Electrochemical Study on Newly Synthesized Chlorocurcumin as an Inhibitor for Mild Steel Corrosion in Hydrochloric Acid
Abstract
:1. Introduction
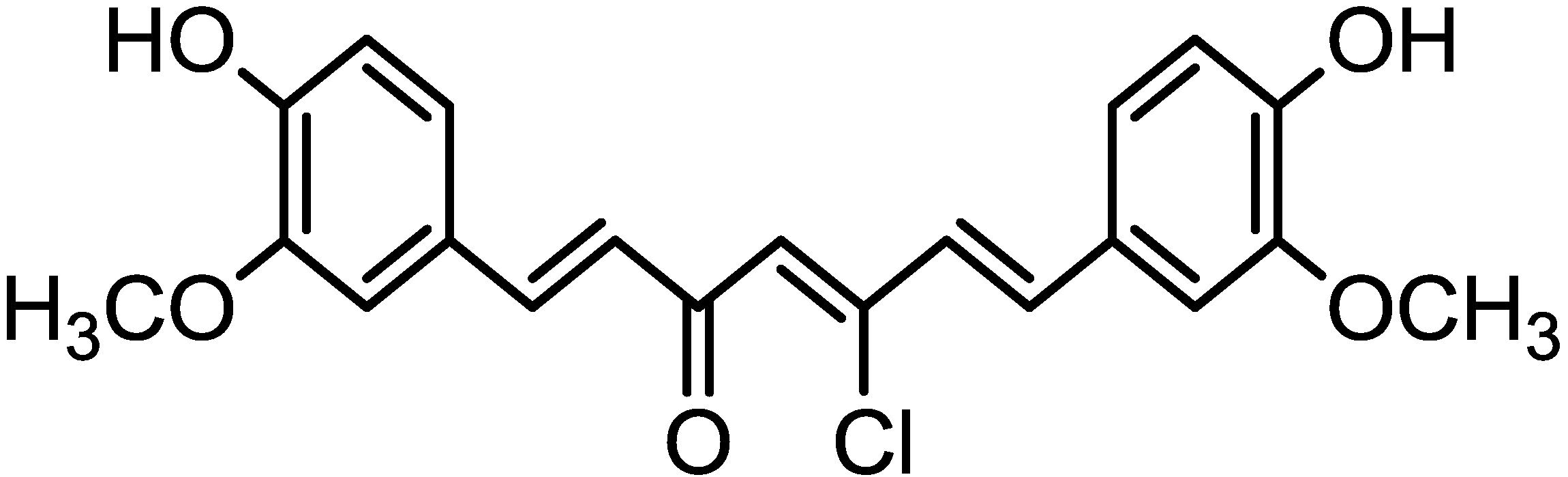
2. Results and Discussion
2.1. Polarization Measurements
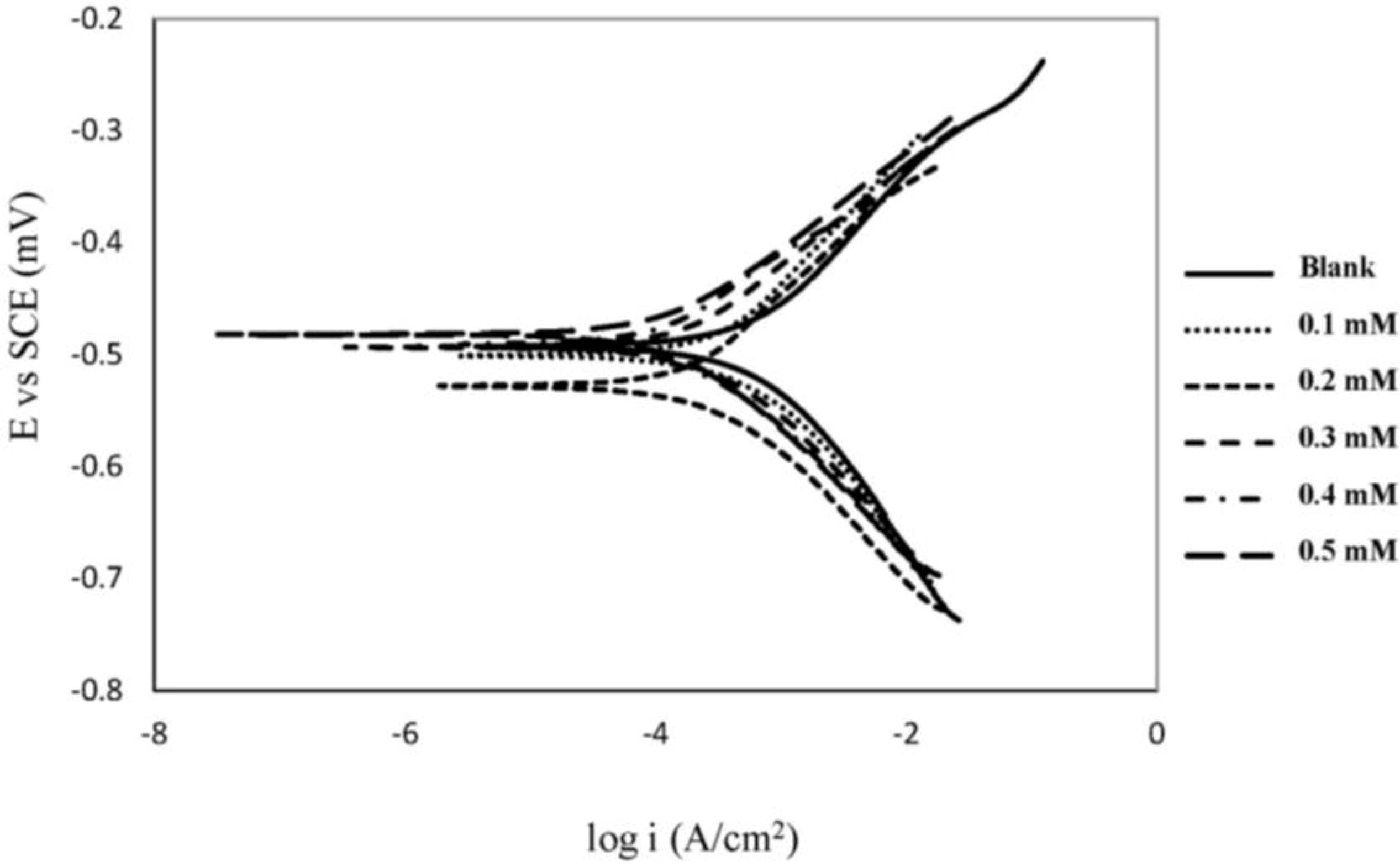
| Concentration (mM) | βa (V dec−1) | βc (V dec−1) | icorr (μA cm−2) | −Ecorr (mV vs. SCE) | IE% |
|---|---|---|---|---|---|
| Blank | 0.12 | 0.14 | 660.1 | 491 | 0.00 |
| 0.1 | 0.15 | 0.13 | 421.0 | 501 | 36.22 |
| 0.2 | 0.12 | 0.11 | 254 | 528 | 61.52 |
| 0.3 | 0.10 | 0.11 | 232 | 493 | 64.85 |
| 0.4 | 0.09 | 0.10 | 150 | 491 | 77.28 |
| 0.5 | 0.09 | 0.11 | 145 | 482 | 78.03 |
2.2. Electrochemical Impedance Spectroscopy (EIS) Measurements
| Concentration (mM) | Rs (ohm cm−2) | Rct (ohm cm−2) | CPEdl YoX10−5 (Ssα/cm2) | α | Cdl (μF cm−2) | IE% |
|---|---|---|---|---|---|---|
| 0 | – | 42.00 | – | 0.78 | 294.47 | 0.00 |
| 0.1 | 2.15 | 61.45 | 60.48 | 0.74 | 190.21 | 31.65 |
| 0.2 | 0.69 | 87.85 | 47.60 | 0.76 | 174.68 | 52.19 |
| 0.3 | 1.12 | 91.55 | 38.86 | 0.81 | 177.69 | 54.12 |
| 0.4 | 0.94 | 121.40 | 27.37 | 0.82 | 159.64 | 65.40 |
| 0.5 | 1.65 | 130.80 | 29.10 | 0.84 | 126.14 | 67.89 |
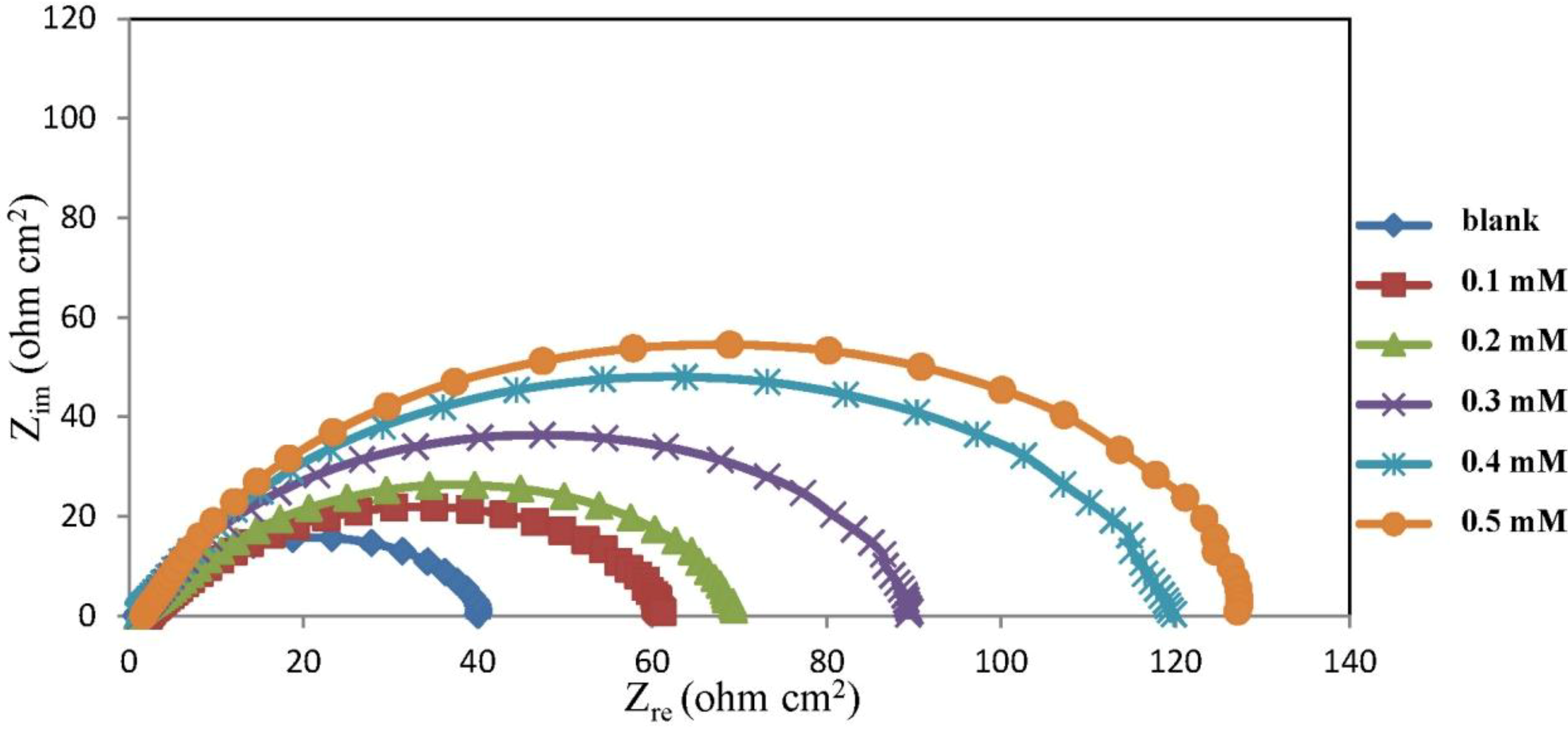
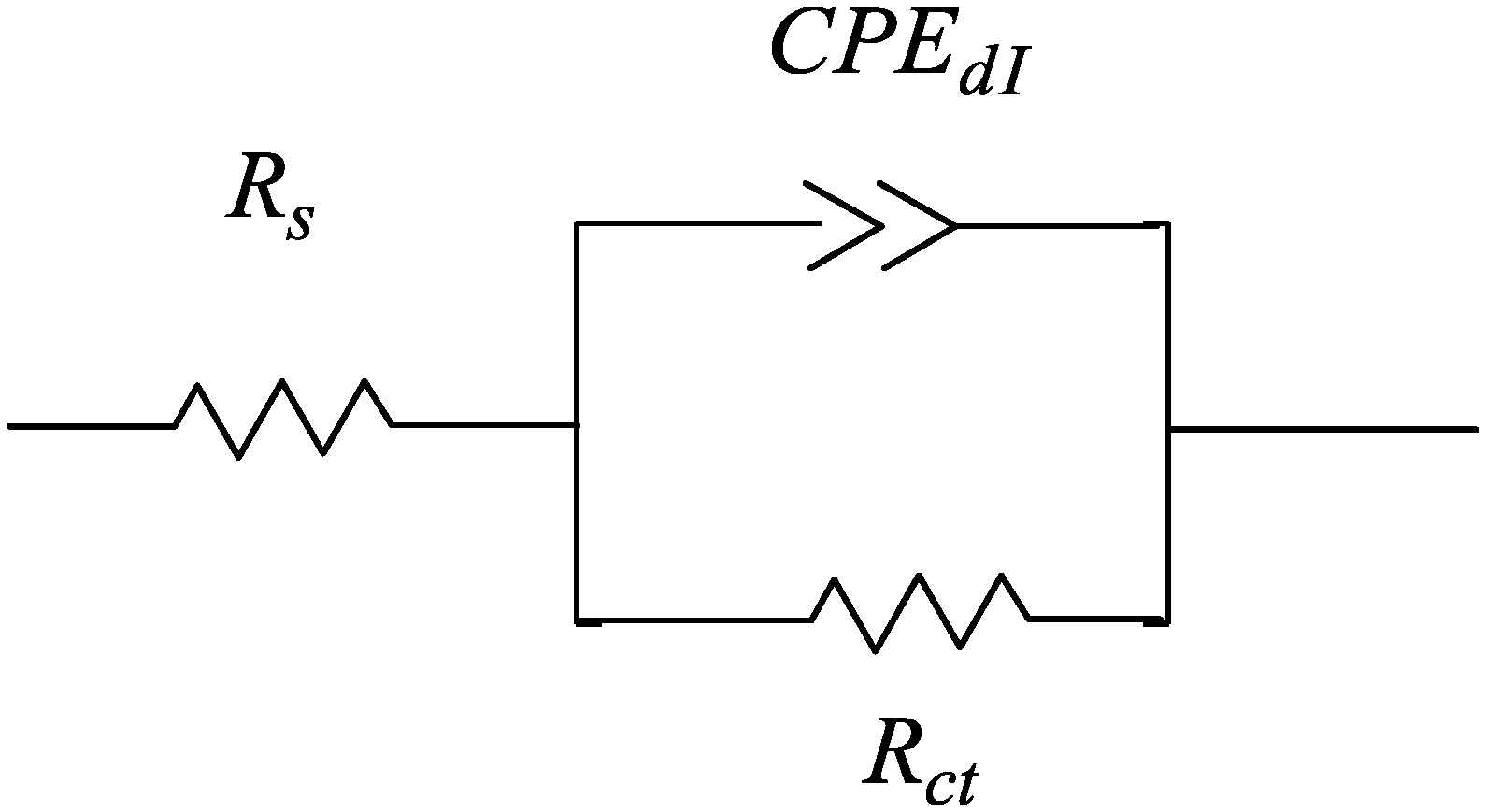
2.3. Corrosion Kinetic Parameters

| Concentration | Ea (kJ·mol−1) | ∆Ha (kJ·mol−1) | ∆Sa (J·mol−1·K−1) |
|---|---|---|---|
| Blank | 77.25 | 74.61 | −60.44 |
| 0.5 mM | 104.74 | 102.10 | 18.91 |
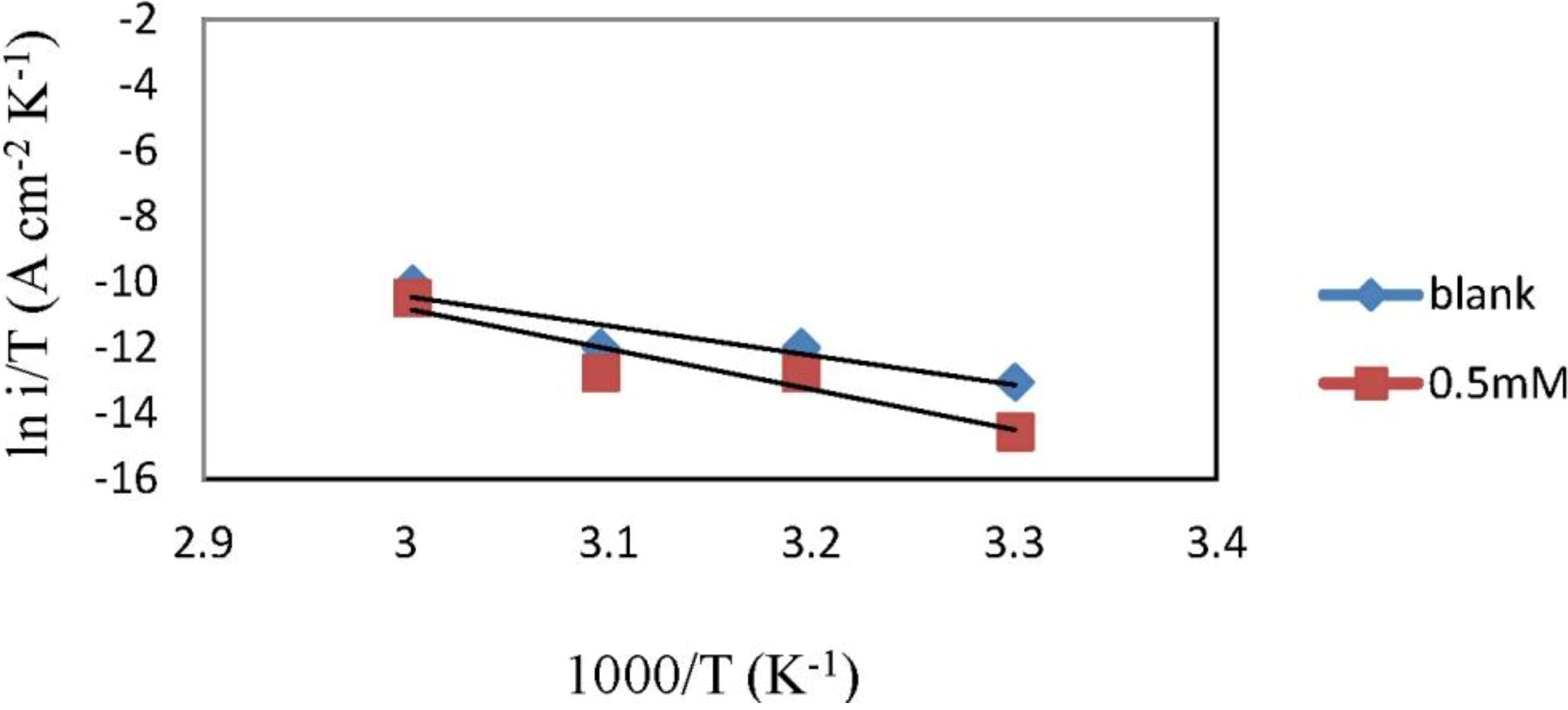
2.4. Adsorption Isotherm
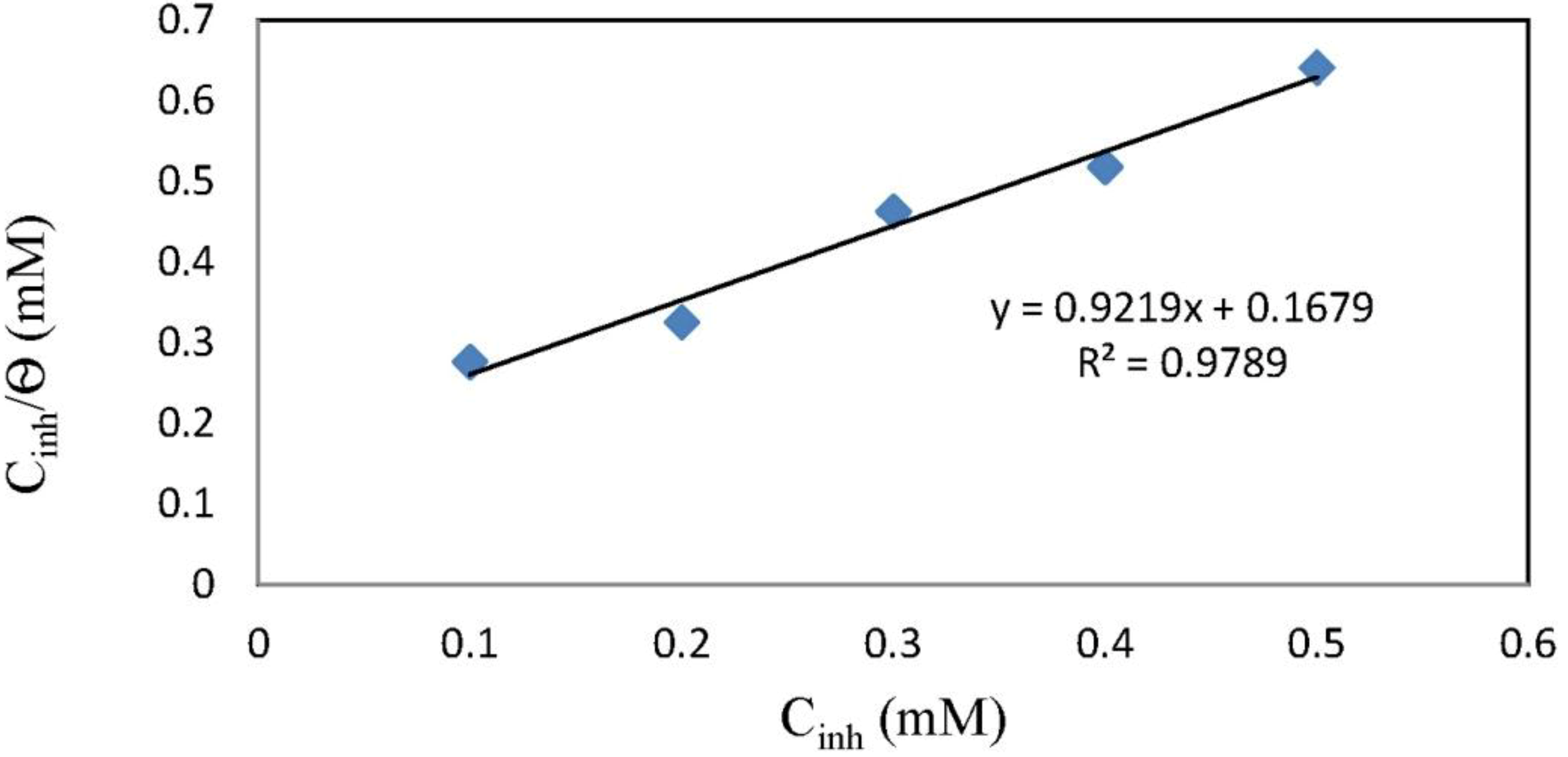
3. Experimental Section
3.1. Synthesis of Chlorocurcumin
3.2. Electrochemical Measurements
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Myint, S.; Daud, W.R.W.; Mohamad, A.B.; Kadhum, A.A.H. Gas chromatographic determination of eugenol in ethanol extract of cloves. J. Chromatogr. Biomed. Appl. 1996, 679, 193–195. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.M.; Gandomi-Niasar, A.; Soltani, N.; Salavati-Niasari, M. The inhibition of mild steel corrosion in hydrochloric acid media by two Schiff base compounds. J. Mater. Sci. 2009, 44, 2444–2453. [Google Scholar] [CrossRef]
- Tang, Y.M.; Yang, X.Y.; Yang, W.Z.; Wan, R.; Chen, Y.Z.; Yin, X.S. A preliminary investigation of corrosion inhibition of mild steel in 0.5 M H2SO4 by 2-amino-5-(n-pyridyl)-1,3,4-thiadiazole: Polarization, EIS and molecular dynamics simulations. Corros. Sci. 2010, 52, 1801–1808. [Google Scholar]
- Cang, H.; Fei, Z.; Shi, W.; Xu, Q. Experimental and theoretical study for corrosion inhibition of mild steel by L-Cysteine. Int. J. Electrochem. Sci. 2012, 7, 10121–10131. [Google Scholar]
- Kumar, H.; Karthikeyan, S. Inhibition of mild steel corrosion in hydrochloric acid solution by cloxacillin drug. J. Mater. Environ. Sci. 2012, 3, 925–934. [Google Scholar]
- Wang, Z. The inhibition effect of bis-benzimidazole compound for mild steel in 0.5 M HCl solution. Int. J. Electrochem. Sci. 2012, 7, 11149–11160. [Google Scholar]
- Ju, H.; Kai, Z.P.; Li, Y. Aminic nitrogen-bearing polydentate Schiff base compounds as corrosion inhibitors for iron in acidic media: A quantum chemical calculation. Corros. Sci. 2008, 50, 865–871. [Google Scholar] [CrossRef]
- El Issami, S.; Hammouti, B.; Kertit, S.; Addi, E.; Salghi, R. Triazolic compounds as corrosion inhibitors for copper in hydrochloric acid. Pigment Resin Technol. 2007, 36, 161–168. [Google Scholar]
- Riggs, O.L., Jr.; Hurd, R.M. Temperature coefficient of coorosion inhibitor. Corrosion 1967, 23, 252–258. [Google Scholar] [CrossRef]
- Durnie, W.; Marco, R.D.; Jefferson, A.; Kinsella, B. Structure activity of oil field corrosion inhibitors. J. Electrochem. Soc. 146, 1751–1756. [CrossRef]
- Bentiss, F.; Lebrini, M.; Lagrenée, M. Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corros. Sci. 2005, 47, 2915–2931. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Okolue, B.N.; Ebenso, E.E.; Onuoha, G.N.; Onuchukwu, A.I. Evaluation of the inhibitory effect of methylene blue dye on corrosion of aluminum in HCl. Mater. Chem. Phys. 2004, 87, 394–401. [Google Scholar] [CrossRef]
- Li, X.; Tang, L. Synergistic inhibition between OP and NaCl on the corrosion of cold-rolled steel in phosphoric acid. Mater. Chem. Phys. 2005, 90, 286–297. [Google Scholar] [CrossRef]
- Sinko, J. Challenges of chromate inhibitor pigments replacement in organic coating. Prog. Org. Coat. 2001, 42, 267–282. [Google Scholar] [CrossRef]
- Popova, A.; Christov, M. Evaluation of impedance measurements on mild steel corrosion in acid media in the presence of heterocyclic compounds. Corros. Sci. 2006, 48, 3208–3221. [Google Scholar] [CrossRef]
- Liu, F.G.; Du, M.; Zhang, J.; Qiu, M. Electrochemical behavior of Q235 steel in saltwater saturated with carbon dioxide based on new imidazoline derivative inhibitor. Corros. Sci. 2009, 51, 102–105. [Google Scholar] [CrossRef]
- Benabdellah, M.; Touzani, R.; Dafali, A.; Hammouti, M.; El Kadiri, S. Ruthenium–ligand complex, an efficient inhibitor of steel corrosion in H3PO4 media. Mater. Lett. 2007, 61, 1197–1204. [Google Scholar] [CrossRef]
- Bayol, E.; Gürten, A.A.; Dursun, M.; Kayakırılmaz, S. Adsorption behavior and inhibition corrosion effect of sodium carboxymethyl cellulose on mild steel in acidic medium. Acta Phys. Chim. Sin. 2008, 24, 2236–2242. [Google Scholar] [CrossRef]
- Popova, A. Temperature effect on mild steel corrosion in acid media in presence of azoles. Corros. Sci. 2007, 49, 2144–2158. [Google Scholar] [CrossRef]
- Li, W.; He, Q.; Zhang, S.; Pei, C.; Hou, B. Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium. J. Appl. Electrochem. 2008, 38, 289–295. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Giacomelli, C.; Giacomelli, F.C.; Spinelli, A. Evaluation of the inhibitor effect of L-ascorbic acid on the corrosion of mild steel. Mater. Chem. Phys. 2004, 83, 129–134. [Google Scholar] [CrossRef]
- Larabi, L.; Harek, Y.; Traisnel, M.; Mansri, A. Synergistic influence of poly(4-vinylpyridine) and potassium iodide on inhibition of corrosion of mild steel in 1 M HCl. J. Appl. Electrochem. 2004, 34, 833–839. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H. Benzyltrimethylammonium iodide as a corrosion inhibitor for steel in phosphoric acid produced by dihydrate wet method process. Corros. Sci. 2011, 53, 664–670. [Google Scholar] [CrossRef]
- Abo El-Khair, M.B.; Abdel Hamed, I.A. Thiosemicarbazide as an inhibitor for the acid corrosion of iron. Corros. Sci. 1976, 16, 163–169. [Google Scholar] [CrossRef]
- Shih, H.; Mansfeld, H. A fitting procedure for impedance data of systems with very low corrosion rates. Corros. Sci. 1989, 29, 1235–1240. [Google Scholar] [CrossRef]
- Elayyachy, M.; El Idrissi, A.; Hammouti, B. New thio-compounds as corrosion inhibitor for steel in 1 M HCl. Corros. Sci. 2006, 48, 2470–2479. [Google Scholar] [CrossRef]
- Jacob, K.S.; Parameswaran, G. Corrosion inhibition of mild steel in hydrochloric acid solution by Schiff base furoin thiosemicarbazone. Corros. Sci. 2010, 52, 224–228. [Google Scholar] [CrossRef]
- Prabhu, R.A.; Venkatesha, T.V.; Shanbhag, A.V.; Kulkarni, G.M.; Kalkhambkar, R.G. Inhibition effects of some Schiff’s bases on the corrosion of mild steel in hydrochloric acid solution. Corros. Sci. 2008, 50, 3356–3362. [Google Scholar] [CrossRef]
- Fu, J.; Pan, J.; Liu, Z.; Li, S.; Wang, Y. Corrosion inhibition of mild steel by benzopyranone derivative in 1.0 M HCl solutions. Int. J. Electrochem. Sci. 2011, 6, 2072–2089. [Google Scholar]
- Babic-Samardzija, K.; Khaled, K.F.; Hackerman, N. Heterocyclic amines and derivatives as corrosion inhibitors for iron in perchloric acid. Anti Corros. Method. Mater. 2005, 52, 11–21. [Google Scholar] [CrossRef]
- Shukla, S.K.; Quraishi, M.A.; Ebenso, E.E. Adsorption and corrosion inhibition properties of cefadroxil on mild steel in hydrochloric acid. Int. J. Electrochem. Sci. 2011, 6, 2912–2931. [Google Scholar]
- Herrag, L.; Hammouti, B.; Elkadiri, S.; Aouniti, A.; Jama, C.; Vezin, H.; Bentiss, F. Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: Experimental and theoretical investigations. Corros. Sci. 2010, 52, 3042–3051. [Google Scholar] [CrossRef]
- Boudalia, M.; Guenbour, A.; Bellaouchou, A.; Laqhaili, A.; Mousaddak, M.; Hakiki, A.; Hammouti, B.; Ebenso, E.E. Corrosion inhibition of organic oil extract of leaves of lanvandula stoekas on stainless steel in concentrated phosphoric acid solution. Int. J. Electrochem. Sci. 2013, 8, 7414–7424. [Google Scholar]
- Musa, A.Y.; Mohamad, A.B.; Kadhum, A.A.H.; Takriff, M.S.; Lim, T.T. Synergistic effect of potassium iodide with phthalazone on the corrosion inhibition of mild steel in 1.0 M HCl. Corros. Sci. 2011, 53, 3672–3677. [Google Scholar] [CrossRef]
- Ell Quali, I.; Hammouti, B.; Aouniti, A.; Ramli, Y.; Azougagh, M.; Essassi, E.M.; Bouachrine, M. Thermodynamic characterisation of steel corrosion in HCl in the presence of 2-phenylthieno (3, 2-b) quinoxaline . J. Mater. Environ. Sci. 2010, 1, 1–8. [Google Scholar]
- Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Daud, A.R.; Kamarudin, S.K. On the inhibition of mild steel corrosion by 4-amino-5-phenyl-4H-1, 2, 4-trizole-3-thiol. Corros. Sci. 2010, 52, 526–433. [Google Scholar] [CrossRef]
- Al-Amiery, A.; Kadhum, A.; Obayes, H.; Mohamad, A. Synthesis and antioxidant activities of novel 5-chlorocurcumin, complemented by semiempirical calculations. Bioinorg. Chem. Appl. 2013, 2013, 354982:1–354982:7. [Google Scholar] [CrossRef]
- Moretti, G.; Guidi, F.; Grion, G. Tryptamine as a green iron corrosion inhibitor in 0.5 M deaerated sulphuric acid. Corros. Sci. 2004, 46, 387–403. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. Available online: http://www.cosasco.com/documents/ASTM_G1_Standard_Practice.pdf (accessed on 11 March 2013).
- Al-amiery, A.A.; Abdul, A.H.K.; Abu, B.M.; Sutiana, J. A novel hydrazinecarbothioamide as a potential corrosion inhibitor for mild steel in HCl. Materials 2013, 6, 1420–1431. [Google Scholar] [CrossRef]
- Junaedi, S.; Al-amiery, A.; Kadihum, A.; Mohamad, A. Inhibition effects of a synthesized novel 4-Aminoantipyrine derivative on the corrosion of mild steel in hydrochloric acid solution together with quantum chemical studies. Int. J. Mol. Sci. 2013, 14, 11915–11928. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B.; Musa, A.Y.; Li, C.J. Electrochemical Study on Newly Synthesized Chlorocurcumin as an Inhibitor for Mild Steel Corrosion in Hydrochloric Acid. Materials 2013, 6, 5466-5477. https://doi.org/10.3390/ma6125466
Al-Amiery AA, Kadhum AAH, Mohamad AB, Musa AY, Li CJ. Electrochemical Study on Newly Synthesized Chlorocurcumin as an Inhibitor for Mild Steel Corrosion in Hydrochloric Acid. Materials. 2013; 6(12):5466-5477. https://doi.org/10.3390/ma6125466
Chicago/Turabian StyleAl-Amiery, Ahmed A., Abdul Amir H. Kadhum, Abu Bakar Mohamad, Ahmed Y. Musa, and Cheong Jiun Li. 2013. "Electrochemical Study on Newly Synthesized Chlorocurcumin as an Inhibitor for Mild Steel Corrosion in Hydrochloric Acid" Materials 6, no. 12: 5466-5477. https://doi.org/10.3390/ma6125466
APA StyleAl-Amiery, A. A., Kadhum, A. A. H., Mohamad, A. B., Musa, A. Y., & Li, C. J. (2013). Electrochemical Study on Newly Synthesized Chlorocurcumin as an Inhibitor for Mild Steel Corrosion in Hydrochloric Acid. Materials, 6(12), 5466-5477. https://doi.org/10.3390/ma6125466






