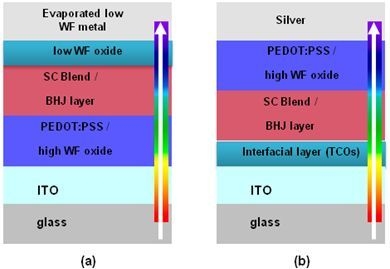
Development of Efficient and Stable Inverted Bulk Heterojunction (BHJ) Solar Cells Using Different Metal Oxide Interfaces
Abstract
:1. Introduction
2. Comparison between the Classical Sol-Gel Method (Precipitation Reactions) and the Non-hydrolytic Sol-Gel Method
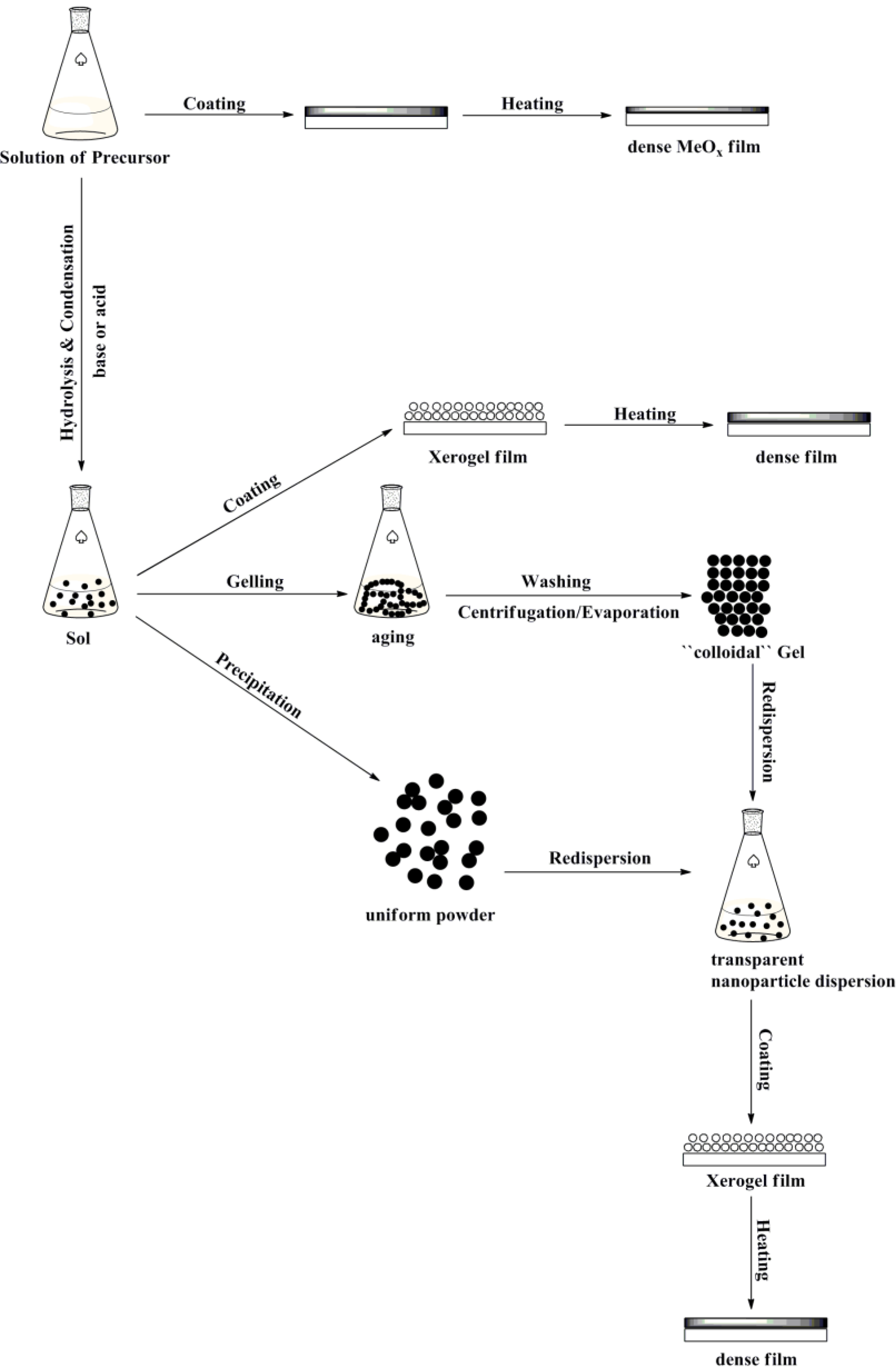
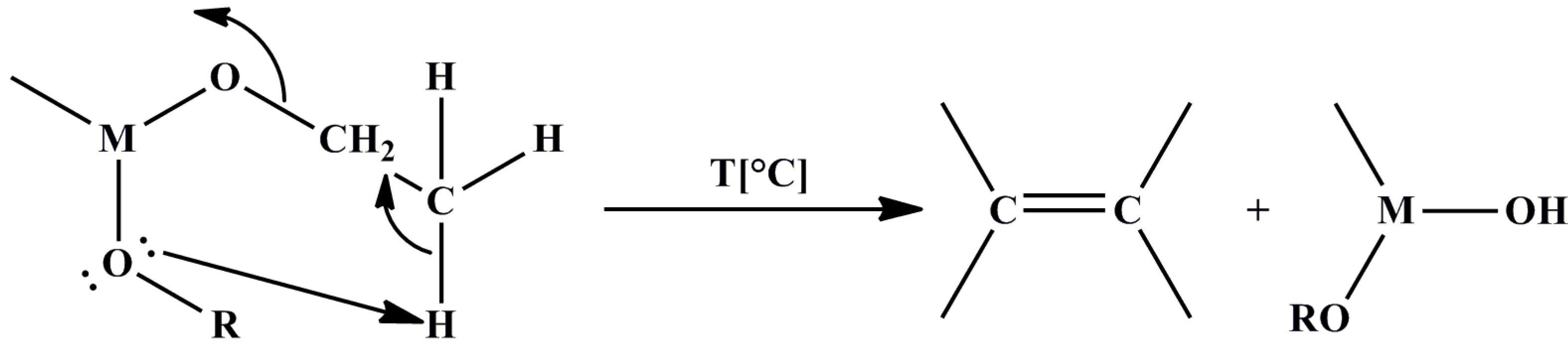
2.1. Classical Sol-Gel Method
2.2. Non-Hydrolytic Sol-Gel Method
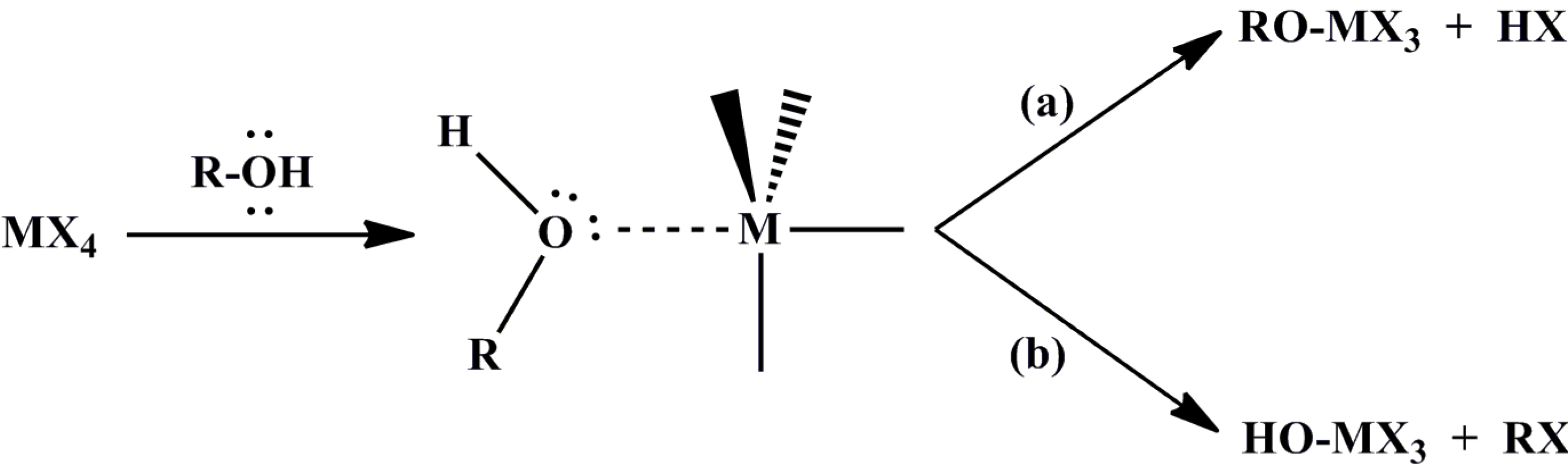
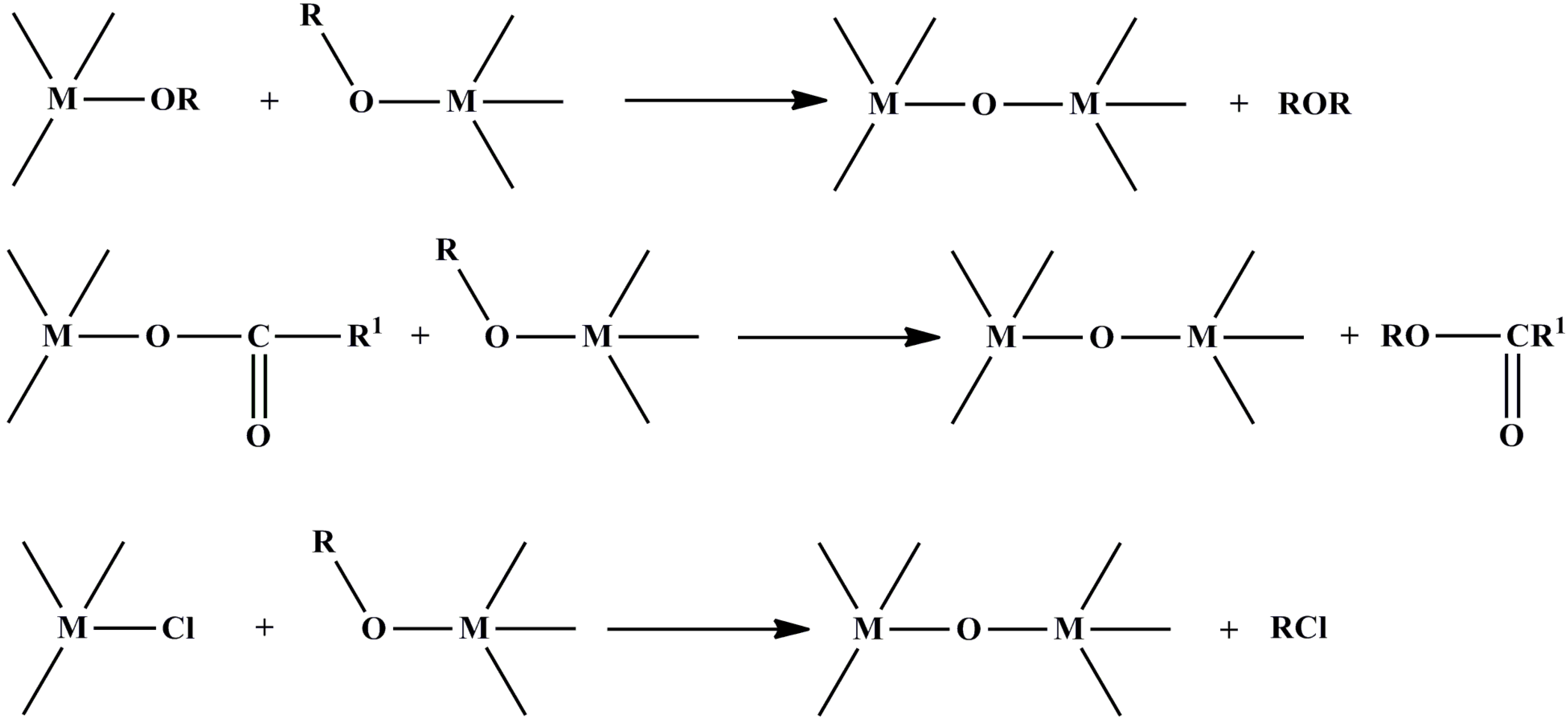
3. Synthesis of n-Type- and p-Type-Like Transition Metal Oxides by Wet Chemical Methods
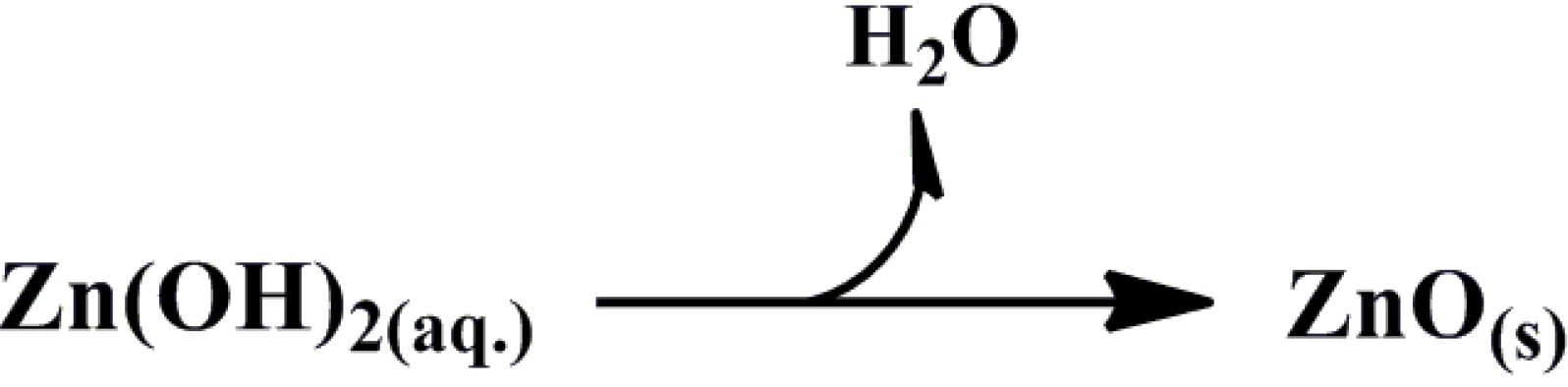
3.1. Zinc Oxide (ZnO)
3.1.1. Synthesis of Colloidal ZnO Nanoparticles (NPs)
3.1.2. Synthesis of Al3+-Doped ZnO Based on Precursor Solutions (sAZO)
3.2. Titanium Dioxide (TiO2)
3.2.1. Synthesis of Colloidal TiO2 Nanoparticles (NPs)
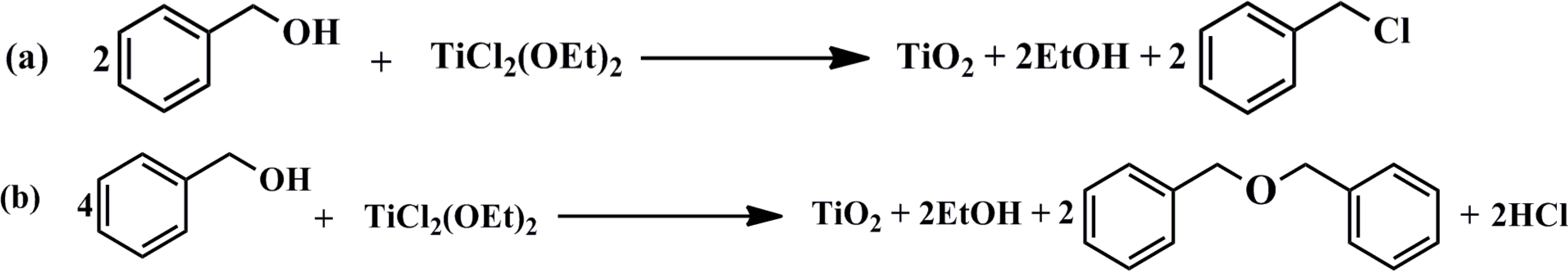
3.2.2. Synthesis of TiO2 Based on Precursor Solution (sTiO2)
3.3. Synthesis of p-Type-Like Transition Metal Oxides by Wet Chemical Methods
4. Requirements for Interface Metal Oxide Materials
5. Development of Inverted BHJ Solar Cell
5.1. Device Structure
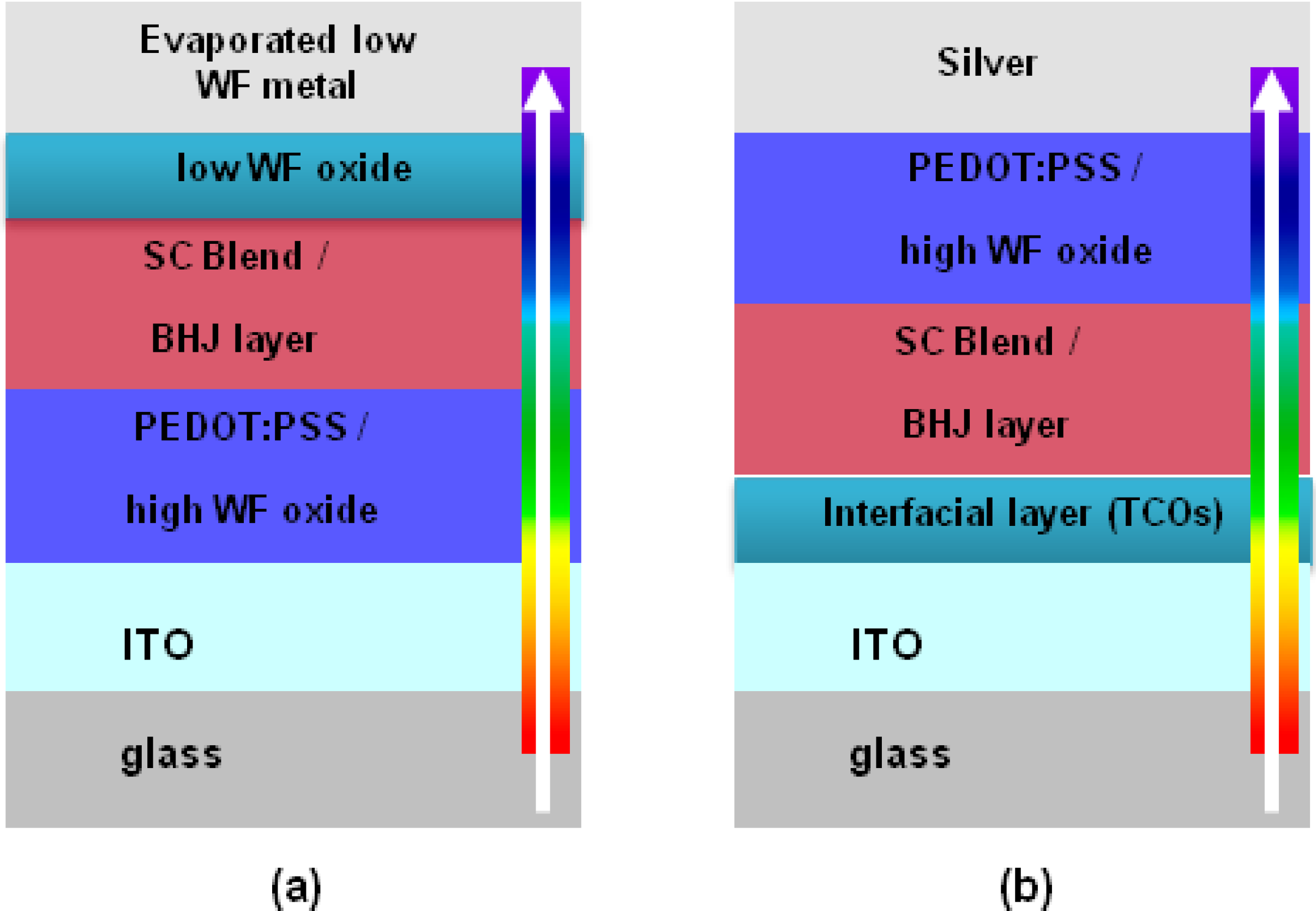
5.2. ZnO, Al3+-Doped ZnO and TiO2 as the Cathode Interlayer in an Inverted Solar Cell
| Reference (N°) | EEL/ETL | VOC (V) | JSC (mA/cm2) | FF% | PCE% |
|---|---|---|---|---|---|
| [17] (P3HT:PCBM) | ZnO NP | 0.62 | 11.17 | 54.3 | 3.78 |
| [23] (P3HT:PCBM) | ZnO NP | 0.56 | 8.33 | 56.5 | 2.62 |
| [24] (P3HT:PCBM) | ZnO NP | 0.56 | 8.36 | 53.9 | 2.56 |
| [16] (P3HT:PCBM) | s(ZnO) | 0.55 | 9.23 | 51.8 | 2.65 |
| [20] (P3HT:PCBM) | s(ZnO) | 0.55 | 8.86 | 45.5 | 2.21 |
| [23] (P3HT:PCBM) | s(AZO) | 0.57 | 9.57 | 47.7 | 2.59 |
| [24] (P3HT:PCBM) | s(AZO) | 0.57 | 8.36 | 50.8 | 2.42 |
| [23] (P3HT:PCBM) | TiO2∙NP | 0.57 | 8.38 | 54.4 | 2.58 |
| [18] (P3HT:PCBM) | s(EA-TiOx) | 0.56 | 6.70 | 55.0 | 2.06 |
| [18] (P3HT:PCBM) | s(DEA-TiOx) | 0.55 | 5.31 | 36.0 | 1.06 |
| [18] (P3HT:PCBM) | s(AA-TiOx) | 0.55 | 7.00 | 60.0 | 2.31 |
| [21] (P3HT:PCBM) | sTiO2 | 0.55 | 9.06 | 51.9 | 2.61 |
5.3. MoO3, WO3 and V2O5 as the Anode Interlayer in an Inverted Solar Cell
| Reference (N°) | HEL/HTL | VOC (V) | JSC (mA/cm2) | FF% | PCE% |
|---|---|---|---|---|---|
| [26] (P3HT:PCBM) | V2O5 NP | 0.56 | 10.4 | 66.0 | 3.80 |
| [26] (P3HT:PCBM) | sV2O5 | 0.52 | 9.50 | 60.0 | 3.00 |
| [22] (P3HT:PCBM) | sVOx | 0.57 | 10.1 | 67.0 | 3.90 |
| [20] (P3HT:PCBM) | eMoO3 | 0.55 | 8.86 | 45.5 | 2.21 |
| [25] (PCDTBT:PC70BM) | eMoO3 | 0.88 | 10.4 | 68.8 | 6.08 |
| [9] con. (P3HT:PCBM) | sMoO3 | 0.57 | 7.96 | 66.7 | 2.92 |
| [11] con. (P3HT:PCBM) | sWO3 | 0.62 | 8.63 | 63.0 | 3.37 |
| [28] (P3HT/PCDTBT:PCBM) | sWO3 | 0.53 | 8.56 | 52.6 | 2.68 |
| [12] (P3HT/PCDTBT:PCBM) | sWO3 | 0.54 | 8.50 | 51.3 | 2.40 |
6. Device Stability
7. Summary and Outlook
Acknowledgments
Conflicts of Interest
References
- Li, J.; Yu, H.-Y. Enhancement of Si-Based Solar Cell Efficiency via Nanostructure Integration. In Energy Eficiency and Renewable Energy through Nanotechnology; Zang, L., Ed.; Springer-Verlag: London, UK, 2011; Volume 2011, pp. 3–55. [Google Scholar]
- Fung, D.D.S.; Choy, W.C.H. Introduction to Organic Solar Cells. In Organic Solar Cells: Materials and Device Physics; Choy, W.C.H., Ed.; Springer-Verlag: London, UK, 2013; Volume 2013, pp. 1–16. [Google Scholar]
- Kim, J.Y.; Kim, S.H.; Lee, H.H.; Lee, K.; Ma, W.; Gong, X.; Heeger, A.J. New architecture for high-efficiency polymer photovoltaic cells using solution-based titanium oxide as an optical spacer. Adv. Mater. 2006, 18, 572–576. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Roy, A.; Park, S.H.; Cowan, S.; Tong, M.H.; Cho, S.; Lee, K.; Heeger, A.J. Titanium suboxide as an optical spacer in polymer solar cells. Appl. Phys. Lett. 2009, 95, 013302:1–013302:3. [Google Scholar]
- Brabec, C.J.; Gowrisanker, S.; Halls, J.J.; Laird, D.; Jia, S.; Williams, S.P. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2010, 22, 3839–3856. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shao, S.; Guo, X.; Zhao, Y.; Xie, Z. Efficient polymer photovoltaic cells using solution-processed MoO3 as anode buffer layer. Sol. Energy Mater. Sol. Cells 2010, 94, 842–845. [Google Scholar] [CrossRef]
- Girotto, C.; Voroshazi, E.; Cheyns, D.; Heremans, P.; Rand, B.P. Solution-processed MoO3 thin films as a hole-injection layer for organic solar cells. ACS Appl. Mater. Interfaces 2011, 3, 3244–3247. [Google Scholar] [CrossRef] [PubMed]
- Stubhan, T.; Ameri, T.; Salinas, M.; Krantz, J.; Machui, F.; Halik, M.; Brabec, C.J. High shunt resistance in polymer solar cells comprising a MoO3 hole extraction layer processed from nanoparticle suspension. Appl. Phys. Lett. 2011, 98, 253308:1–253308:3. [Google Scholar] [CrossRef]
- Zilberberg, K.; Trost, S.; Schmidt, H.; Riedl, T. Solution processed vanadium pentoxide as charge extraction layer for organic solar cells. Adv. Energy Mater. 2011, 1, 377–381. [Google Scholar] [CrossRef]
- Choi, H.; Kim, B.; Ko, M.J.; Lee, D.-K.; Kim, H.; Kim, S.H.; Kim, K. Solution processed WO3 layer for the replacement of PEDOT:PSS layer in organic photovoltaic cells. Org. Electron. 2012, 13, 959–968. [Google Scholar] [CrossRef]
- Stubhan, T.; Li, N.; Luechinger, N.A.; Halim, S.C.; Matt, G.J.; Brabec, C.J. High fill factor polymer solar cells incorporating a low temperature solution processed WO3 hole extraction layer. Adv. Energy Mater. 2012, 2, 1433–1438. [Google Scholar] [CrossRef]
- Zilberberg, K.; Gharbi, H.; Behrendt, A.; Trost, S.; Riedl, T. Low-temperature, solution-processed MoOx for efficient and stable organic solar cells. ACS Appl. Mater. Interfaces 2012, 4, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Hancox, I.; Rochford, L.A.; Clare, D.; Walker, M.; Mudd, J.J.; Sullivan, P.; Schumann, S.; McConville, C.F.; Jones, T.S. Optimization of a high work function solution processed vanadium oxide hole-extracting layer for small molecule and polymer organic photovoltaic cells. J. Phys. Chem. C 2013, 117, 49–57. [Google Scholar] [CrossRef]
- Waldauf, C.; Morana, M.; Denk, P.; Schilinsky, P.; Coakley, K.; Choulis, S.A.; Brabec, C.J. Highly efficient inverted organic photovoltaics using solution based titanium oxide as electron selective contact. Appl. Phys. Lett. 2006, 89, 233517:1–233517:3. [Google Scholar] [CrossRef]
- White, M.S.; Olson, D.C.; Shaheen, S.E.; Kopidakis, N.; Ginley, D.S. Inverted bulk-heterojunction organic photovoltaic device using a solution-derived ZnO underlayer. Appl. Phys. Lett. 2006, 89, 143517:1–143517:3. [Google Scholar]
- Hau, S.K.; Yip, H.-L.; Baek, N.S.; Zou, J.; O’Malley, K.; Jen, A.K.Y. Air-stable inverted flexible polymer solar cells using zinc oxide nanoparticles as an electron selective layer. Appl. Phys. Lett. 2008, 92, 253301:1–253301:3. [Google Scholar] [CrossRef]
- Kuwabara, T.; Nakayama, T.; Uozumi, K.; Yamaguchi, T.; Takahashi, K. Highly durable inverted-type organic solar cell using amorphous titanium oxide as electron collection electrode inserted between ITO and organic layer. Sol. Energy Mater. Sol. Cells 2008, 92, 1476–1482. [Google Scholar] [CrossRef]
- Tao, C.; Ruan, S.; Xie, G.; Kong, X.; Shen, L.; Meng, F.; Liu, C.; Zhang, X.; Dong, W.; Chen, W. Role of tungsten oxide in inverted polymer solar cells. Appl. Phys. Lett. 2009, 94, 043311:1–043311:3. [Google Scholar]
- Park, H.-J.; Lee, K.-H.; Kumar, B.; Shin, K.-S.; Jeong, S.-W.; Kim, S.-W. Inverted organic solar cells with ZnO thin films prepared by sol–gel method. J. Nanoelectron. Optoelectron. 2010, 5, 135–138. [Google Scholar] [CrossRef]
- Bolognesi, M.; Sanchez-Diaz, A.; Ajuria, J.; Pacios, R.; Palomares, E. The effect of selective contact electrodes on the interfacial charge recombination kinetics and device efficiency of organic polymer solar cells. Phys. Chem. Chem. Phys. PCCP 2011, 13, 6105–6109. [Google Scholar] [CrossRef]
- Chen, C.-P.; Chen, Y.-D.; Chuang, S.-C. High-performance and highly durable inverted organic photovoltaics embedding solution-processable vanadium oxides as an interfacial hole-transporting layer. Adv. Mater. 2011, 23, 3859–3863. [Google Scholar] [PubMed]
- Oh, H.; Krantz, J.; Litzov, I.; Stubhan, T.; Pinna, L.; Brabec, C.J. Comparison of various sol–gel derived metal oxide layers for inverted organic solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 2194–2199. [Google Scholar] [CrossRef]
- Stubhan, T.; Oh, H.; Pinna, L.; Krantz, J.; Litzov, I.; Brabec, C.J. Inverted organic solar cells using a solution processed aluminum-doped zinc oxide buffer layer. Org. Electron. 2011, 12, 1539–1543. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted polymer solar cells integrated with a low-temperature-annealed sol-gel-derived ZnO film as an electron transport layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, K.; Trost, S.; Meyer, J.; Kahn, A.; Behrendt, A.; Lützenkirchen-Hecht, D.; Frahm, R.; Riedl, T. Inverted organic solar cells with sol-gel processed high work-function vanadium oxide hole-extraction layers. Adv. Funct. Mater. 2011, 21, 4776–4783. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.-H.; Pugazhendi, I.; Jeong, J.K.; Kim, S.-W. Inverted polymer solar cells with an electron transport layer treated by ultraviolet light irradiation. J. Photonic Sci. Technol. 2012, 2, 22–24. [Google Scholar]
- Li, N.; Stubhan, T.; Luechinger, N.A.; Halim, S.C.; Matt, G.J.; Ameri, T.; Brabec, C.J. Inverted structure organic photovoltaic devices employing a low temperature solution processed WO3 anode buffer layer. Org. Electron. 2012, 13, 2479–2484. [Google Scholar] [CrossRef]
- Li, N.; Stubhan, T.; Baran, D.; Min, J.; Wang, H.; Ameri, T.; Brabec, C.J. Design of the solution-processed intermediate layer by engineering for inverted organic multi junction solar cells. Adv. Energy Mater. 2013, 3, 301–307. [Google Scholar] [CrossRef]
- Chen, S.; Manders, J.R.; Tsang, S.-W.; So, F. Metal oxides for interface engineering in polymer solar cells. J. Mater. Chem. 2012, 22, 24202–24212. [Google Scholar] [CrossRef]
- Meyer, J.; Hamwi, S.; Kroger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Transition metal oxides for organic electronics: Energetics, device physics and applications. Adv. Mater. 2012, 24, 5408–5427. [Google Scholar] [CrossRef] [PubMed]
- Stadler, A. Transparent conducting oxides—An up-to-date overview. Materials 2012, 5, 661–683. [Google Scholar] [CrossRef]
- Minami, T. New n-type transparent conducting oxides. MRS Bull. 2000, 25, 38–44. [Google Scholar] [CrossRef]
- Bahnemann, D.W.; Kormann, C.; Hoffmann, M.R. Preparation and characterization of quantum size zinc oxide: A detailed spectroscopic study. J. Phys. Chem. 1987, 91, 3789–3798. [Google Scholar] [CrossRef]
- Spanhel, L.; Anderson, M.A. Semiconductor clusters in the sol-gel process: Quantized aggregation, gelation, and crystal growth in concentrated ZnO colloids. J. Am. Chem. Soc. 1991, 113, 2826–2833. [Google Scholar] [CrossRef]
- Vioux, A. Nonhydrolytic sol-gel routes to oxides. Chem. Mater. 1997, 9, 2292–2299. [Google Scholar] [CrossRef]
- Meulenkamp, E.A. Synthesis and growth of ZnO nanoparticles. J. Phys. Chem. B 1998, 102, 5566–5572. [Google Scholar] [CrossRef]
- Dong, W.; Dunn, B. Sol–gel synthesis and characterization of molybdenum oxide gels. J. Non-Cryst. Solids 1998, 225, 135–140. [Google Scholar] [CrossRef]
- Kruis Einar, F.; Fissan, H.; Peled, A. Synthesis of nanoparticles in the gas phase for electronic, optical and magnetic applications—A review. J. Aerosol Sci. 1998, 29, 511–535. [Google Scholar] [CrossRef]
- Ohyama, M.; Kozuka, H.; Yoko, T. Sol-gel preparation of transparent and conductive aluminum-doped zinc oxide films with highly preferential crystal orientattion. J. Am. Ceram. Soc. 1998, 81, 1622–1632. [Google Scholar] [CrossRef]
- Dong, W.; Dunn, B. Sol–gel synthesis of monolithic molybdenum oxide aerogels and xerogels. J. Mater. Chem. 1998, 8, 665–670. [Google Scholar] [CrossRef]
- Antonelli, D.M.; Trudeau, M. Phasenumwandlungen und elektronische Eigenschaften mesoporöser Molybdänoxide mit ringförmiger Struktur (in German). Angew. Chem. 1999, 111, 1555–1559. [Google Scholar] [CrossRef]
- Alam, M.J.; Cameron, D.C. Preparation and properties of transparent conductive aluminum-doped zinc oxide thin films by sol–gel process. J. Vac. Sci. Technol. Vac. Surf. Films 2001, 19, 1642–1646. [Google Scholar] [CrossRef]
- Pacholski, C.; Kornowski, A.; Weller, H. Self-assembly of ZnO: From nanodots to nanorods. Angew. Chem. Int. Ed. 2002, 41, 1188–1191. [Google Scholar] [CrossRef]
- Noack, V.; Eychmüller, A. Annealing of nanometer-sized zinc oxide particles. Chem. Mater. 2002, 14, 1411–1417. [Google Scholar] [CrossRef]
- Pinna, N.; Wild, U.; Urban, J.; Schlögl, R. Divanadium pentoxide nanorods. Adv. Mater. 2003, 15, 329–331. [Google Scholar] [CrossRef]
- Fukumura, T.; Toyosaki, H.; Yamada, Y. Magnetic oxide semiconductors. Semicond. Sci. Technol. 2005, 20, 103–111. [Google Scholar] [CrossRef]
- Joo, J.; Kwon, S.G.; Yu, J.A.; Hyeon, T. Synthesis of ZnO nanocrystals with cone, hexagonal cone, and rod shapes via non-hydrolytic ester elimination sol-gel reactions. Adv. Mater. 2005, 17, 1873–1877. [Google Scholar] [CrossRef]
- Cheng, B.; Shi, W.; Russell-Tanner, J.M.; Zhang, L.; Samulski, E.T. Synthesis of variable-aspect-ratio, single-crystalline ZnO nanostructures. Inorg. Chem. 2006, 45, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, Y.; Rong, G.; Jing, M.; Xie, Y. Vanadium pentoxide nanobelts and nanorolls: From controllable synthesis to investigation of their electrochemical properties and photocatalytic activities. Nanotechnology 2006, 17, 2560–2566. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, X.; Li, Y.; Sulieman, K.M.; Sun, F.; He, X. Selective growth and properties of zinc oxide nanostructures. Scr. Mater. 2006, 55, 795–798. [Google Scholar] [CrossRef]
- Spanhel, L. Colloidal ZnO nanostructures and functional coatings: A survey. J. Sol-Gel Sci. Technol. 2006, 39, 7–24. [Google Scholar] [CrossRef]
- Clavel, G.; Willinger, M.G.; Zitoun, D.; Pinna, N. Solvent dependent shape and magnetic properties of doped ZnO nanostructures. Adv. Funct. Mater. 2007, 17, 3159–3169. [Google Scholar] [CrossRef]
- Wang, G.; Ji, Y.; Zhang, L.; Zhi, Y.; Gouma, P.-I.; Dudley, M. Synthesis of molybdenum oxide nanoplatelets during crystallization of the precursor gel from its hybrid nanocomposites. Chem. Mater. 2007, 19, 979–981. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous sol-gel routes to metal oxide nanoparticles. Acc. Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Pinna, N.; Niederberger, M. Tensidfreie nichtwässrige synthese von metalloxid–nanostrukturen (in German). Angew. Chem. 2008, 120, 5372–5385. [Google Scholar] [CrossRef]
- Kim, W.-S.; Kim, H.-C.; Hong, S.-H. Gas sensing properties of MoO3 nanoparticles synthesized by solvothermal method. J. Nanopart. Res. 2009, 12, 1889–1896. [Google Scholar] [CrossRef]
- Wei, G.; Qin, W.; Zhang, D.; Wang, G.; Kim, R.; Zheng, K.; Wang, L. Synthesis and field emission of MoO3 nanoflowers by a microwave hydrothermal route. J. Alloy. Compd. 2009, 481, 417–421. [Google Scholar]
- Yu, X.; Xu, Z.; Han, S.; Che, H.; Yan, X.; Liu, A. Synthesis of well-ordered lamellar mesoporous molybdenum oxide. Colloids Surf. Physicochem. Eng. Asp. 2009, 333, 194–198. [Google Scholar] [CrossRef]
- Grote, C.; Garnweitner, G. Die nicht-wässrige sol-gel-synthese-eine alternative für die herstellung hochqualitativer metalloxid- nanopartikel (in German). Chem. Ing. Tech. 2010, 82, 615–622. [Google Scholar] [CrossRef]
- Pinna, N.; Karmaoui, M.; Willinger, M.-G. The “benzyl alcohol route”: An elegant approach towards doped and multimetal oxide nanocrystals. J. Sol-Gel Sci. Technol. 2010, 57, 323–329. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Madler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef] [PubMed]
- Tari, O.; Aronne, A.; Addonizio, M.L.; Daliento, S.; Fanelli, E.; Pernice, P. Sol–gel synthesis of ZnO transparent and conductive films: A critical approach. Sol. Energy Mater. Sol. Cells 2012, 105, 179–186. [Google Scholar] [CrossRef]
- Niederberger, M.; Pinna, N. Metal Oxide Nanoparticles in Organic Solvents: Synthesis, Formation, Assembly and Application; Springer-Verlag: London, UK, 2009; pp. 7–18. [Google Scholar]
- Bhat, J.S.; Patil, A.S.; Swami, N.; Mulimani, B.G.; Gayathri, B.R.; Deshpande, N.G.; Kim, G.H.; Seo, M.S.; Lee, Y.P. Electron irradiation effects on electrical and optical properties of sol-gel prepared ZnO films. J. Appl. Phys. 2010, 108, 043513:1–043513:8. [Google Scholar] [CrossRef]
- Djurisic, A.B.; Leung, Y.H. Optical properties of ZnO nanostructures. Small 2006, 2, 944–961. [Google Scholar] [PubMed]
- Morkoc, H.; Özgür, Ü. Zinc Oxide: Fundamentals, Materials and Device Technology; WILEY-VCH Verlag GmbH&Co: Weinheim, Germany, 2009; pp. 1–70. [Google Scholar]
- Oprea, O.; Andronescu, E.; Vasile, B.C.; Voicu, G.; Covaliu, C. Synthesis and characterization of ZnO nanopowder by non-basic route. Dig. J. Nanomater. Biostruct. 2011, 6, 1393–1401. [Google Scholar]
- Briois, V.; Giorgetti, C.H.; Baudelet, F.; Blanchandin, S.; Tokumoto, M.S.; Pulcinelli, S.H.; Santilli, C.V. Dynamical study of ZnO nanocrystal and Zn-HDS layered basic zinc acetate formation from sol-gel route. J. Phys. Chem. C 2007, 111, 3253–3258. [Google Scholar]
- Krebs, F.C.; Thomann, Y.; Thomann, R.; Andreasen, J.W. A simple nanostructured polymer/ZnO hybrid solar cell-preparation and operation in air. Nanotechnology 2008, 19, 424013:1–424013:12. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zeng, H. ZnO-based transparent conductive thin films: Doping, performance, and processing. J. Nanomater. 2013, 2013, 1–9. [Google Scholar]
- Banerjee, S.; Gopal, J.; Muraleedharan, P.; Tyagi, A.K.; Raj, B. Physics and chemistry of photocatalytic titanium dioxide: Visualization of bactericidal activity using atomic force microscopy. Curr. Sci. 2006, 90, 1378–1383. [Google Scholar]
- Jing, L.; Li, S.; Song, S.; Xue, L.; Fu, H. Investigation on the electron transfer between anatase and rutile in nano-sized TiO2 by means of surface photovoltage technique and its effects on the photocatalytic activity. Sol. Energy Mater. Sol. Cells 2008, 92, 1030–1036. [Google Scholar] [CrossRef]
- Banerjee, A.N. The design, fabrication, and photocatalytic utility of nanostructured semiconductors: Focus on TiO2-based nanostructures. Nanotechnol. Sci. Appl. 2011, 4, 35–65. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.R.; Meyer, J.; Widjonarko, N.E.; Ndione, P.F.; Sigdel, A.K.; Garcia, A.; Miedaner, A.; Lloyd, M.T.; Kahn, A.; Ginley, D.S. Low-temperature, solution-processed molybdenum oxide hole-collection layer for organic photovoltaics. J. Mater. Chem. 2012, 22, 3249–3254. [Google Scholar] [CrossRef]
- Coutt, T.J.; Mason, T.O.; Perkins, J.D.; Ginley, D.S. Transparent Conducting Oxides: Status and Opportunities in Basic Research. In Photovoltaics for the 21st Century: Proceedings of the International Symposium; Kapur, V.K., McConnell, R.D., Carlson, D., Ceasar, G.P., Rohatgi, A., Eds.; The Electrochemical Society: Philadelphia, PA, USA, 1999; Volume 1999, pp. 274–286. [Google Scholar]
- Yip, H.-L.; Jen, A.K.Y. Recent advances in solution-processed interfacial materials for efficient and stable polymer solar cells. Energy Environ. Sci. 2012, 5, 5994–6011. [Google Scholar] [CrossRef]
- Ecker, B.; Egelhaaf, H.-J.; Steim, R.; Parisi, J.; von Hauff, E. Understanding S-shaped current–voltage characteristics in organic solar cells containing a TiOx interlayer with impedance spectroscopy and equivalent circuit analysis. J. Phys. Chem. 2012, 116, 16333–16337. [Google Scholar]
- Meyer, J.; Zilberberg, K.; Riedl, T.; Kahn, A. Electronic structure of Vanadium pentoxide: An efficient hole injector for organic electronic materials. J. Appl. Phys. 2011, 110, 033710:1–033710:5. [Google Scholar] [CrossRef]
- Meyer, J.; Khalandovsky, R.; Gorrn, P.; Kahn, A. MoO3 films spin-coated from a nanoparticle suspension for efficient hole-injection in organic electronics. Adv. Mater. 2011, 23, 70–73. [Google Scholar]
- Hauch, J.A.; Schilinsky, P.; Choulis, S.A.; Rajoelson, S.; Brabec, C.J. The impact of water vapor transmission rate on the lifetime of flexible polymer solar cells. Appl. Phys. Lett. 2008, 93, 103306:1–103306:3. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Litzov, I.; Brabec, C.J. Development of Efficient and Stable Inverted Bulk Heterojunction (BHJ) Solar Cells Using Different Metal Oxide Interfaces. Materials 2013, 6, 5796-5820. https://doi.org/10.3390/ma6125796
Litzov I, Brabec CJ. Development of Efficient and Stable Inverted Bulk Heterojunction (BHJ) Solar Cells Using Different Metal Oxide Interfaces. Materials. 2013; 6(12):5796-5820. https://doi.org/10.3390/ma6125796
Chicago/Turabian StyleLitzov, Ivan, and Christoph J. Brabec. 2013. "Development of Efficient and Stable Inverted Bulk Heterojunction (BHJ) Solar Cells Using Different Metal Oxide Interfaces" Materials 6, no. 12: 5796-5820. https://doi.org/10.3390/ma6125796