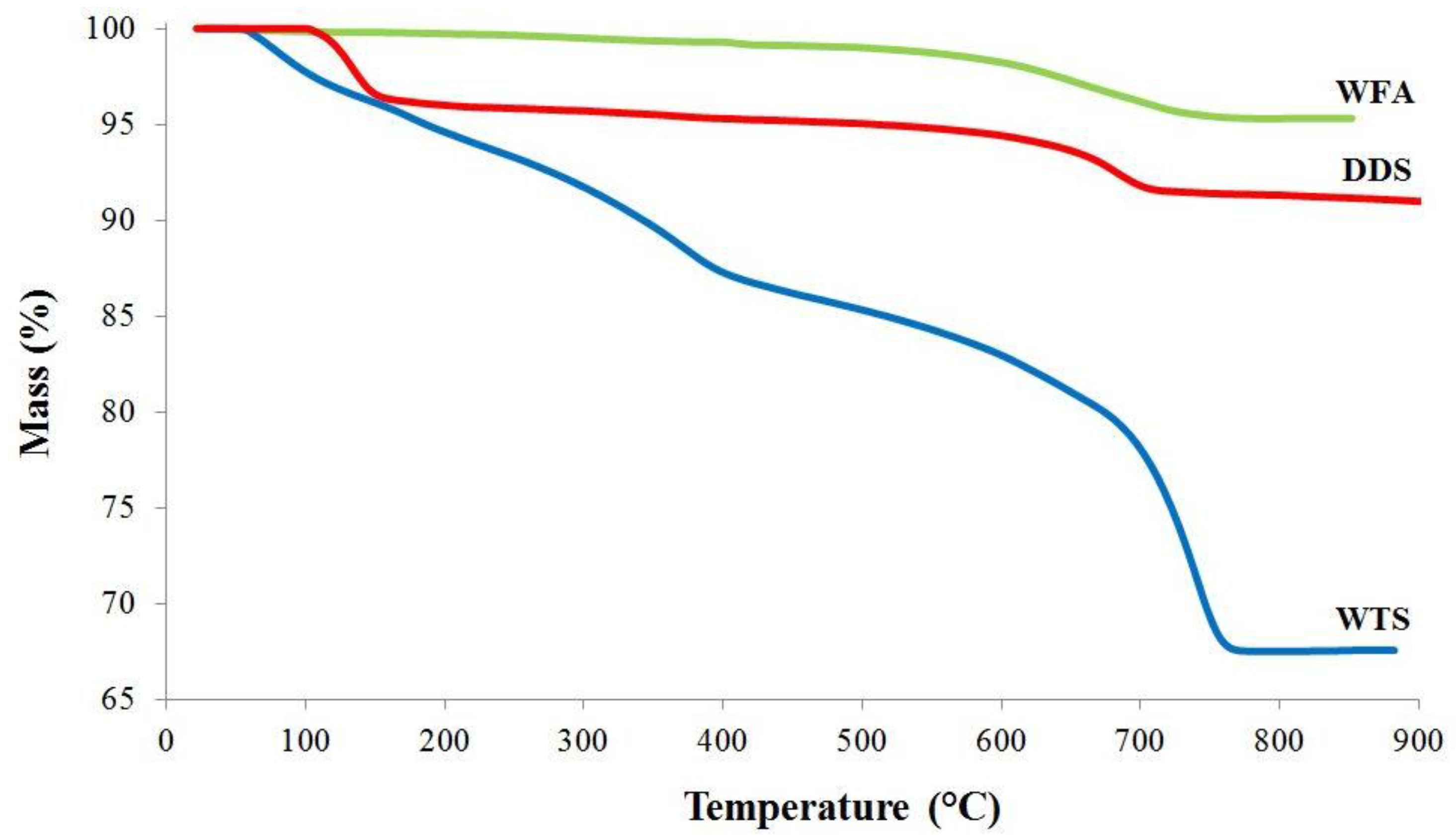
| Analyte | Cd | Cr | Cu | Ni | Pb | Se | V | Zn |
|---|---|---|---|---|---|---|---|---|
| WFA | 0.04 | 8.37 | 13.15 | 13.71 | 0.31 | 1.23 | 0.35 | 0.67 |
| WTS | 0.10 | 26.22 | 34.76 | 74.38 | 0.12 | 7.10 | 0.01 | 3.51 |
| DDS | 0.57 | 4.37 | 7.67 | 10.98 | 0.23 | 4.37 | 12.25 | 2.15 |
| Limits * | 5 | 50 | 50 | 10 | 50 | 10 | 250 | 3000 |
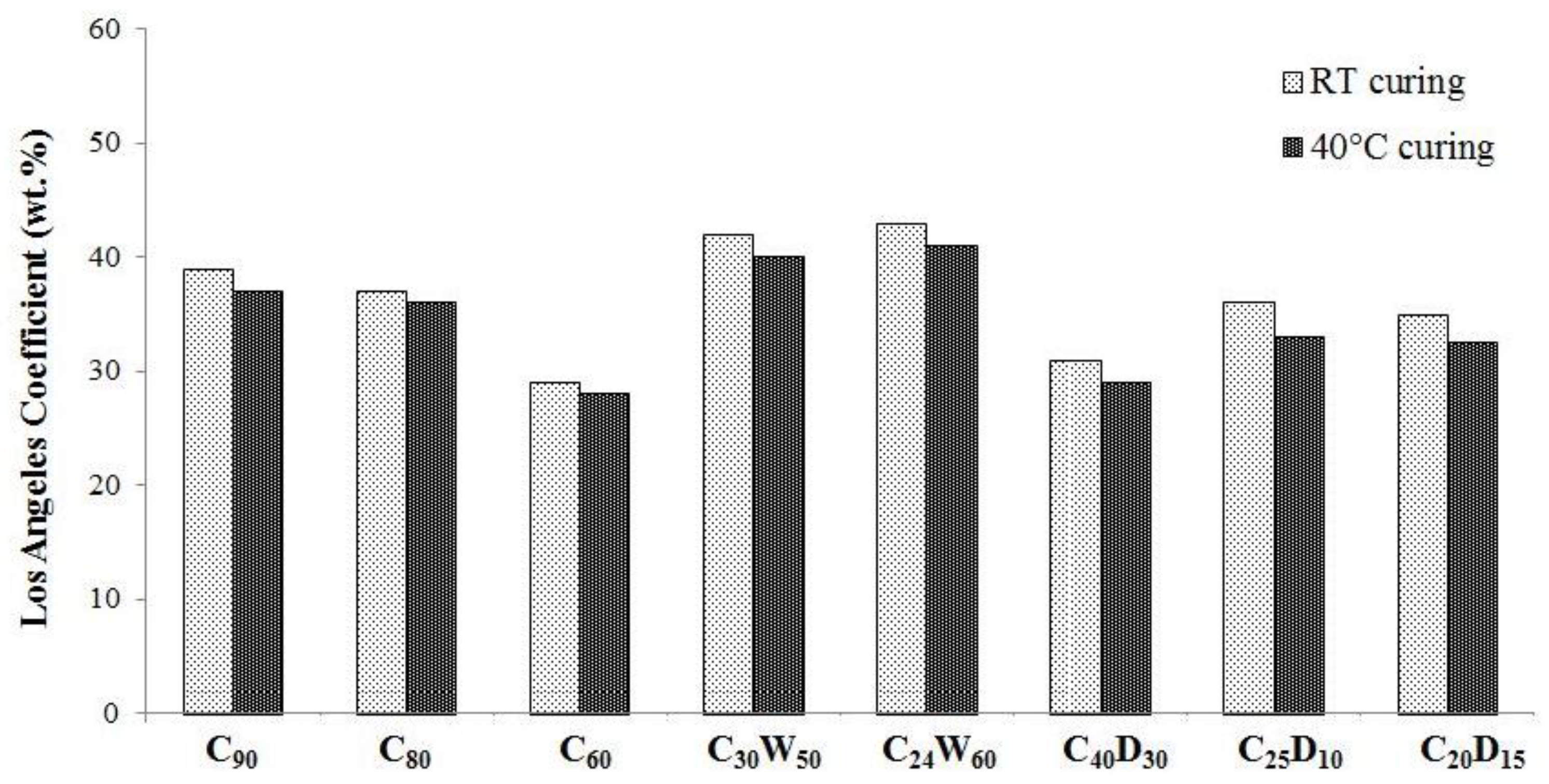
3.2. Physical, Mechanical Properties, and Heavy Metals Release of Artificial Aggregates
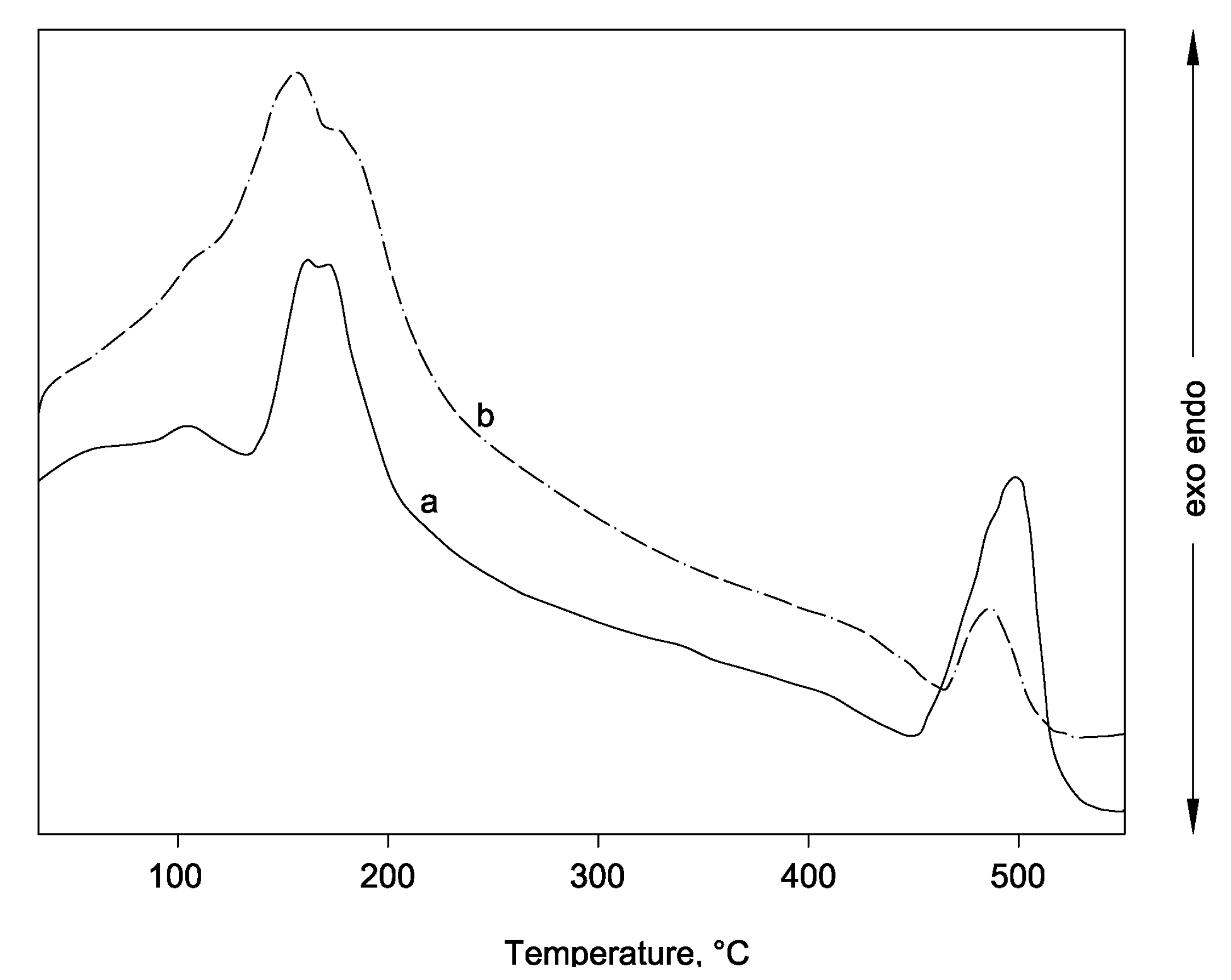

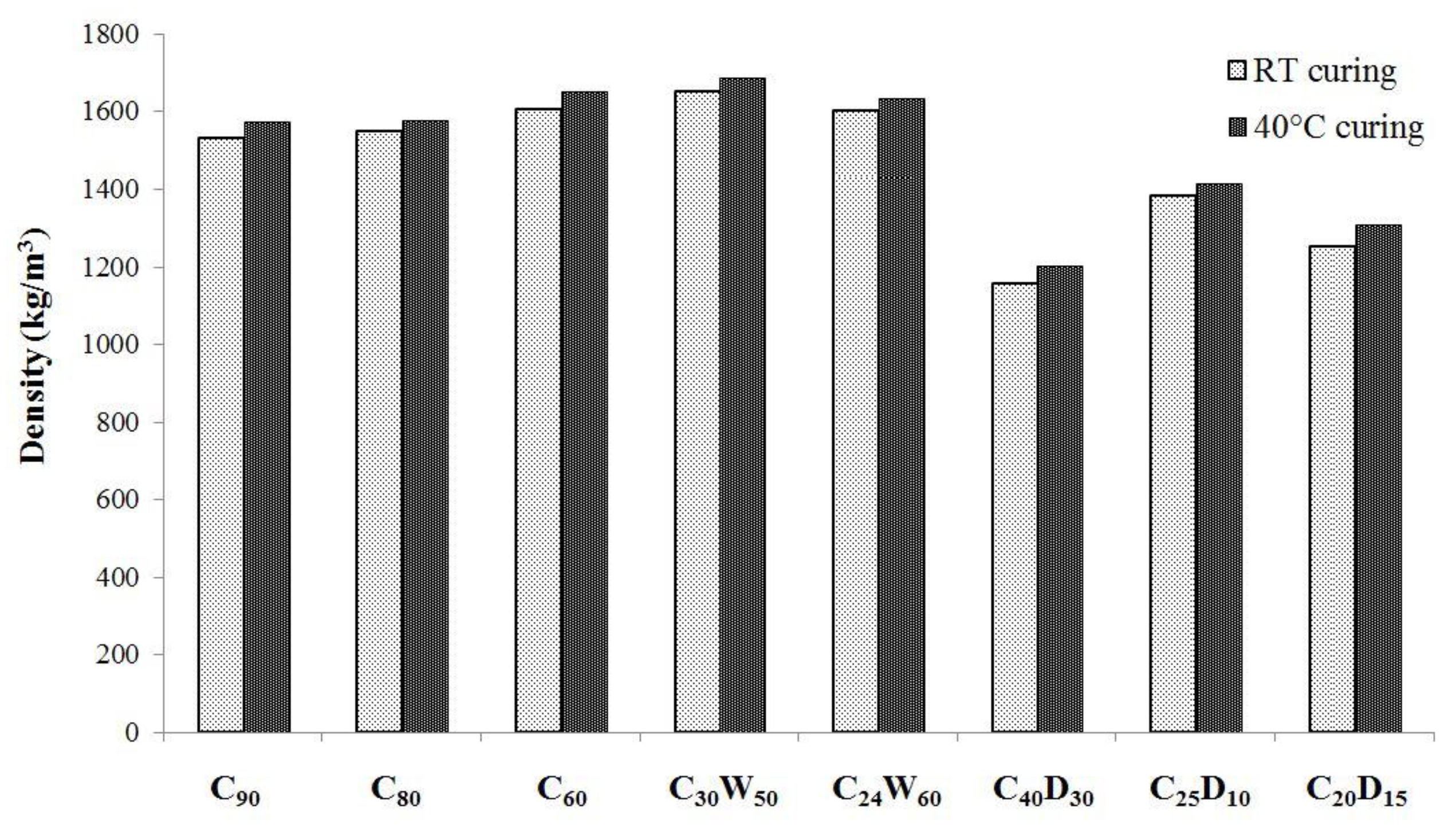
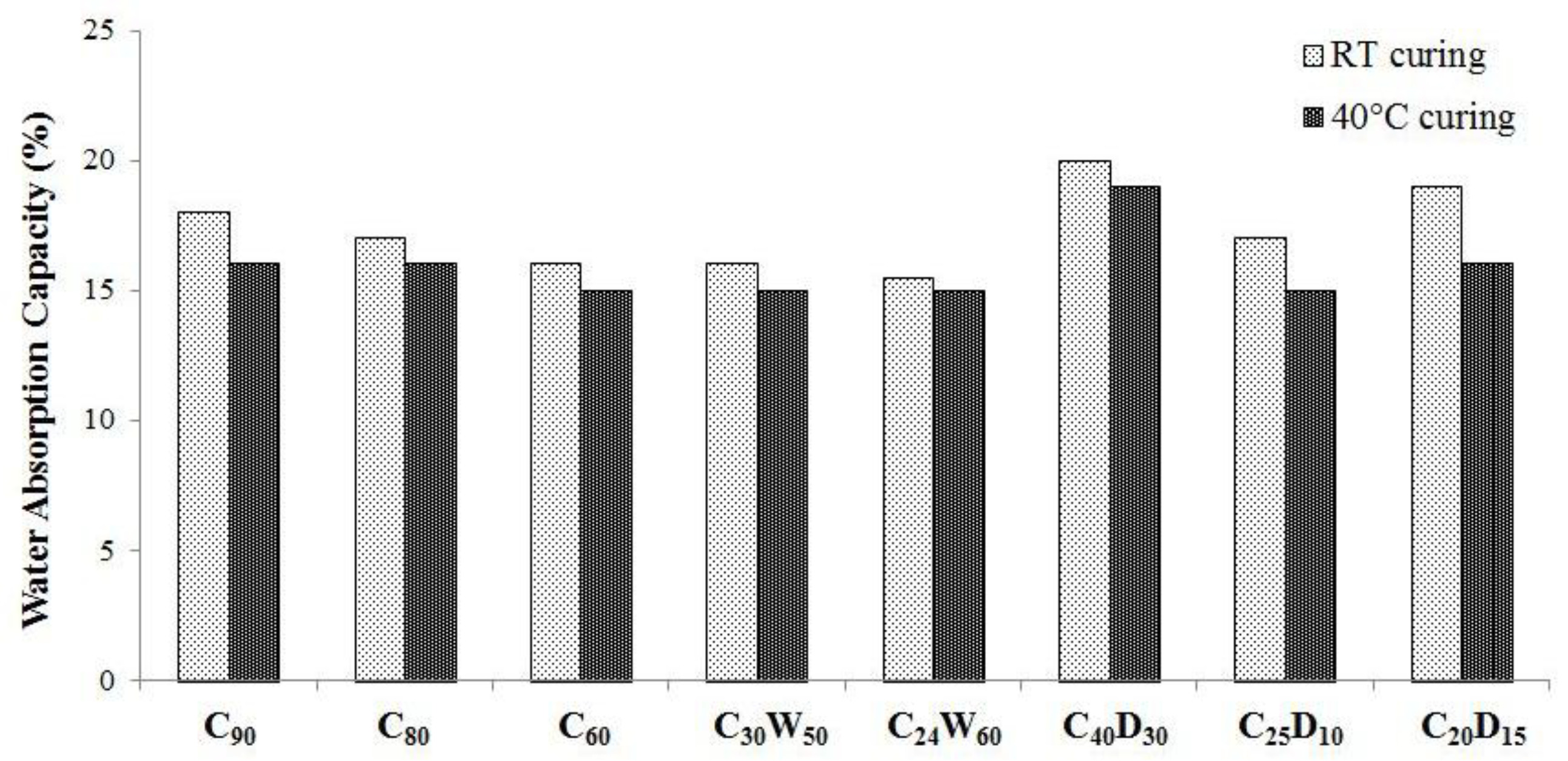
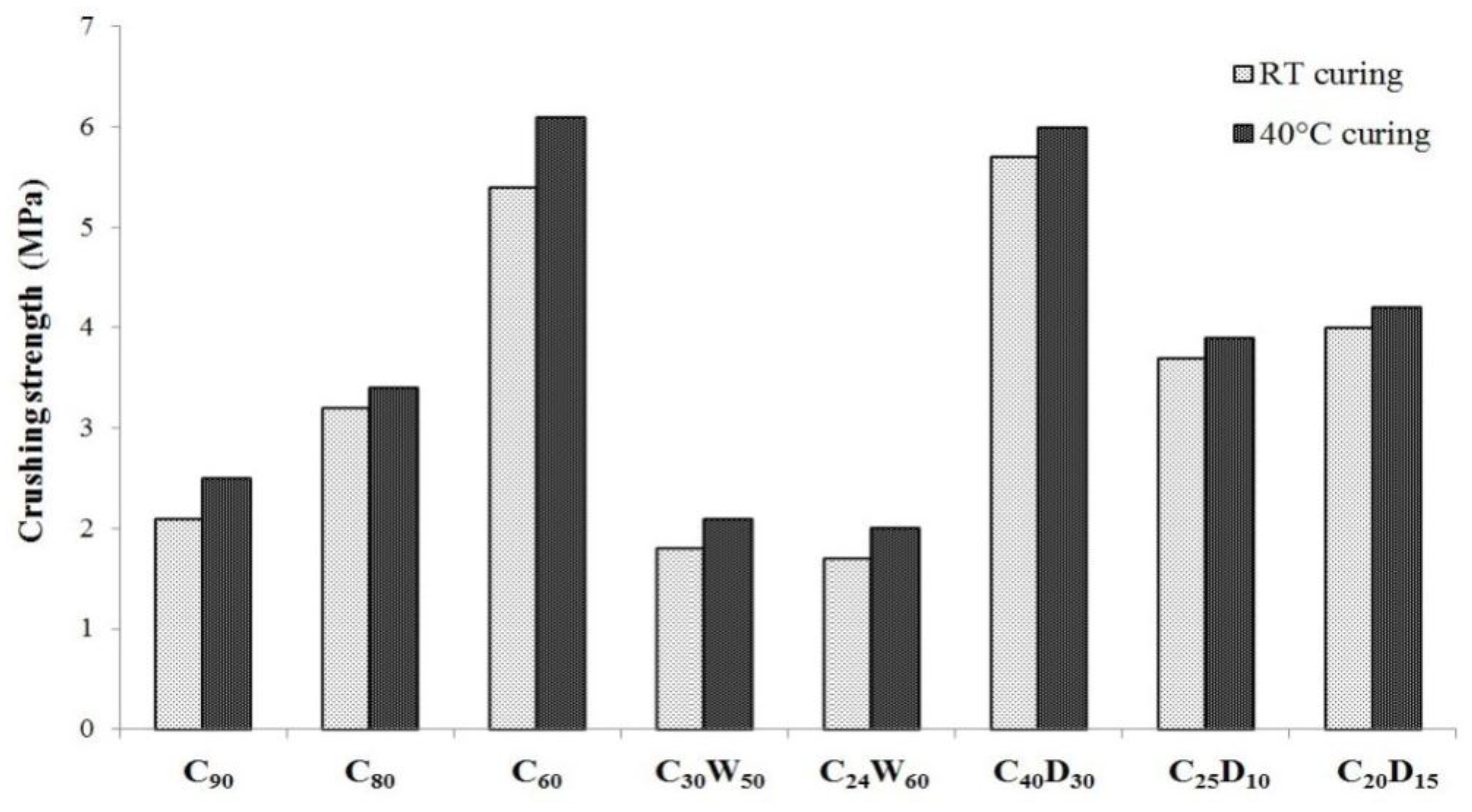
| Analyte | Cr | Cu | Ni | Se | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renewal times, days | 3 | 14 | 28 | 3 | 14 | 28 | 3 | 14 | 28 | 3 | 14 | 28 | |
| C30W50 | RT | 3.10 | 3.44 | 3.49 | 4.06 | 4.38 | 4.47 | 6.99 | 7.61 | 7.72 | 0.78 | 0.85 | 0.89 |
| 40 °C | 2.95 | 3.22 | 3.28 | 3.85 | 4.11 | 4.28 | 6.58 | 7.39 | 7.51 | 0.71 | 0.82 | 0.87 | |
| C24W60 | RT | 3.26 | 3.57 | 3.65 | 4.11 | 4.43 | 4.51 | 8.59 | 8.98 | 9.13 | 0.76 | 0.87 | 0.92 |
| 40 °C | 3.09 | 3.37 | 3.52 | 3.87 | 4.09 | 4.21 | 7.63 | 8.11 | 8.25 | 0.68 | 0.81 | 0.88 | |
| C25D10 | RT | 2.23 | 2.45 | 2.51 | 2.16 | 2.31 | 2.42 | 4.15 | 4.53 | 4.61 | 0.43 | 0.51 | 0.53 |
| 40 °C | 2.12 | 2.36 | 2.43 | 1.94 | 2.33 | 2.41 | 4.09 | 4.41 | 4.57 | 0.41 | 0.48 | 0.50 | |
| C20D15 | RT | 2.39 | 2.73 | 2.81 | 2.09 | 2.54 | 2.62 | 4.62 | 5.17 | 5.35 | 0.49 | 0.57 | 0.61 |
| 40 °C | 2.25 | 2.61 | 2.78 | 1.87 | 2.07 | 2.15 | 4.48 | 4.87 | 4.96 | 0.47 | 0.56 | 0.62 | |
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Bribián, I.Z.; Capilla, A.V.; Usón, A.A. Life cycle assessment of building materials: Comparative analysis of energy and environmental impacts and evaluation of the eco-efficiency improvement potential. Build. Environ. 2011, 46, 1133–1140. [Google Scholar] [CrossRef]
- Flatt, R.J.; Roussel, N.; Cheeseman, C.R. Concrete: An eco material that needs to be improved. J. Eur. Ceram. Soc. 2012, 32, 2787–2798. [Google Scholar] [CrossRef]
- Bignozzi, M.C. Sustainable cements for green buildings construction. Procedia Eng. 2011, 21, 915–921. [Google Scholar] [CrossRef]
- Andini, S.; Montagnaro, F.; Santoro, L.; Accardo, G.; Cioffi, R.; Colangelo, F. Mechanochemical processing of blast furnace slag for its reuse as adsorbent. Chem. Eng. Trans. 2013, 32, 2299–2304. [Google Scholar]
- Baykal, G.; Döven, A.G. Utilization of fly ash by pelletization process; theory, application areas and research results. Resour. Conserv. Recycl. 2000, 30, 59–77. [Google Scholar] [CrossRef]
- Cioffi, R.; Colangelo, F.; Montagnaro, F.; Santoro, L. Manufacture of artificial aggregate using MSWI bottom ash. Waste Manag. 2011, 31, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, F.; Cioffi, R.; Lavorgna, M.; Verdolotti, L.; De Stefano, L. Treatment and recycling of asbestos-cement containing waste. J. Hazard. Mater. 2011, 195, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Soluble salt removal from MSWI fly ash and its stabilization for safer disposal and recovery as road basement material. Waste Manag. 2012, 32, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Geetha, S.; Ramamurthy, K. Environmental friendly technology of cold-bonded bottom ashaggregate manufacture through chemical activation. J. Clean. Pro. 2010, 18, 1563–1569. [Google Scholar]
- Gesoglu, M.; Güneyisi, E.; Öz, H.Ö. Properties of lightweight aggregates produced with cold-bonding pelletization of fly ash and ground granulated blast furnace slag. Mater. Struct. 2012, 45, 1535–1546. [Google Scholar] [CrossRef]
- Limbachiya, M.C.; Leelawat, T.; Dhir, R.K. Use of recycled concrete aggregate in high-strength concrete. Mater. Struct. 2000, 33, 574–580. [Google Scholar] [CrossRef]
- Beretka, J.; Cioffi, R.; Marroccoli, M.; Valenti, G.L. Energy-saving cements obtained from chemical gypsum and other industrial wastes. Waste Manag. 1996, 16, 231–235. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modeling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Andini, S.; Cioffi, R.; Colangelo, F.; Grieco, T.; Montagnaro, F.; Santoro, L. Coal fly ash as raw material for the manufacture of geopolymer-based products. Waste Manag. 2008, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, R.; Maffucci, L.; Santoro, L. Optimization of geopolymer synthesis by calcinations and polycondensation of a kaolinitic residue. Resour. Conserv. Recycl. 2003, 40, 27–38. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S. The role of inorganic polymer technology in the development of “green concrete”. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Mechanical performances of weathered coal fly ash based geopolymer bricks. Procedia Eng. 2011, 21, 745–752. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Use of reservoir clay sediments as raw materials for geopolymer binders. Adv. Appl. Ceram. 2013, 112, 184–189. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Messina, F.; Santoro, L.; Cioffi, R. Recycling of pre-washed municipal solid waste incinerator fly ash in the manufacturing of low temperature setting geopolymer materials. Materials 2013, 6, 3420–3437. [Google Scholar] [CrossRef]
- Colangelo, F.; Roviello, G.; Ricciotti, L.; Ferone, C.; Cioffi, R. Preparation and characterization of new geopolymer-epoxy resin hybrid mortars. Materials 2013, 6, 2989–3006. [Google Scholar] [CrossRef]
- Ferone, C.; Roviello, G.; Colangelo, F.; Cioffi, R.; Tarallo, O. Novel hybridorganic geopolymer materials. Appl. Clay Sci. 2013, 73, 42–50. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Roviello, G.; Asprone, D.; Menna, C.; Balsamo, A.; Prota, A.; Cioffi, R.; Manfredi, G. Application-oriented chemical optimization of a metakaolin based geopolymer. Materials 2013, 6, 1920–1939. [Google Scholar] [CrossRef] [Green Version]
- Iucolano, F.; Liguori, B.; Caputo, D.; Colangelo, F.; Cioffi, R. Recycled plastic aggregate in mortars composition: Effect on physical and mechanical properties. Mater. Des. 2013, 52, 916–922. [Google Scholar] [CrossRef]
- Lancellotti, I.; Kamseu, E.; Michelazzi, M.; Barbieri, L.; Corradi, A.; Leonelli, C. Chemical stability of geopolymers containing municipal solid waste incinerator fly ash. Waste Manag. 2010, 30, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Menna, C.; Asprone, D.; Ferone, C.; Colangelo, F.; Balsamo, A.; Prota, A.; Cioffi, R.; Manfredi, G. Use of geopolymers for composite external reinforcement of RC members. Compos. Part B Eng. 2012, 45, 1667–1676. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lorenzen, L. The potential use of geopolymeric materials to immobilise toxic metals: Part I. Theory and applications. Miner. Eng. 1997, 10, 659–669. [Google Scholar] [CrossRef]
- Asprone, D.; Durante, M.; Prota, A.; Manfredi, G. Potential of structural pozzolanic matrix–hemp fiber grid composites. Constr. Build. Mater. 2011, 25, 2867–2874. [Google Scholar] [CrossRef]
- Brandt, A.M. Fibre Reinforced Cement-Based (FRC) composites after over 40 years of development in building and civil engineering. Compos. Struct. 2008, 86, 3–9. [Google Scholar] [CrossRef]
- Asokan, P.; Saxena, M.; Asolekar, S.R. Coal combustion residues—Environmental implications and recycling potentials. Resour. Conserv. Recycl. 2005, 43, 239–262. [Google Scholar] [CrossRef]
- National Agency for Electric Energy, Environmental Report 2012. Available online: https://www.enel.com/it-IT/doc/report_2012/rapporto_ambientale_2012.pdf (accessed on 13 July 2013).
- Qiao, X.C.; Poon, C.S.; Cheeseman, C. Use of flue gas desulphurisation (FGD) waste and rejected fly ash in waste stabilization/solidification systems. Waste Manag. 2006, 26, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Italian Standardization Organization (UNI). Tests for Geometrical Properties of Aggregates—Part 1: Determination of Particle Size Distribution—Sieving Method; UNI EN 933-1:2012; Italian Standardization Organization (UNI): Milano, Italy, 2012. [Google Scholar]
- Italian Standardization Organization (UNI). Tests for Geometrical Properties of Aggregates–Part 2: Determination of Particle Size Distribution–Test Sieves, Nominal Size of Apertures; UNI EN 933-2:1997; Italian Standardization Organization (UNI): Milano, Italy, 1997. [Google Scholar]
- American Society for Testing and Materials (ASTM) International. Standard Practice for Acid-Extraction of Elements from Sediments Using Closed Vessel Microwave Heating; ASTM D 5258-92 (Reapproved 1996); American Society for Testing and Materials (ASTM) International: West Conshohocken, PA, USA, 1996. [Google Scholar]
- Italian Standardization Organization (UNI). Characterisation of Waste–Leaching–Compliance Test for Leaching of Granular Waste Materials and Sludges–Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 l/kg for Materials with Particle Size Below 4 mm (without or with Size Reduction); UNI EN 12457-2:2004; Italian Standardization Organization (UNI): Milano, Italy, 2004. [Google Scholar]
- Cioffi, R.; Marroccoli, M.; Martone, G.; Santoro, L. Utilization of zeolite-rich tuff for the manufacture of building materials based on calcium silicate and trisulphoaluminate hydrates. Thermochim. Acta 1997, 306, 93–98. [Google Scholar] [CrossRef]
- Italian Standardization Organization (UNI). Tests for Mechanical and Physical Properties of Aggregates—Part 6: Determination of Particle Density and Water Absorption; UNI EN 1097-6:2013; Italian Standardization Organization (UNI): Milano, Italy, 2013. [Google Scholar]
- Italian Standardization Organization (UNI). Lightweight Aggregates—Part 1: Lightweight Aggregates for Concrete, Mortar and Grout; UNI EN 13055-1:2003; Italian Standardization Organization (UNI): Milano, Italy, 2003. [Google Scholar]
- Saeedi, M.; Amini, H.R. Stabilization of heavy metals in wastewater treatment sludge from power plants air heater washing. Waste Manag. Res. 2009, 27, 274–280. [Google Scholar] [CrossRef]
- Ministro dell’Ambiente e della Tutela del Territorio. Individuazione dei Rifiuti non Pericolosi Sottoposti Alle Procedure Semplificate di Recupero; (in Italian). Ministro dell’Ambiente e della Tutela del Territorio: Roma, Italy, 2006. Available online: http://www.gazzettaufficiale.biz/atti/2006/20060115/006G0202.htm (accessed on 30 October 2013).
- Santoro, L.; Valenti, G.; Volpicelli, G. Application of differential scanning calorimetry to the study of the system phosphogypsum-lime-aluminium hydroxide-water. Thermochim. Acta 1984, 74, 35–44. [Google Scholar] [CrossRef]
- Gougar, M.L.D.; Scheetz, B.E.; Roy, D.M. Ettringite and C–S–H Portland cement phases for waste ion immobilization: A review. Waste Manag. 1996, 16, 295–303. [Google Scholar] [CrossRef]
- Italian Standardization Organization (UNI). Aggregates for Unbound and Hydraulically Bound Materials for Use in Civil Engineering Work and Road Construction; UNI EN 13242; Italian Standardization Organization (UNI): Milano, Italy, 2003. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ferone, C.; Colangelo, F.; Messina, F.; Iucolano, F.; Liguori, B.; Cioffi, R. Coal Combustion Wastes Reuse in Low Energy Artificial Aggregates Manufacturing. Materials 2013, 6, 5000-5015. https://doi.org/10.3390/ma6115000
Ferone C, Colangelo F, Messina F, Iucolano F, Liguori B, Cioffi R. Coal Combustion Wastes Reuse in Low Energy Artificial Aggregates Manufacturing. Materials. 2013; 6(11):5000-5015. https://doi.org/10.3390/ma6115000
Chicago/Turabian StyleFerone, Claudio, Francesco Colangelo, Francesco Messina, Fabio Iucolano, Barbara Liguori, and Raffaele Cioffi. 2013. "Coal Combustion Wastes Reuse in Low Energy Artificial Aggregates Manufacturing" Materials 6, no. 11: 5000-5015. https://doi.org/10.3390/ma6115000
APA StyleFerone, C., Colangelo, F., Messina, F., Iucolano, F., Liguori, B., & Cioffi, R. (2013). Coal Combustion Wastes Reuse in Low Energy Artificial Aggregates Manufacturing. Materials, 6(11), 5000-5015. https://doi.org/10.3390/ma6115000










