Biosynthesis of Silver Nanoparticles Using Brown Marine Macroalga, Sargassum Muticum Aqueous Extract
Abstract
:1. Introduction
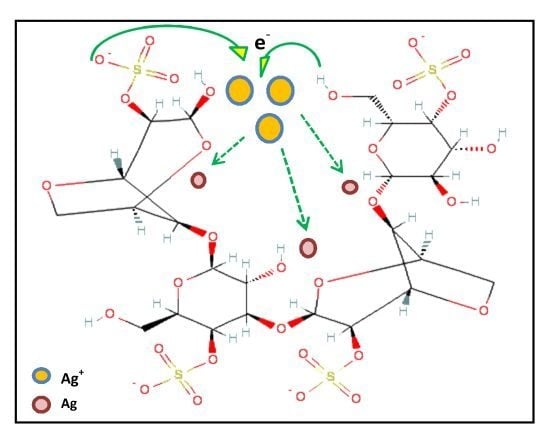
2. Results and Discussion
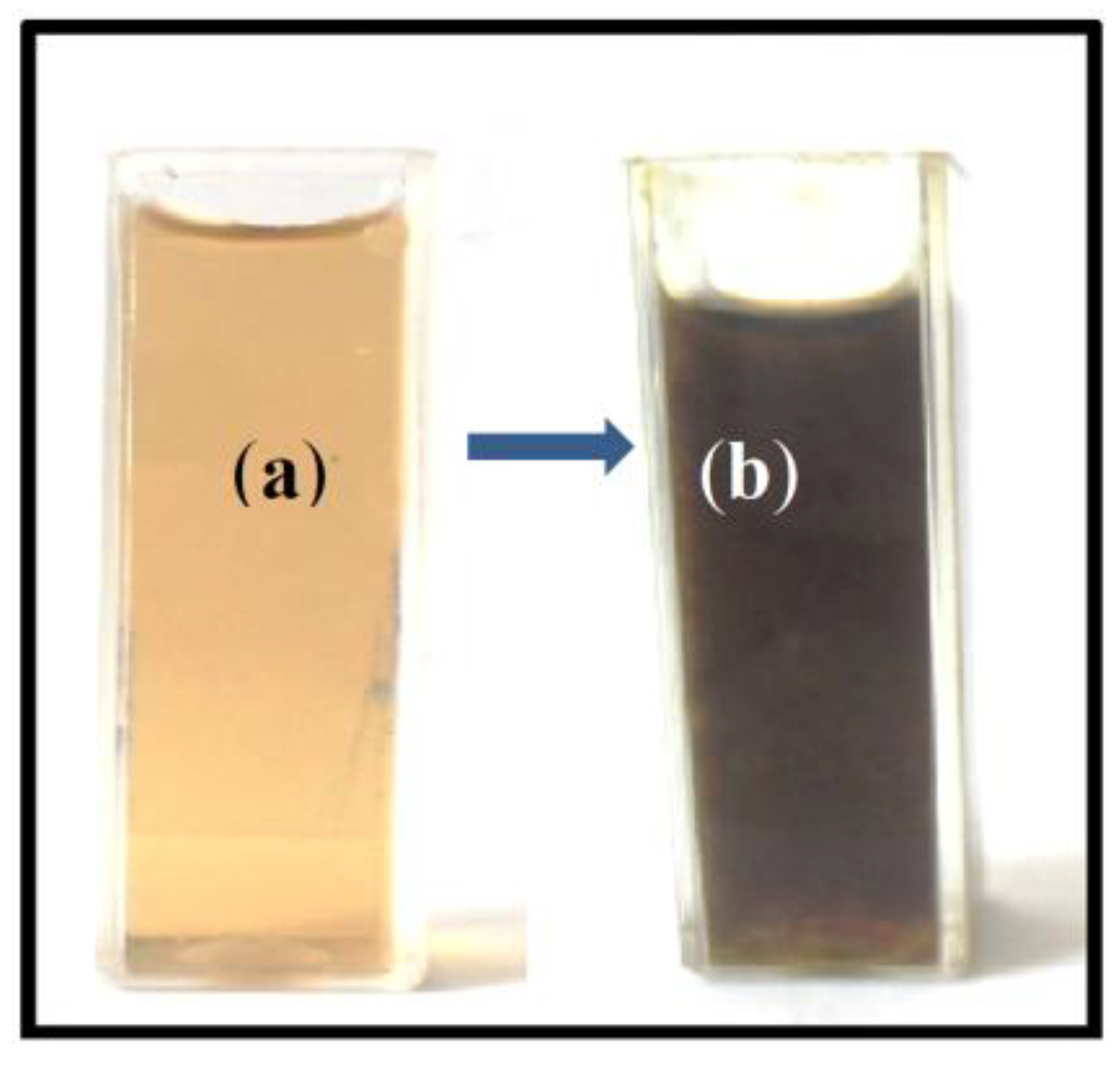
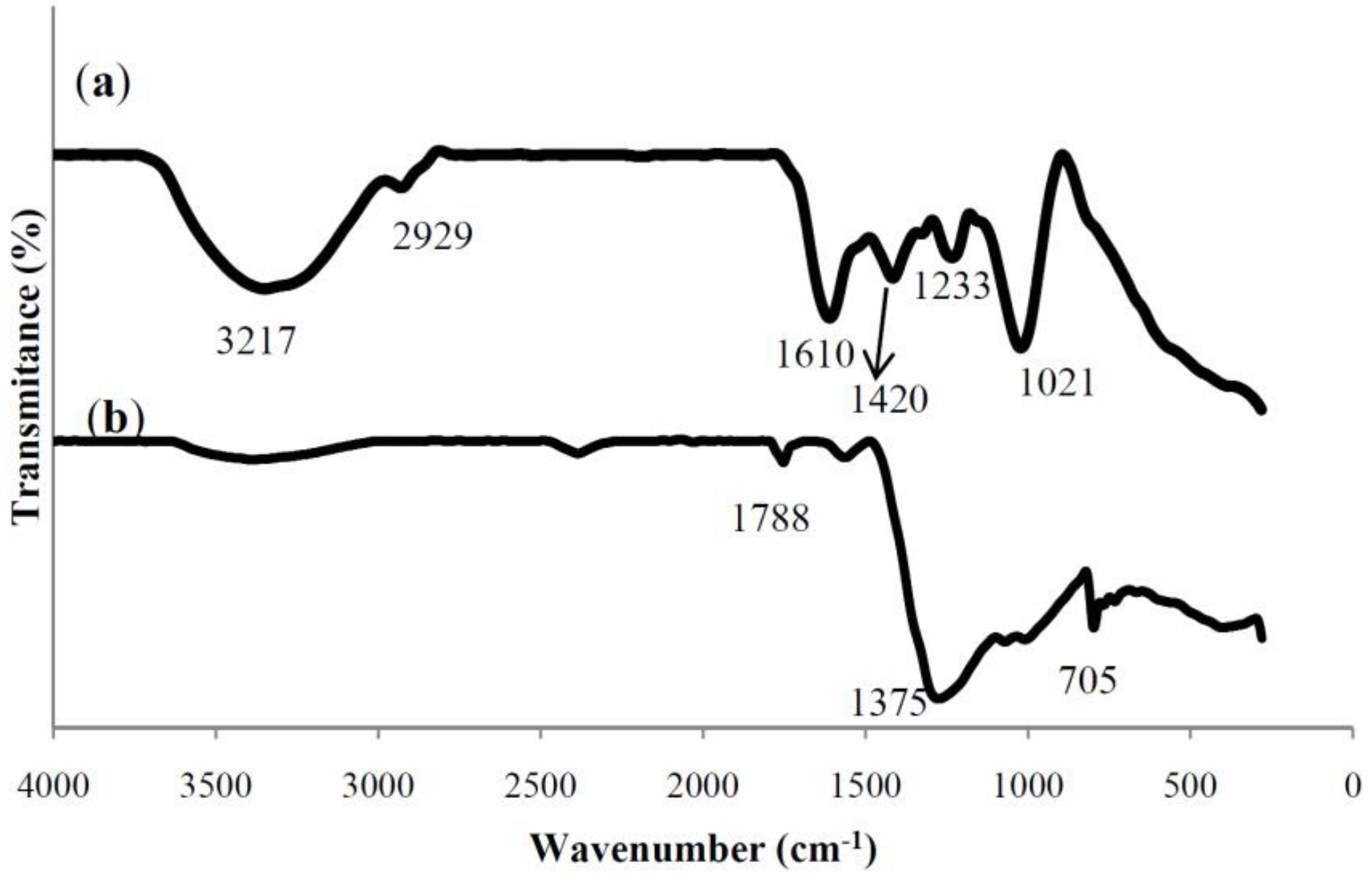
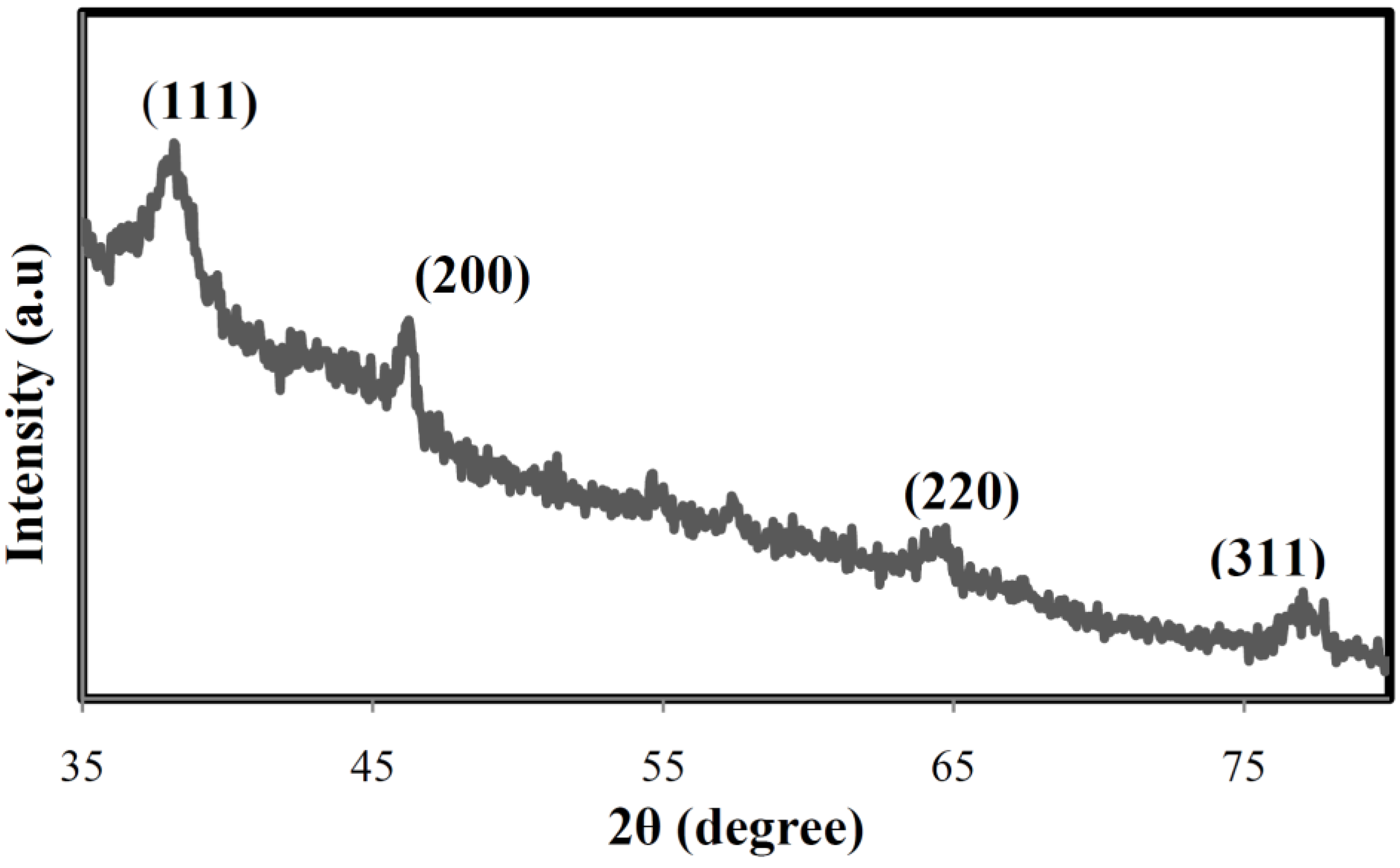
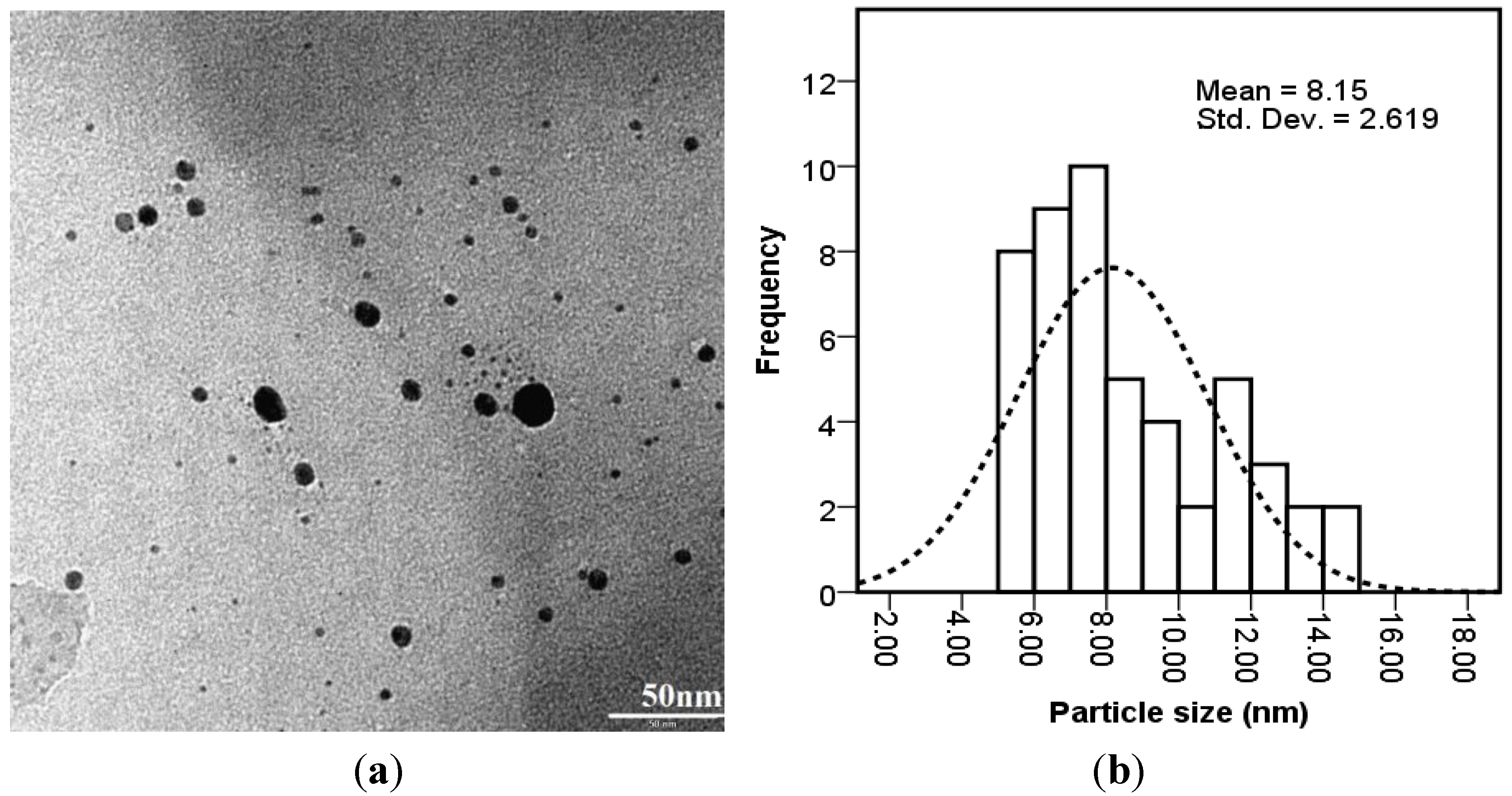
Characterization of Ag Nanoparticles

3. Experimental Section
3.1. Materials
3.2. Preparation of S. muticum Extracts
3.3. Biosynthesis of Ag-NPs
3.4. Characterization of Ag-NPs
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gopalakrishnan, K.; Ramesh, C.; Ragunathan, V.; Thamilselvan, M. Antibacterial activity of Cu2O nanoparticles on E.coli synthesized from tridax procumbens leaf extract and surface coating with polyaniline. Dig. J. Nanomater. Biostructure 2012, 7, 833–839. [Google Scholar]
- Darroudi, M.; Zak, A.M.; Muhamad, M.R.; Huang, N.M.; Hakimi, M. Green synthesis of colloidal silver nanoparticles by sonochemical method. Mater. Lett. 2012, 66, 117–120. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Ab Rahman, M.Z.; Amin, J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arockiya Aarthi Rajathi, F.; Parthiban, C.; Ganesh Kumar, V.; Anantharaman, P. Biosynthesis of antibacterial gold nanoparticles using brown alga, stoechospermum marginatum (kützing). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 99, 166–173. [Google Scholar]
- Shameli, K.; Ahmad, M.B.; Zamanian, A.; Sangpour, P.; Shabanzadeh, P.; Abdollahi., Y.; Zargar, M. Green biosynthesis of silver nanoparticles using Curcuma longa tuber powder. Int. J. Nanomed. 2012, 7, 5603–5610. [Google Scholar] [CrossRef]
- Govindaraju, K.; Kiruthiga, V.; Kumar, V.G.; Singaravelu, G. Extracellular synthesis of silver nanoparticles by a marine alga, Sargassum wightii Grevilli and their antibacterial effects. J. Nanosci. Nanotechnol. 2009, 9, 5497–5501. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, N.; Wong, C.W.; Yasumira, A.A.N.E. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. E. J. Chem. 2009, 6, 61–70. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’Souza, S.F. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf. B. Biointerf. 2006, 47, 160–164. [Google Scholar] [CrossRef]
- Willner, I.; Basnar, B.; Willner, B. Nanoparticle-enzyme hybrid systems for nanobiotechnology. FEBS J. 2007, 4, 302–309. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.; Adil, S.F.; Tahir, M.N.; Tremel, W.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R. Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int. J. Nanomed. 2013, 8, 1507–1516. [Google Scholar]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Namvar, F.; Suhaila, M.; Fard, S.G.; Behravan, J. Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chem. 2012, 130, 376–382. [Google Scholar] [CrossRef]
- Zuercher, A.W.; Fritsché, R.; Corthésy, B.; Mercenier, A. Food products and allergy development, prevention and treatment. Curr. Opin. Biotechnol. 2006, 17, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.R.M.; Zavala, S.M.; Perez, G.S.; Perez, G.C. Antidiabetic effect of compounds isolated from plants. Phytomedicine 1998, 5, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Fukuda, A.; Nagumo, T.; Fujihara, M.; Kaji, E. Inhibition of the generation of thrombin and factor Xa by a fucoidan from the brown seaweed Ecklonia kurome. Thromb. Res. 1999, 96, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K. The carotenoid fucoxanthin from brown seaweed affects obesity. Lipid Technol. 2009, 21, 186–190. [Google Scholar] [CrossRef]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Wada, K.; Nakamura, K.; Tamai, Y. Seaweed intake and blood pressure levels in healthy pre-school Japanese children. Nutr. J. 2011, 10, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Namvar, F.; Ahmad, M.B.; Mohamad, R. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 2013, 18, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Cheng, C.; Ma, Y.L.; Zhao, C.S. Preparation of silver nanoparticles with antimicrobial activities and the researches of their biocompatibilities. J. Mater. Sci. Mater. Med. 2010, 21, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Mohan, Y.M.; Lee, K.J.; Premkumar, T.; Geckeler, K.E. Hydrogel networks as nanoreactors: A novel approach to silver nanoparticles for antibacterial applications. Polymer 2007, 48, 158–164. [Google Scholar] [CrossRef]
- Feng, X.; Qi, X.; Li, J.; Yang, L.W.; Qiu, M.C.; Yin, J.J.; Lu, F.; Zhong, J.X. Preparation structure and photo-catalytic performances of hybrid Bi2SiO5 modified Si nanowire arrays. Appl. Surf. Sci. 2011, 257, 5571–5575. [Google Scholar] [CrossRef]
- Hayward, R.C.; Saville, D.A.; Aksay, I.A. Electrophoretic assembly of colloidal crystals with optically tunable micropatterns. Nature 2000, 404, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Lohse, S.E.; Murphy, C.J. Applications of colloidal inorganic nanoparticles: From medicine to energy. J. Am. Chem. Soc. 2012, 134, 15607–15620. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Han, C.; Wang, X.; Zheng, Y.; Li, Q.; Hu, X.; Sun, H. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol. Biol. Rep. 2012, 39, 9193–9201. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.R.; Silva, S.; Negri, M.; Gorup, L.F.; de Camargo, E.R.; Oliveira, R.; Barbosa, D.B.; Henriques, M. Silver nanoparticles: Influence of stabilizing agent and diameter on antifungal activity against Candida albicans and Candida glabrata biofilms. Lett. Appl. Microbiol. 2012, 54, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gutierrez, F.; Thi, E.P.; Silverman, J.M.; de Oliveira, C.C.; Svensson, S.L.; Vanden Hoek, A.; Sánchez, E.M.; Reiner, N.E.; Gaynor, E.C.; Pryzdial, E.L.; et al. Antibacterial activity, inflammatory response, coagulation and cytotoxicity effects of silver nanoparticles. Nanomedicine 2012, 8, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Im, J.; Shin, M.; Min, Y.; Park, J.; Cho, H.; Park, S.; Shim, M.-B.; Jeon, S.; Chung, D.-Y.; et al. Highly stretchable electric circuits from a composite material of silver nanoparticles and elastomeric fibres. Nat. Nanotechnol. 2012, 7, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.; Shukla, S.; Perkas, N.; Solovyov, L.A.; Nitzan, Y.; Gedanken, A. Sonochemical coating of paper by microbiocidal silver nanoparticles. Langmuir 2011, 27, 720–726. [Google Scholar] [PubMed]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Lee, Ch.J.; Karim, M.R.; Vasudevan, T.; Kim, H.J.; Raushan, K.; Jung, M.J.; Kim, D.Y.; Lee, M.S. A comparison method of silver nanoparticles prepared by the gamma irradiation and in situ reduction methods. Bull. Korea Chem. Soc. 2010, 31, 1993–1996. [Google Scholar] [CrossRef]
- Camara, R.B.G.; Costa, S.L.; Fidelis, G.P. Heterofucans from the brown seaweed canistrocarpus cervicornis with anticoagulant and antioxidant activities. Marine Drugs 2011, 9, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. lett 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Venkatpurwar, V.; Pokharkar, V. Green synthesis of silver nanoparticles using marine polysaccharide: Study of in-vitro antibacterial activity. Mater. Lett. 2011, 65, 999–1002. [Google Scholar] [CrossRef]
- Shen, L.M.; Bao, N.Z.; Yanagisawa, K. Direct synthesis of ZnO nanoparticles by a solution-free mechanochemical reaction. Nanotechnology 2006, 17, 5117–5123. [Google Scholar] [CrossRef]
- Shao, Y.; Jin, Y.; Dong, S. Synthesis of gold nanoparticles by aspartate reduction of gold chloride. Chem. Commun. 2004, 9, 1104–1105. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Azizi, S.; Namvar, F.; Mahdavi, M.; Ahmad, M.B.; Mohamad, R. Biosynthesis of Silver Nanoparticles Using Brown Marine Macroalga, Sargassum Muticum Aqueous Extract. Materials 2013, 6, 5942-5950. https://doi.org/10.3390/ma6125942
Azizi S, Namvar F, Mahdavi M, Ahmad MB, Mohamad R. Biosynthesis of Silver Nanoparticles Using Brown Marine Macroalga, Sargassum Muticum Aqueous Extract. Materials. 2013; 6(12):5942-5950. https://doi.org/10.3390/ma6125942
Chicago/Turabian StyleAzizi, Susan, Farideh Namvar, Mahnaz Mahdavi, Mansor Bin Ahmad, and Rosfarizan Mohamad. 2013. "Biosynthesis of Silver Nanoparticles Using Brown Marine Macroalga, Sargassum Muticum Aqueous Extract" Materials 6, no. 12: 5942-5950. https://doi.org/10.3390/ma6125942