Electron Density Modification of Single Wall Carbon Nanotubes (SWCNT) by Liquid-Phase Molecular Adsorption of Hexaiodobenzene
Abstract
:1. Introduction
2. Results and Discussion
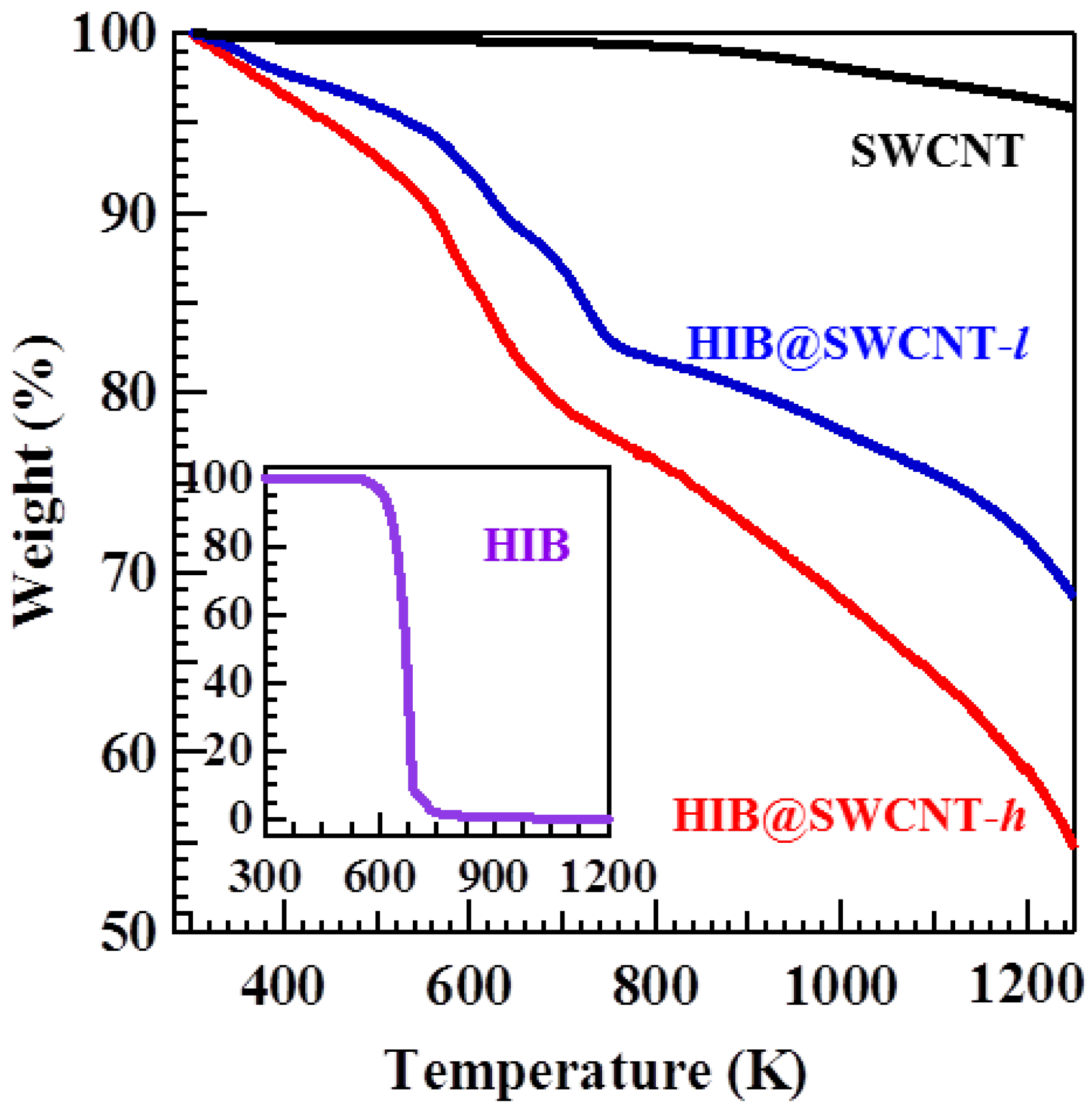
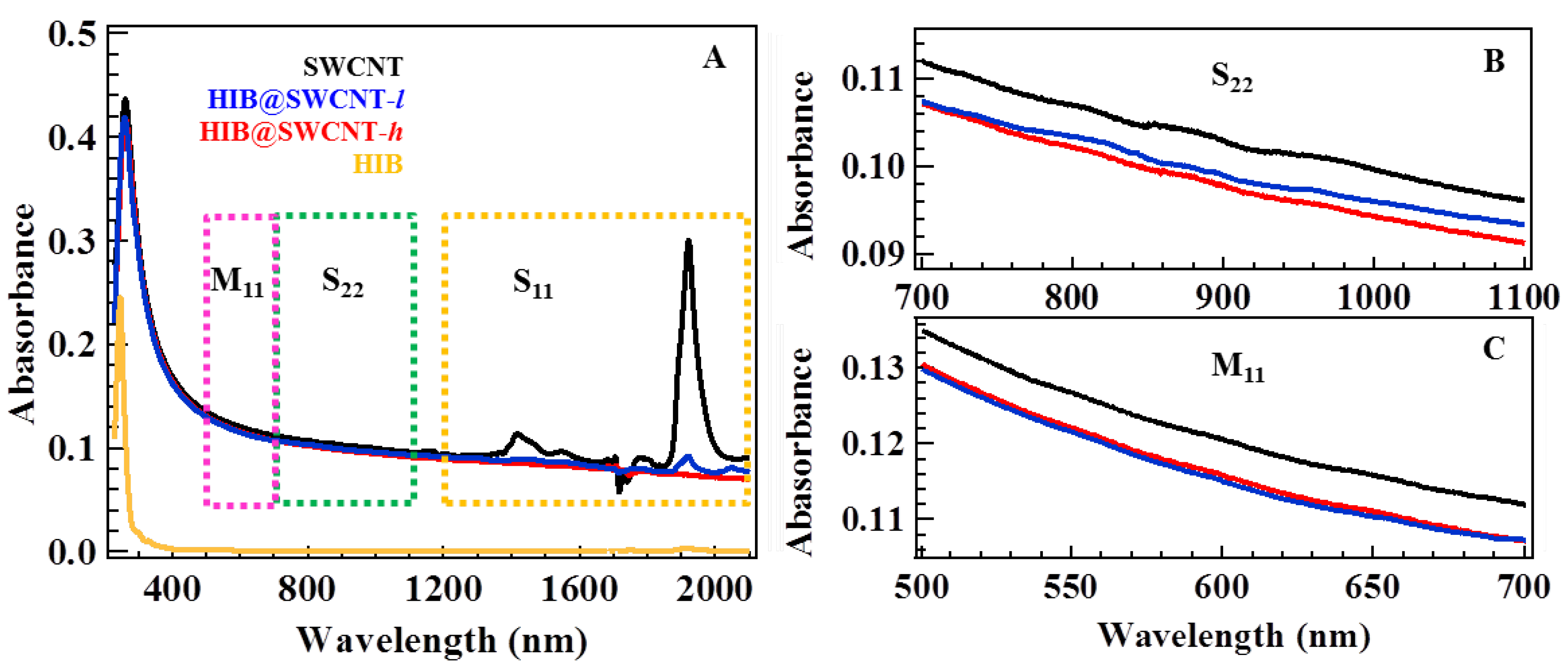
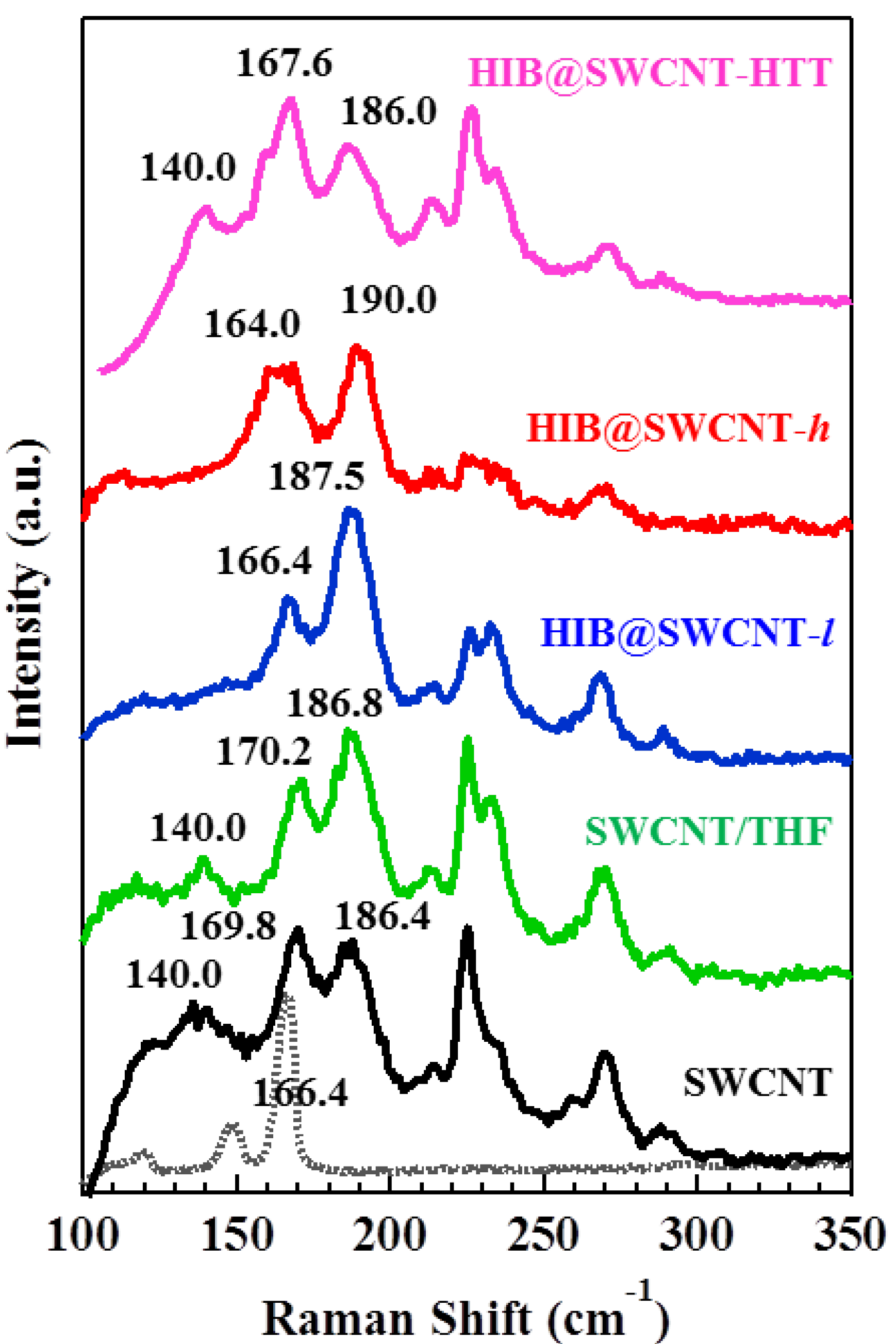

3. Experimental Section
4. Conclusions
Supplementary materials
Supplementary File 1Acknowledgments
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Odom, T.W.; Huang, J.L.; Kim, P.; Lieber, C.M. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 1998, 391, 62–64. [Google Scholar] [CrossRef]
- He, R.R.; Jin, H.Z.; Zhu, J.; Yan, Y.J.; Chen, X.H. Physical and electronic structure in carbon nanotubes. Chem. Phys. Lett. 1998, 298, 170–176. [Google Scholar] [CrossRef]
- Chen, P.; Wu, X.; Sun, X.; Lin, J.; Ji, W.; Tan, K.L. Electronic structure and optical limiting behavior of carbon nanotubes. Phys. Rev. Lett. 1999, 82, 2548–2551. [Google Scholar] [CrossRef]
- Shiraishi, M.; Swaraj, S.; Takenobu, T.; Iwasa, Y.; Ata, M.; Unger, W.E.S. Spectroscopic characterization of single-walled carbon nanotubes carrier-doped by encapsulation of TCNQ. Phys. Rev. B 2005, 71, 125419–125425. [Google Scholar] [CrossRef]
- Voggu, R.; Rout, C.S.; Franklin, A.D.; Fisher, T.S.; Rao, C.N.R. Extraordinary sensitivity of the electronic structure and properties of single-walled carbon nanotubes to molecular charge-transfer. Phys. Chem. C 2008, 112, 13053–13056. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Voggu, R. Charge-transfer with graphene and nanotubes. Mater. Today 2010, 13, 34–40. [Google Scholar] [CrossRef]
- Hayakawa, C.; Urita, K.; Ohba, T.; Kanoh, H.; Kaneko, K. Physico-chemical properties of iodine-adsorbed single-walled carbon nanotubes. Langmuir 2009, 25, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.M.; Eklund, P.C.; Bandow, S.; Thess, A.; Smalley, R.E. Evidence for charge transfer in doped carbon nanotube bundles from Raman scattering. Nature 1997, 388, 257–259. [Google Scholar] [CrossRef]
- Minami, N.; Kazaoui, S.; Jacquemin, R.; Yamawaki, H.; Aoki, K.; Kataura, H.; Achiba, Y. Optical properties of semiconducting and metallic single wall carbon nanotubes: Effects of doping and high pressure. Synth. Met. 2001, 116, 405–409. [Google Scholar] [CrossRef]
- Khoerunnisa, F.; Fujimori, T.; Itoh, T.; Kanoh, H.; Ohba, T.; Yudasaka, M.; Iijima, S.; Kaneko, K. Electronically modified single wall carbon nanohorns with iodine adsorption. Chem. Phys. Lett. 2001, 501, 485–490. [Google Scholar] [CrossRef]
- Eliseev, A.A.; Yashina, L.V.; Brzhezinskaya, M.M.; Chernysheva, M.V.; Kharlamova, M.V.; Verbitsky, N.I.; Lukashin, A.V.; Kiselev, N.A.; Kumskov, A.S.; Zakalyuhin, R.M.; Hutchison, J.L.; Freitag, B.; Vinogradov, A.S. Structure and electronic properties of AgX (X = Cl, Br, I)-intercalated single-walled carbon nanotubes. Carbon 2010, 48, 2708–2721. [Google Scholar] [CrossRef]
- Ghosh, S.; Yamijala, S.S.; Pati, S.K.; Rao, C.N.R. The interaction of halogen molecules with SWNTs and graphene. RSC Adv. 2012, 2, 1181–1188. [Google Scholar] [CrossRef]
- Jung, Y.; Hwang, S.J.; Kim, S.J. Spectroscopic evidence on weak electron transfer from intercalated iodine molecules to single-walled carbon nanotubes. J. Phys. Chem. C 2007, 111, 10181–10184. [Google Scholar] [CrossRef]
- Sagl, D.J.; Martin, J.C. The stable singlet ground state dication of hexaiodobenzene: Possibly a σ-delocalized dication. J. Am. Chem. Soc. 1988, 110, 5827–5833. [Google Scholar] [CrossRef]
- Havenith, R.W.A.; Fowler, P.W.; Fias, S.; Bultinck, P. Evidence from current-density mapping for σ-delocalisation in the aromatic hexaiodobenzene cation. Tetrahedron Lett. 2008, 49, 1421–1424. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical properties of single-wall carbon nanotubes. Synth. Met. 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Alvarez, L.; Righi, A.; Guillard, T.; Rols, S.; Anglaret, E.; Laplaze, D. Resonant raman study of the structure and electronic properties of single-wall carbon nanotubes. Chem. Phys. Lett. 2000, 316, 186–190. [Google Scholar] [CrossRef]
- Eliseev, A.A.; Kharlamova, M.V.; Chernysheva, M.V.; Lukashin, A.V.; Tretyakov, Y.D.; Kumskov, A.S.; Kiselev, N.A. Preparation and properties of single-walled nanotubes filled with inorganic compounds. Russ. Chem. Rev. 2009, 78, 833–854. [Google Scholar] [CrossRef]
- Gotovac, S.; Honda, H.; Hattori, Y.; Takahashi, K.; Kanoh, H.; Kaneko, K. Effect of nanoscale curvature of single-walled carbon nanotubes on adsorption of polycyclic aromatic hydrocarbons. Nano Lett. 2007, 7, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Botka, B.; Pekker, Á.; Botos, Á.; Kamarás, K.; Hackl, R. A systematic study of optical and Raman spectra of peapod-based DWNTs. Phys. Status Solidi B 2010, 247, 2843–2846. [Google Scholar] [CrossRef]
- Loi, M.A.; Gao, J.; Cordella, F.; Blondeau, P.; Menna, E.; Bártová, B.; Hébert, C.; Lazar, S.; Botton, G.A.; Milko, M.; Ambrosch-Draxl, C. Encapsulation of conjugated oligomers in single-walled carbon nanotubes: Towards nanohybrids for photonic devices. Adv. Mater. 2010, 22, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, S.; Honda, H.; Hattori, Y.; Kanoh, H.; Takahashi, K.; Sakai, H.; Abe, M.; Yudasaka, M.; Iijima, S.; Kaneko, K. Direct evidence on C–C single bonding in single-wall carbon nanohorn aggregates. J. Phys. Chem. C 2007, 111, 5572–5575. [Google Scholar] [CrossRef]
- Liu, J.; Zubiri, M.R.I.; Dossot, M.; Vigolo, B.; Hauge, R.H.; Fort, Y.; Ehrhardt, J.J.; McRae, E. Sidewall functionalization of single-wall carbon nanotubes (SWNTs) through aryl free radical addition. Chem. Phys. Lett. 2006, 430, 93–96. [Google Scholar] [CrossRef]
- Rosario-Castro, B.I.; Contes, E.J.; Perez-Davis, M.E.; Cabrera, C.R. Attachment of single-wall carbon nanotubes on platinum surfaces by self-assembling techniques. Rev. Adv. Mater. Sci. 2005, 10, 381–386. [Google Scholar]
- Wang, P.C.; Liao, Y.C.; Lai, Y.L.; Lin, Y.C.; Su, C.Y.; Tsai, C.H.; Hsu, Y.J. Conversion of pristine and p-doped sulfuric-acid-treated single-walled carbon nanotubes to n-type materials by a facile hydrazine vapor exposure process. Mater. Chem. Phys. 2012, 134, 325–332. [Google Scholar] [CrossRef]
- Mistry, K.S.; Larsen, B.A.; Bergeson, J.D.; Barnes, T.M.; Teeter, G.; Engtrakul, C.; Blackburn, J.L. n-Type transparent conducting films of small molecule and polymer amine doped single-walled carbon nanotubes. ACS Nano 2011, 5, 3714–3723. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-assisted highly efficient synthesis of impurity-free single-walled carbon nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lu, M.; Ohba, T.; Kaneko, K.; Hata, K.; Yumura, M.; Iijima, S.; Komatsu, H.; Sakuma, A.; Kanoh, H. Electron Density Modification of Single Wall Carbon Nanotubes (SWCNT) by Liquid-Phase Molecular Adsorption of Hexaiodobenzene. Materials 2013, 6, 535-543. https://doi.org/10.3390/ma6020535
Lu M, Ohba T, Kaneko K, Hata K, Yumura M, Iijima S, Komatsu H, Sakuma A, Kanoh H. Electron Density Modification of Single Wall Carbon Nanotubes (SWCNT) by Liquid-Phase Molecular Adsorption of Hexaiodobenzene. Materials. 2013; 6(2):535-543. https://doi.org/10.3390/ma6020535
Chicago/Turabian StyleLu, Mingxia, Tomonori Ohba, Katsumi Kaneko, Kenji Hata, Motoo Yumura, Sumio Iijima, Hiroto Komatsu, Akira Sakuma, and Hirofumi Kanoh. 2013. "Electron Density Modification of Single Wall Carbon Nanotubes (SWCNT) by Liquid-Phase Molecular Adsorption of Hexaiodobenzene" Materials 6, no. 2: 535-543. https://doi.org/10.3390/ma6020535
APA StyleLu, M., Ohba, T., Kaneko, K., Hata, K., Yumura, M., Iijima, S., Komatsu, H., Sakuma, A., & Kanoh, H. (2013). Electron Density Modification of Single Wall Carbon Nanotubes (SWCNT) by Liquid-Phase Molecular Adsorption of Hexaiodobenzene. Materials, 6(2), 535-543. https://doi.org/10.3390/ma6020535




