Modified Titanium Surface-Mediated Effects on Human Bone Marrow Stromal Cell Response
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Characterization
| Group | Contact Angle (°) | Topography parameter | ||
|---|---|---|---|---|
| Sa (µm) | Str | Sdr (%) | ||
| Ti | 75.53 ± 0.51 | 0.29 ± 0.005 | 0.54 ± 0.02 | 0.35 ± 0.01 |
| AMS | 36.56 ± 0.21 * | 0.61 ± 0.01 * | 0.32 ± 0.06 * | 0.91 ± 0.03 * |
| Ti + BMP | ≈0 | 0.61 ± 0.02 * | 0.24 ± 0.03 * | 1.58 ± 0.11 * |
| AMS + BMP | ≈0 | 0.64 ± 0.02 * | 0.25 ± 0.03 * | 1.68 ± 0.10 * |
| TiO2 | 45.10 ± 0.67 * | 0.31 ± 0.01 | 0.53 ± 0.05 | 0.37 ± 0.01 |
| BAG | ≈0 | 3.25 ± 0.13 * | 0.79 ± 0.02 * | 97.23 ± 7.53 * |
| T1 | 99.22 ± 0.51 * | 7.86 ± 0.21 *,† | 0.70 ± 0.03 * | 102.50 ± 6.26 *,† |
| T2 | 101.27 ± 0.32 * | 9.06 ± 0.21 *,† | 0.72 ± 0.03 * | 188.98 ± 6.81 *,† |

2.2. Proliferation of hMSC on Modified Ti Surfaces
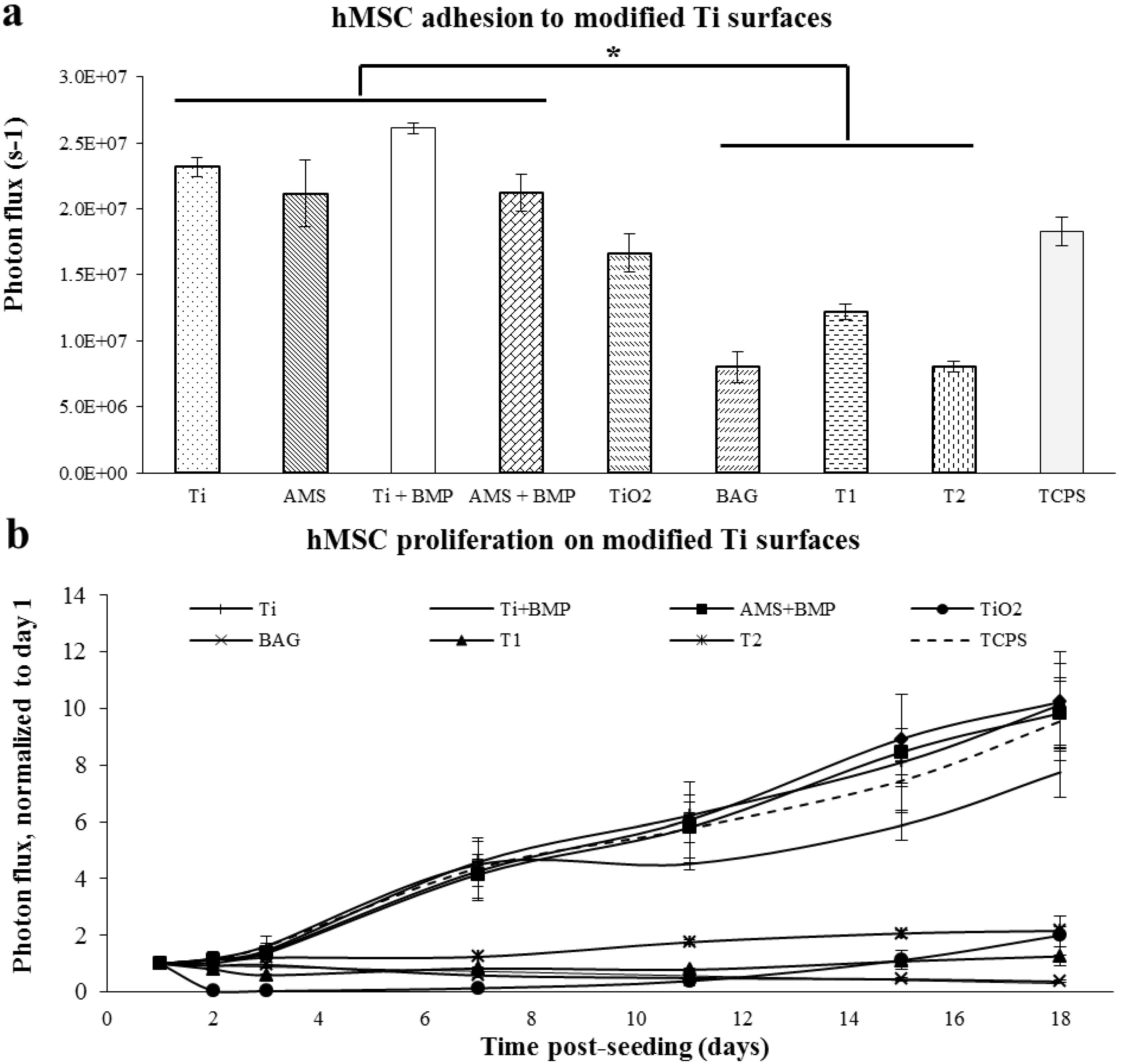
2.3. Differentiation of hMSC on Modified Ti Surfaces
2.3.1. Alkaline Phosphatase Activity
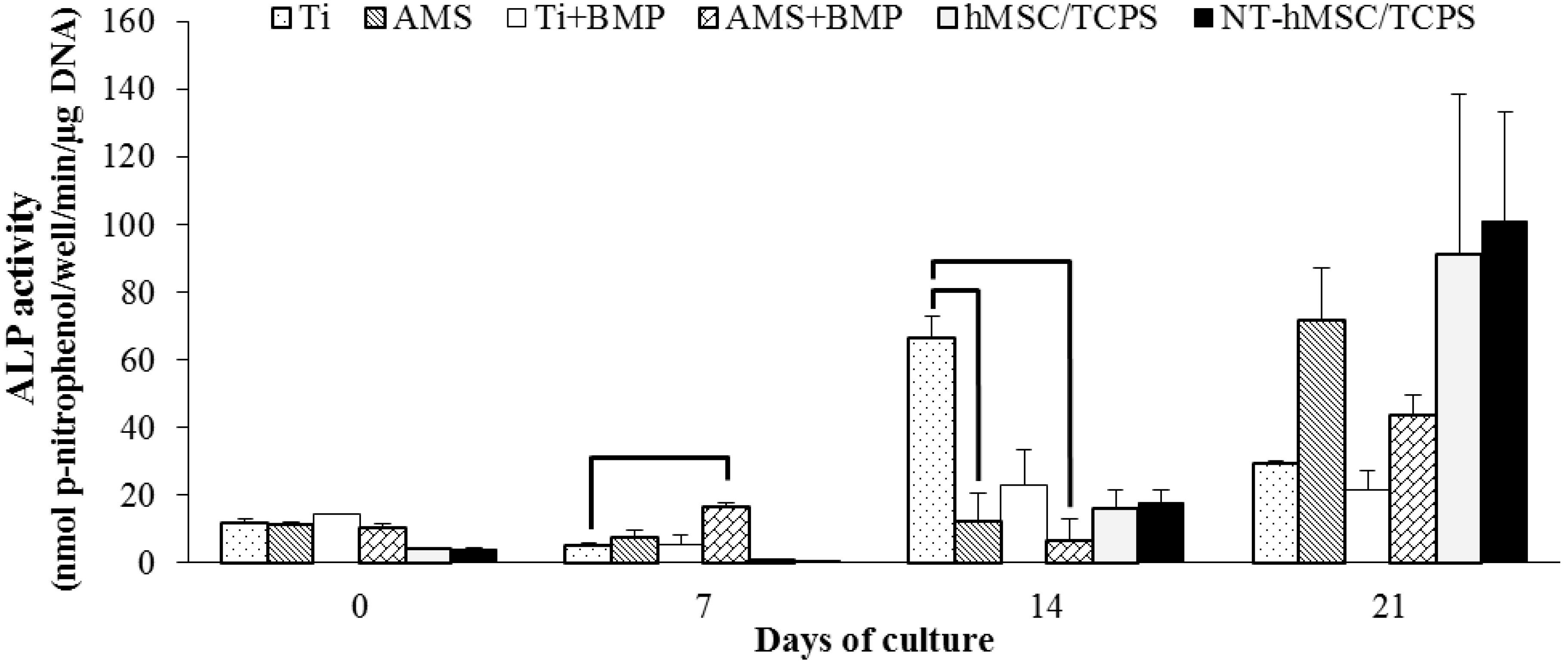
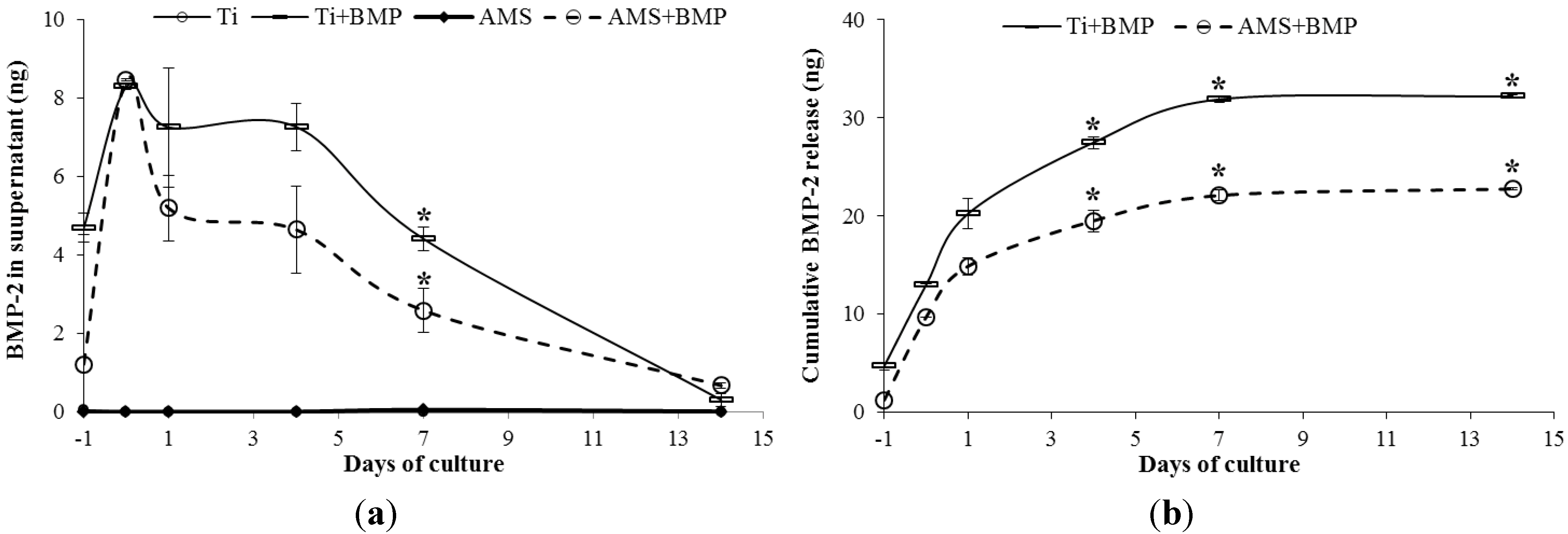
2.3.2. OC, OPG, VEGF-A and BMP-2 Release
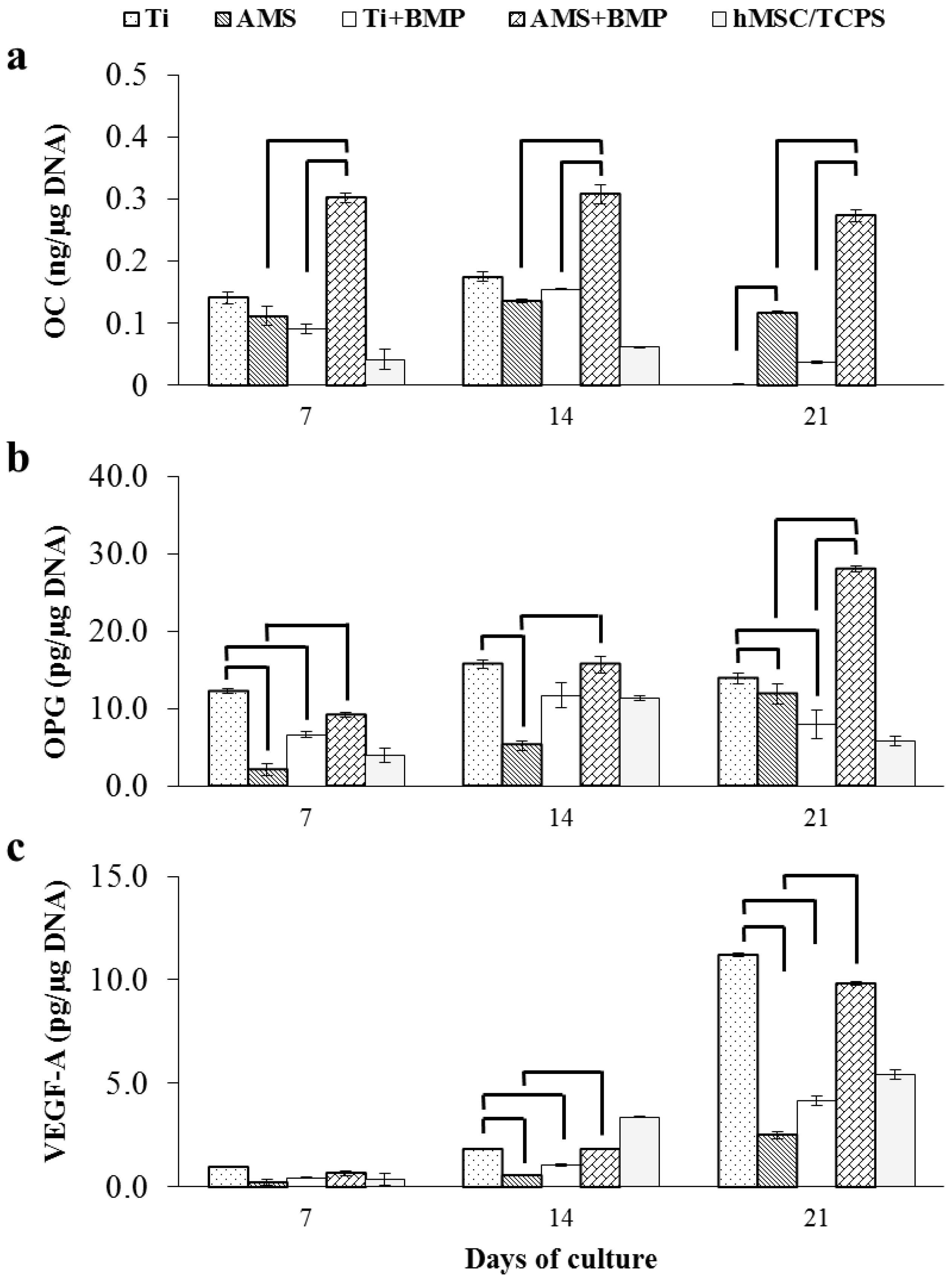
3. Experimental Section
3.1. Titanium Discs as Substrate
3.2. Titanium Surface Modification
3.2.1. Amorphous Microporous Silica Coating
3.2.2. BMP-2 Adsorption on AMS-Coating
3.2.3. Bio-Active Glass Coating
3.2.4. Porous Ti Coatings (T1; T2)
3.2.5. Control Surfaces
3.3. Surface Characterization
3.3.1. Static Contact Angle Measurement
3.3.2. Surface Topography
3.4. The Proliferation of hMSC on Modified Ti Surfaces
3.5. The Osteogenic Differentiation of hMSC on Modified Ti Surface
3.5.1. Cell Culture Conditions
3.5.2. DNA Content
3.5.3. ALP Activity
3.5.4. Enzyme-Linked Immunosorbent Assays (ELISA) of Cells’ Supernatants
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef] [PubMed]
- Adell, R.; Eriksson, B.; Lekholm, U.; Branemark, P.I.; Jemt, T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int. J. Oral Maxillofac. Implants 1990, 5, 347–359. [Google Scholar] [PubMed]
- Misch, C.E.; Perel, M.L.; Wang, H.L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Paquette, D.W.; Brodala, N.; Williams, R.C. Risk factors for endosseous dental implant failure. Dent. Clin. North Am. 2006, 50, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Polyzos, I.P.; Felice, P.; Worthington, H.V. Interventions for replacing missing teeth: Dental implants in fresh extraction sockets (immediate, immediate-delayed and delayed implants). Cochrane Database Syst. Rev. 2010, 9. [Google Scholar] [CrossRef]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early wound healing around endosseous implants: A review of the literature. Int. J. Oral Maxillofac. Implants 2005, 20, 425–431. [Google Scholar] [PubMed]
- Dohan Ehrenfest, D.M.; Coelho, P.G.; Kang, B.S.; Sul, Y.T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Kruse-Losler, B.; Wiesmann, H.P. Principles of bone formation driven by biophysical forces in craniofacial surgery. Br. J. Oral Maxillofac. Surg. 2006, 44, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Romanos, G.E. The role of primary stability for successful immediate loading of dental implants. A literature review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 2009, 9, 61–71. [Google Scholar] [PubMed]
- Vitte, J.; Benoliel, A.M.; Pierres, A.; Bongrand, P. Is there a predictable relationship between surface physical-chemical properties and cell behaviour at the interface? Eur. Cells Mater. 2004, 7, 52–63. [Google Scholar]
- Le Guehennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar]
- Mendonca, G.; Mendonca, D.B.; Aragao, F.J.; Cooper, L.F. Advancing dental implant surface technology from micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Verraedt, E.; Pendela, M.; Adams, E.; Hoogmartens, J.; Martens, J.A. Controlled release of chlorhexidine from amorphous microporous silica. J. Control. Release 2010, 142, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Balas, F. Silica materials for medical applications. Open Biomed. Eng. J. 2008, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Denoyel, R.; Meneses, J.M.; Armatas, G.S.; Rouquerol, J.; Unger, K.K.; Pomonis, P.J. Comparative study of morphometric properties characterizing the complexity of silicate pore networks probed by adsorption of nitrogen and methanol. Langmuir 2006, 22, 5350–5357. [Google Scholar] [CrossRef] [PubMed]
- Scheufler, C.; Sebald, W.; Hulsmeyer, M. Crystal structure of human bone morphogenetic protein-2 at 2.7 A resolution. J. Mol. Biol. 1999, 287, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Aerts, C.A.; Verraedt, E.; Depla, A.; Follens, L.; Froyen, L.; van Humbeeck, J.; Augustijns, P.; van den Mooter, G.; Mellaerts, R.; Martens, J.A. Potential of amorphous microporous silica for ibuprofen controlled release. Int. J. Pharm. 2010, 397, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.; Vanmellaert, L.; Bauwens, M.; Vermaelen, P.; Deroose, C.M.; Naert, I.; Cardoso, M.V.; Martens, J.A.; Duyck, J. In vitro and in vivo investigation of the potential of amorphous microporous silica as a protein delivery vehicle. BioMed Res. Int. 2013, 2013, 306418:1–306418:10. [Google Scholar] [CrossRef]
- Groeneveld, E.H.; Burger, E.H. Bone morphogenetic proteins in human bone regeneration. Eur. J. Endocrinol. 2000, 142, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Vallet-Regi, M. Sol-gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics and the origin of life. J. Biomed. Mater. Res. 1989, 23, 685–703. [Google Scholar] [CrossRef] [PubMed]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, H.; Bram, M.; Buchkremer, H.P.; Stover, D. Mechanical examinations on dental implants with porous titanium coating. J. Mater. Sci. Mater. Med. 2009, 20, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.; Gauthier, M.; Lefebvre, L.P.; Dunbar, M.; Filiaggi, M. Fibroblastic interactions with high-porosity Ti-6Al-4V metal foam. J. Biomed. Mater. Res. Appl. Biomater. 2007, 82, 440–449. [Google Scholar] [CrossRef] [Green Version]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Mattheys, T.; Braem, A.; Neirinck, B.; van der Biest, O.; Vleugels, J. Ti coatings with macropores for improved implant fixation obtained by electrophoretic deposition of TiH2 stabilized emulsions. Adv. Eng. Mater. 2012, 14, 371–376. [Google Scholar] [CrossRef]
- Braem, A.; Mattheys, T.; Neirinck, B.; Schrooten, J.; van der Biest, O.; Vleugels, J. Porous titanium coatings through electrophoretic deposition of TiH2 suspensions. Adv. Eng. Mater. 2011, 13, 509–515. [Google Scholar] [CrossRef]
- Vercaigne, S.; Wolke, J.G.; Naert, I.; Jansen, J.A. Bone healing capacity of titanium plasma-sprayed and hydroxylapatite-coated oral implants. Clin. Oral Implants Res. 1998, 9, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.F.; Masuda, T.; Whitson, S.W.; Yliheikkila, P.; Felton, D.A. Formation of mineralizing osteoblast cultures on machined, titanium oxide grit-blasted, and plasma-sprayed titanium surfaces. Int. J. Oral Maxillofac. Implants 1999, 14, 37–47. [Google Scholar] [PubMed]
- Suzuki, K.; Aoki, K.; Ohya, K. Effects of surface roughness of titanium implants on bone remodeling activity of femur in rabbits. Bone 1997, 21, 507–514. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Eitenmuller, J.; Muhr, G.; Pommer, A.; Bar, H.F.; Ostermann, P.A.; Schildhauer, T.A. Mechanical and histological evaluation of hydroxyapatite-coated, titanium-coated and grit-blasted surfaces under weight-bearing conditions. Arch. Orthop. Trauma Surg. 1995, 114, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. 2005, 74, 49–58. [Google Scholar] [CrossRef]
- Lamolle, S.F.; Monjo, M.; Rubert, M.; Haugen, H.J.; Lyngstadaas, S.P.; Ellingsen, J.E. The effect of hydrofluoric acid treatment of titanium surface on nanostructural and chemical changes and the growth of MC3T3-E1 cells. Biomaterials 2009, 30, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.; Braem, A.; Vleugels, J.; Martens, J.A.; Naert, I.; Cardoso, M.V.; Duyck, J. Bone tissue response to porous and functionalized titanium and silica based coatings. PLoS One 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Hosogane, N.; Huang, Z.; Rawlins, B.A.; Liu, X.; Boachie-Adjei, O.; Boskey, A.L.; Zhu, W. Stromal derived factor-1 regulates bone morphogenetic protein 2-induced osteogenic differentiation of primary mesenchymal stem cells. Int. J. Biochem. Cell Biol. 2010, 42, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Thorey, F.; Menzel, H.; Lorenz, C.; Gross, G.; Hoffmann, A.; Windhagen, H. Enhancement of endoprosthesis anchoring using BMP-2. Technol. Health Care 2010, 18, 217–229. [Google Scholar] [PubMed]
- Liu, Y.; Huse, R.O.; de Groot, K.; Buser, D.; Hunziker, E.B. Delivery mode and efficacy of BMP-2 in association with implants. J. Dent. Res. 2007, 86, 84–89. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chaudhari, A.; Duyck, J.; Braem, A.; Vleugels, J.; Petite, H.; Logeart-Avramoglou, D.; Naert, I.; Martens, J.A.; Vandamme, K. Modified Titanium Surface-Mediated Effects on Human Bone Marrow Stromal Cell Response. Materials 2013, 6, 5533-5548. https://doi.org/10.3390/ma6125533
Chaudhari A, Duyck J, Braem A, Vleugels J, Petite H, Logeart-Avramoglou D, Naert I, Martens JA, Vandamme K. Modified Titanium Surface-Mediated Effects on Human Bone Marrow Stromal Cell Response. Materials. 2013; 6(12):5533-5548. https://doi.org/10.3390/ma6125533
Chicago/Turabian StyleChaudhari, Amol, Joke Duyck, Annabel Braem, Jozef Vleugels, Hervé Petite, Delphine Logeart-Avramoglou, Ignace Naert, Johan A. Martens, and Katleen Vandamme. 2013. "Modified Titanium Surface-Mediated Effects on Human Bone Marrow Stromal Cell Response" Materials 6, no. 12: 5533-5548. https://doi.org/10.3390/ma6125533




