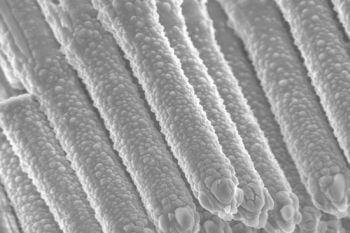
Structural and Electrochemical Investigation during the First Charging Cycles of Silicon Microwire Array Anodes for High Capacity Lithium Ion Batteries
Abstract
:1. Introduction
2. Results and Discussion
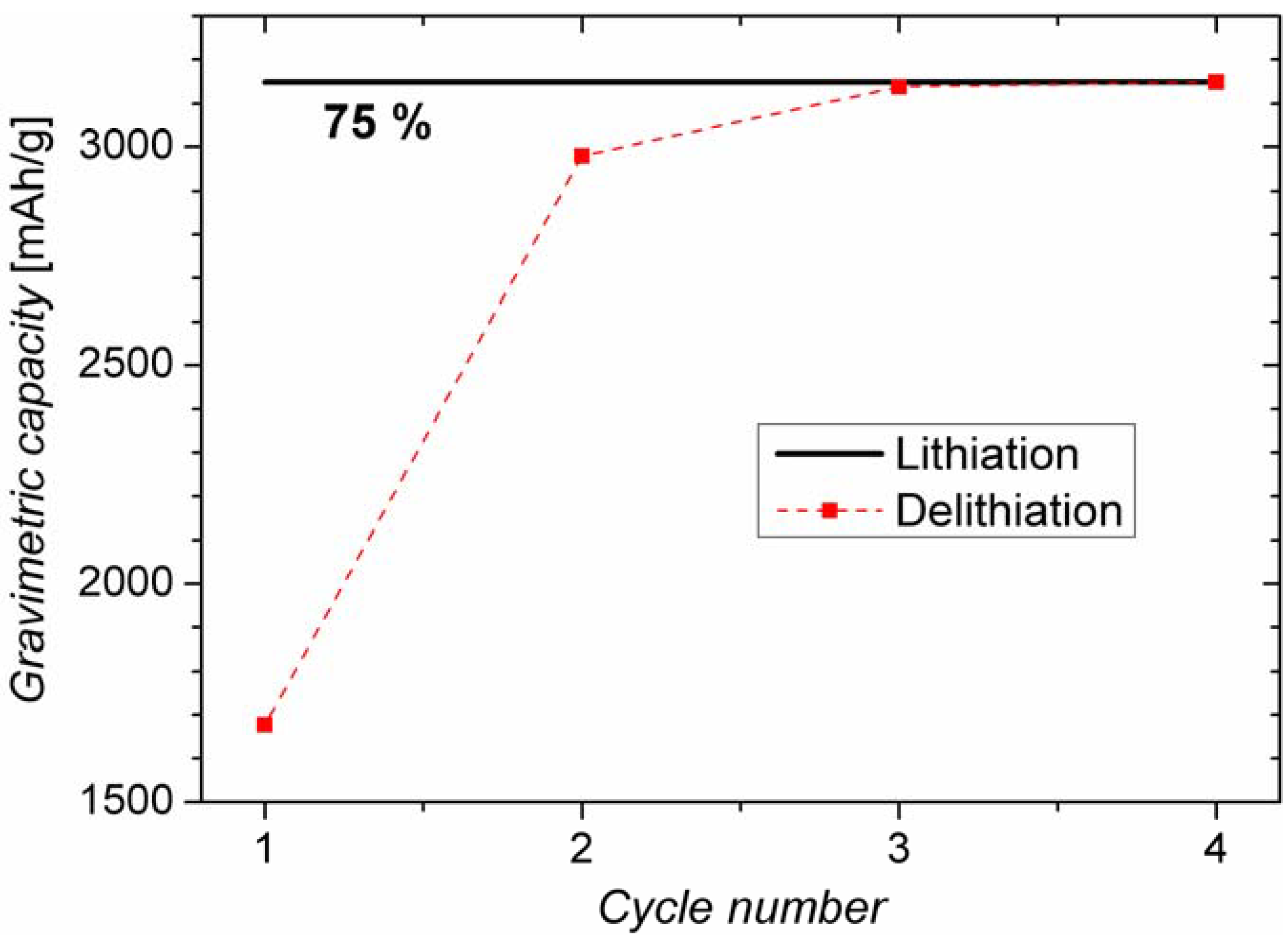
2.1. Battery Cycling Test
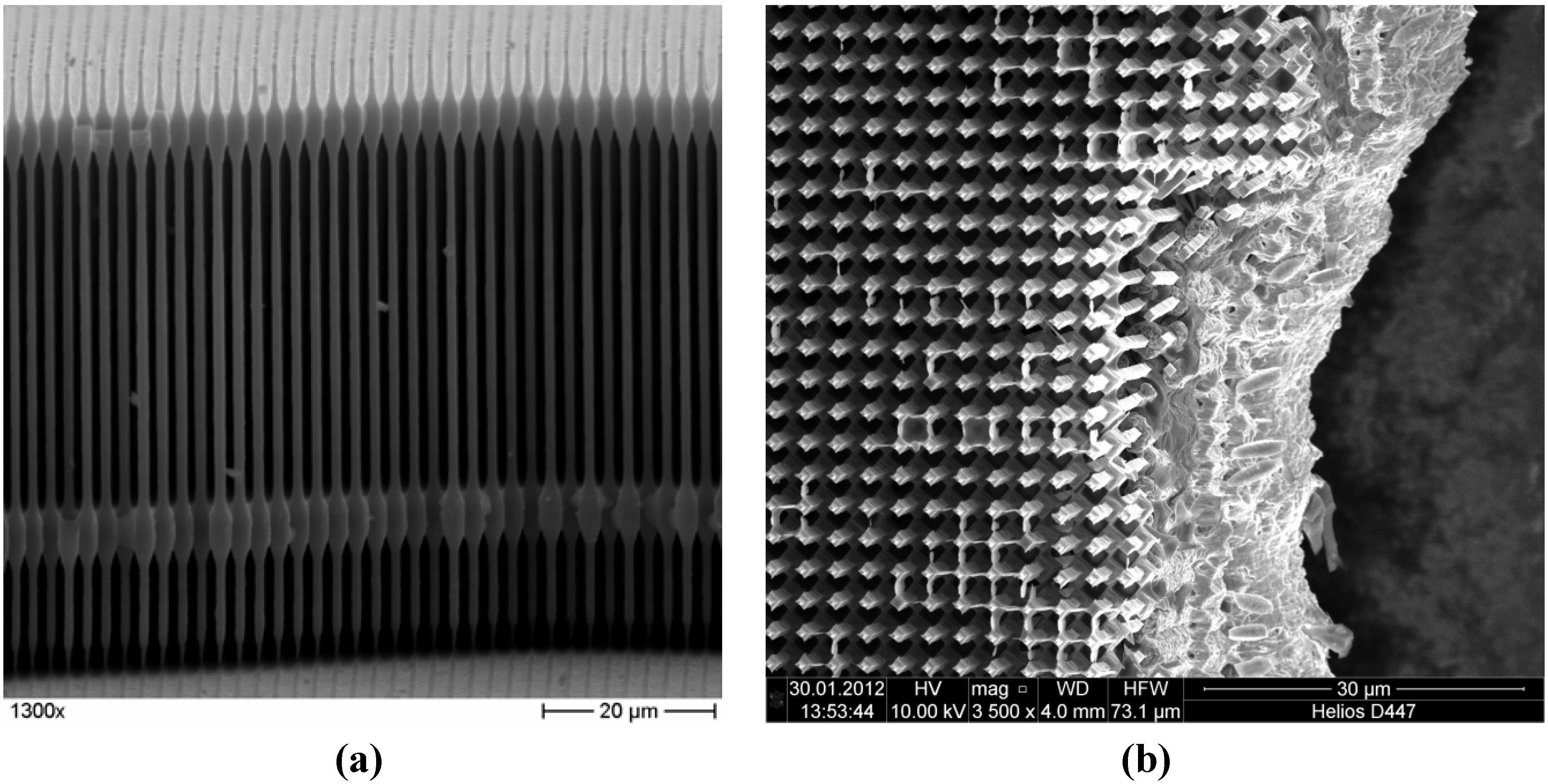
2.2. Structural Characterization
2.2.1. Unlithiated Anodes
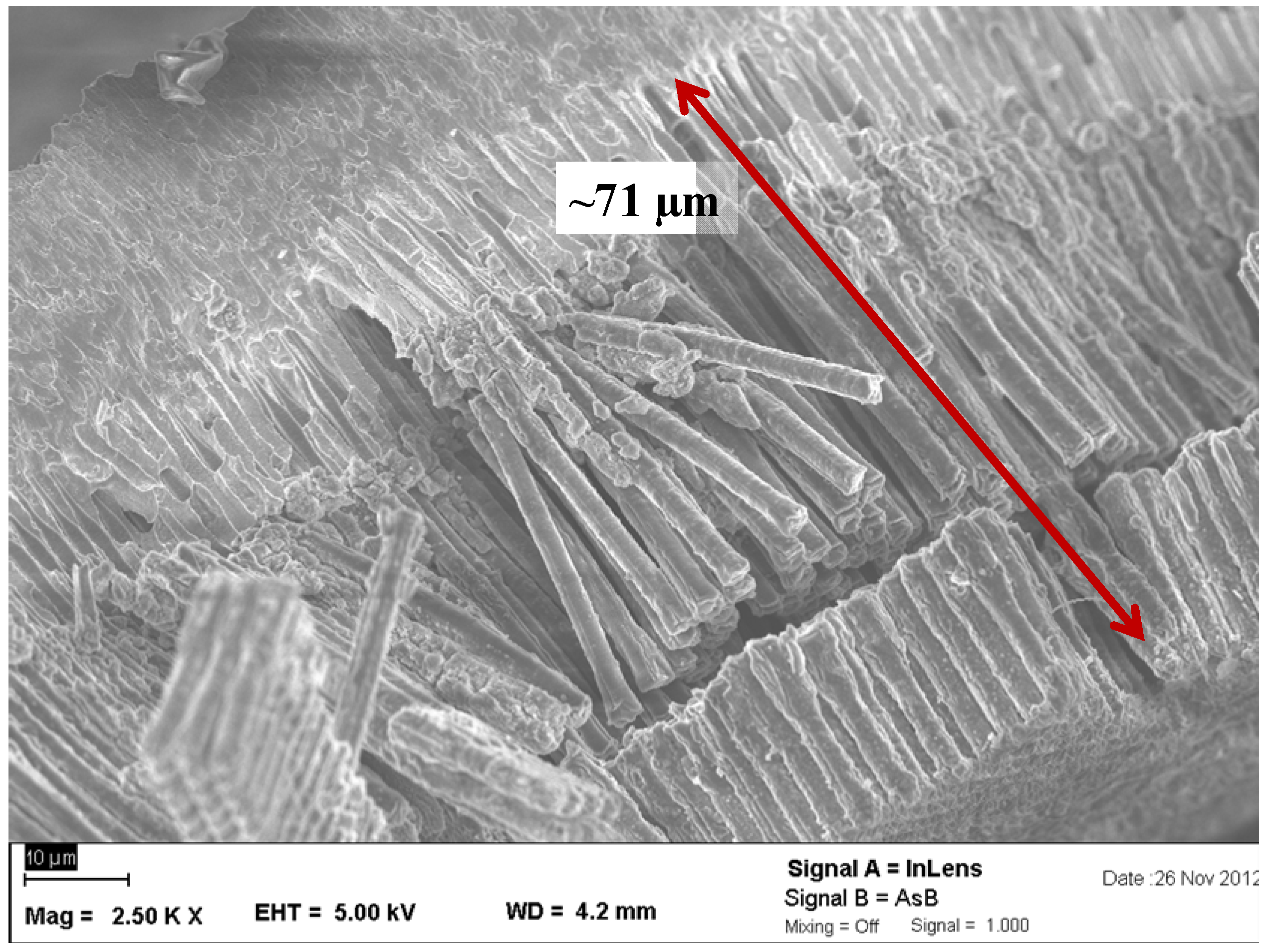
2.2.2. Lithiated Anodes

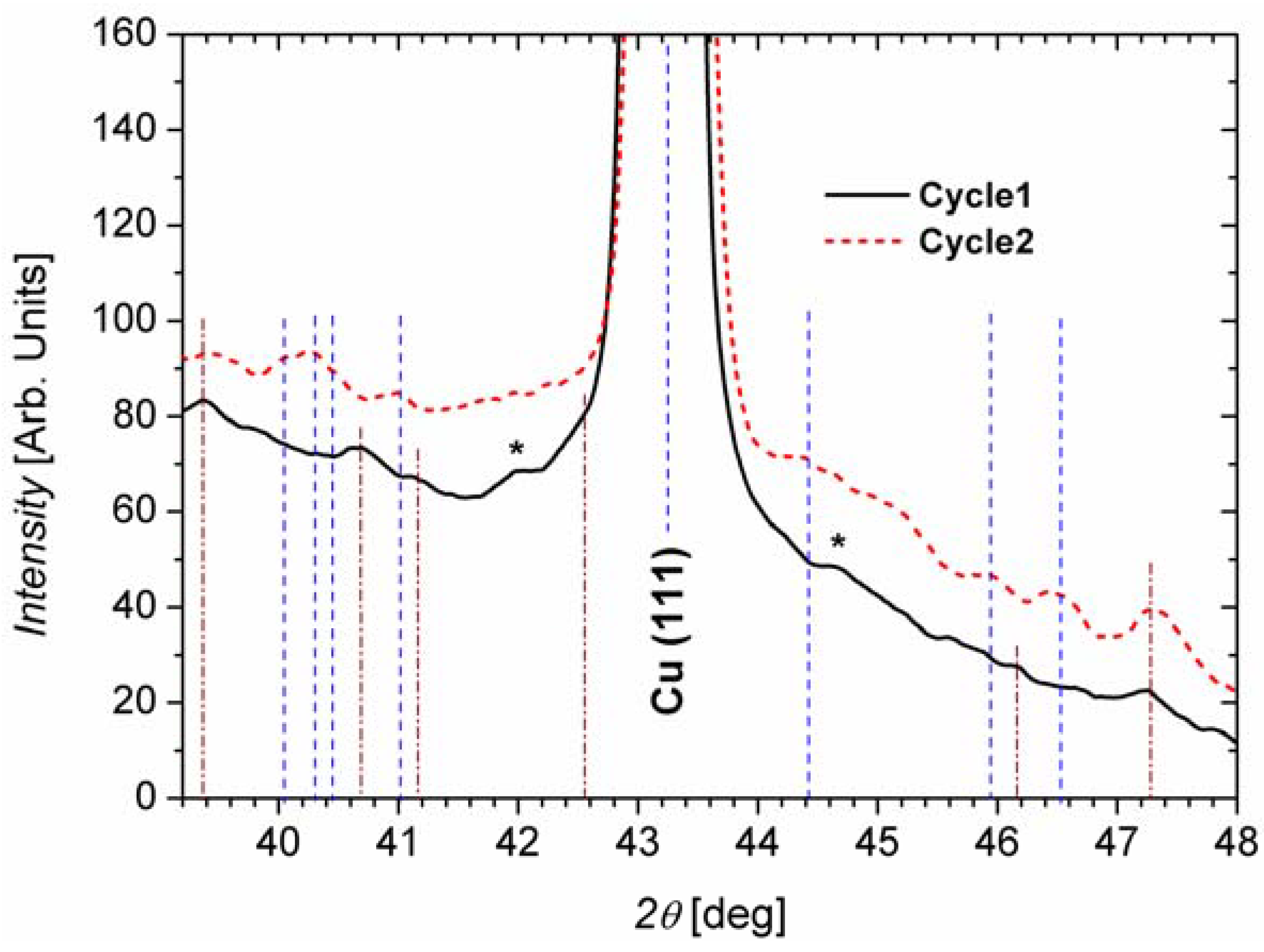
| Cycle 1 | Cycle 2 | ||||
|---|---|---|---|---|---|
| Measured 2θ [deg] | Literature 2θ [deg] | Origin (hkl) | Measured 2θ [deg] | Literature 2θ [deg] | Origin (hkl) |
| 39.4 | 39.5 | Li5SiF6 (002) | 39.44 | 39.5 | Li5SiF6 (002) |
| 40.72 | 40.81, 40.71 | Li22Si5 (660), Li13Si4 (002) | 39.97 | 39.88 | Li12Si7 (254) |
| 41.16 | 41.27 | Li13Si4 (321) | 40.28 | 40.22 | Li12Si7 (272) |
| 42.5 | 42.55 | Li13Si4 (251) | 40.5 | 40.64 | Li2Si (−311) |
| 46.16 | 46.13 | Li13Si4 (132) | 40.96 | 40.9 | Li2Si (020) |
| 47.24 | 47.06 | Si (220) | 44.44 | 44.54 | Li12Si7 (084) |
| – | – | – | 45.9 | 45.9 | Li12Si7 (010 0) |
| – | – | – | 46.45 | 46.39, 46.6 | Li12Si7 (037), Li2Si (−113) |
| – | – | – | 47.29 | 47.06 | Si (220) |
2.3. In Situ Impedance Spectroscopy
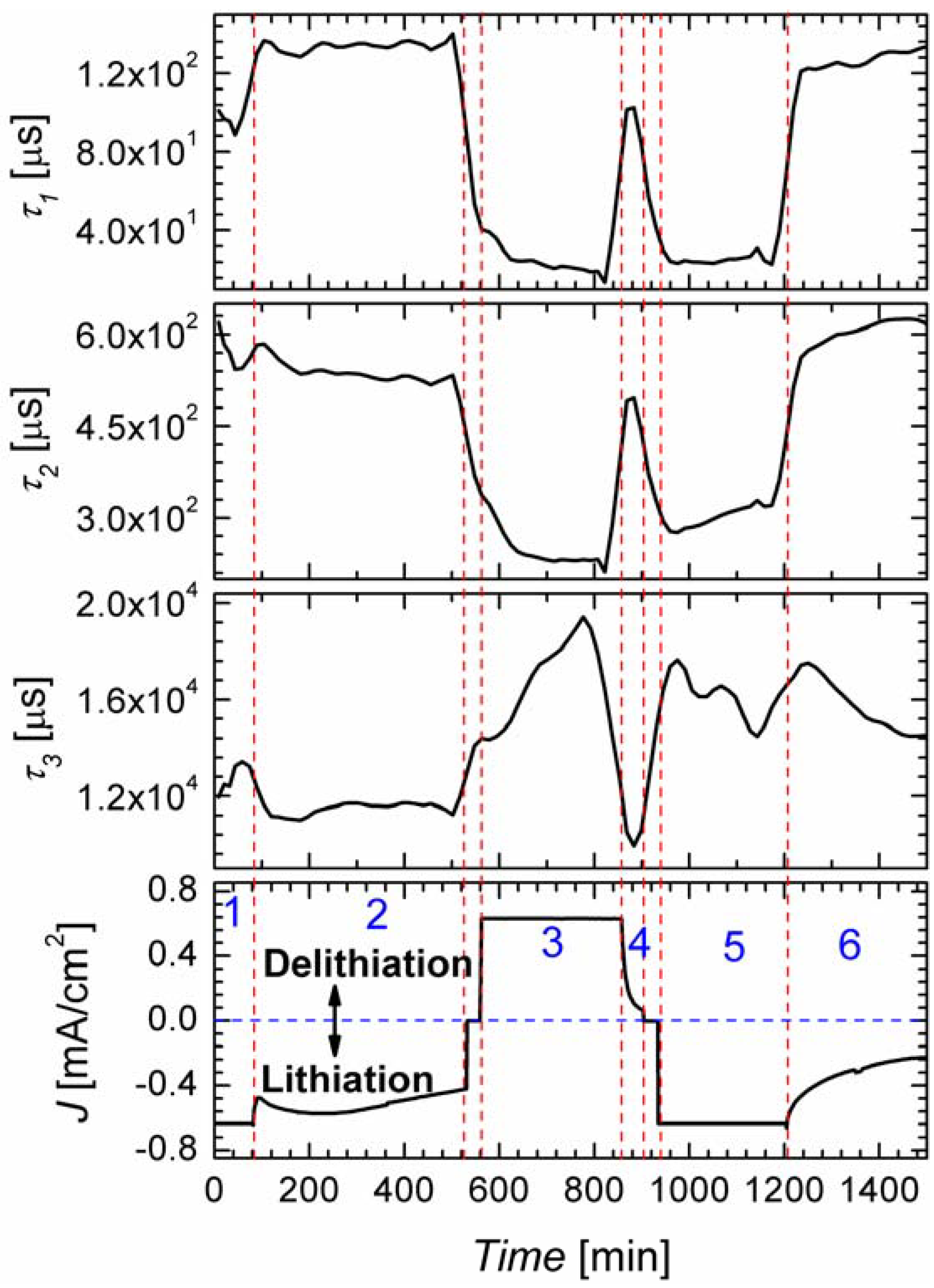
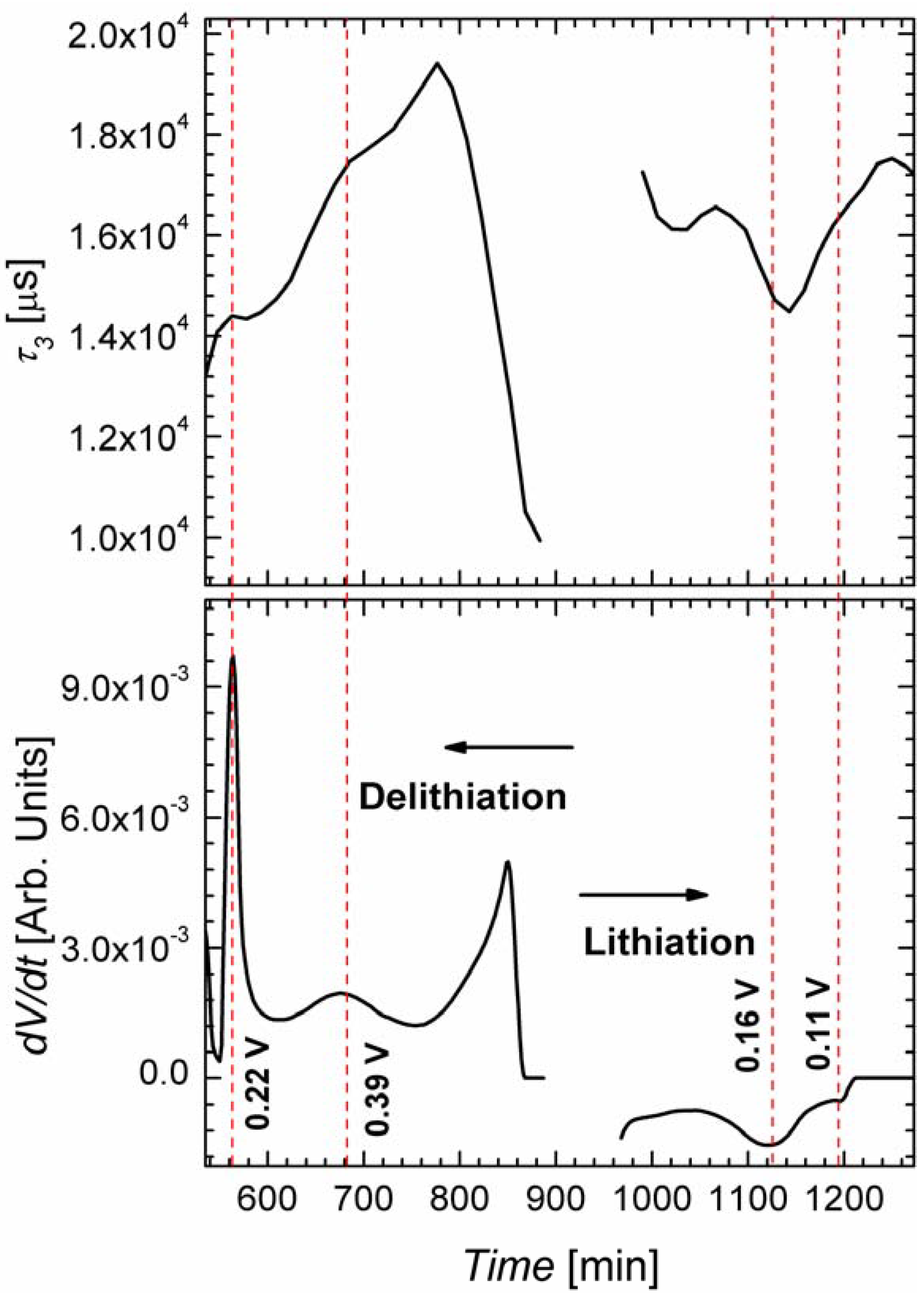
3. Experimental Section
4. Conclusions
Supplementary materials
Supplementary File 1Acknowledgements
References
- Kang, K.; Lee, H.S.; Han, D.W.; Kim, G.S.; Lee, D.; Lee, G.; Kang, Y.M.; Jo, M.H. Maximum Li storage in Si nanowires for the high capacity three dimensional Li-ion battery. Appl. Phys. Lett. 2010, 96, 053110:1–053110:3. [Google Scholar]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Föll, H.; Carstensen, J.; Ossei-Wusu, E.; Cojocaru, A.; Quiroga-González, E.; Neumann, G. Optimized Cu contacted Si nanowire anodes for Li ion batteries made in a production near process. J. Electrochem. Soc. 2011, 158, A580–A584. [Google Scholar] [CrossRef]
- Quiroga-González, E.; Carstensen, J.; Föll, H. Good cycling performance of high-density arrays of Si microwires as anodes for Li ion batteries. Electrochim. Acta 2012. [Google Scholar] [CrossRef]
- Graetz, J.; Ahn, C.C.; Yazami, R.; Fultz, B. Highly reversible lithium storage in nanostructured silicon. Electrochem. Solid State Lett. 2003, 6, A194–A197. [Google Scholar] [CrossRef]
- Green, M.; Fielder, E.; Scrosati, B.; Wachtler, M.; Moreno, J.S. Structured silicon anodes for lithium battery applications. Electrochem. Solid State Lett. 2003, 6, A75–A79. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L. Structural changes in silicon anodes during lithium insertion/extraction. Electrochem. Solid State Lett. 2004, 7, A93–A96. [Google Scholar] [CrossRef]
- Chevrier, V.L.; Dahn, J.R. First principles model of amorphous silicon lithiation. J. Electrochem. Soc. 2009, 156, A454–A458. [Google Scholar] [CrossRef]
- Quiroga-González, E.; Ossei-Wusu, E.; Carstensen, J.; Föll, H. How to make optimized arrays of Si nanowires suitable as superior anode for Li-ion batteries. J. Electrochem. Soc. 2011, 158, E119–E123. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Smith, A.J.; Burns, J.C.; Zhao, X.; Xiong, D.; Dahn, J.R. A high precision coulometry study of the SEI growth in Li/graphite cells. J. Electrochem. Soc. 2011, 158, A447–A452. [Google Scholar] [CrossRef]
- Waldo, A.W. Identification of the copper ore minerals by means of X-ray powder diffraction patterns. Am. Miner. 1935, 20, 587–590. [Google Scholar]
- Axel, H.; Schaefer, H.; Weiss, A. Zur Kenntnis der phase Li22Si5. Z. Naturforsch. B 1966, 21, 115–117. [Google Scholar]
- Frank, U.; Müller, W.; Schäfer, H. Zur Kenntnis der phase Li13Si4. Z. Naturforsch. B 1975, 30, 10–13. [Google Scholar]
- Axel, H.; Schaefer, H.; Weiss, A. Die kristallstruktur von lithiumsilicid Li2Si. Angew. Chem. 1965, 77, 379–380. [Google Scholar] [CrossRef]
- Hull, A.W. The crystal structure of aluminium and silicon. Phys. Rev. 1917, 9, 564–566. [Google Scholar]
- Cox, B.; Sharpe, A.G. Complex fluorides. Part 1. J. Chem. Soc. 1954, 3251–3252. [Google Scholar]
- Ruffo, R.; Hong, S.S.; Chan, C.K.; Huggins, R.A.; Cui, Y. Impedance analysis of silicon nanowire lithium ion battery anodes. J. Phys. Chem. C 2009, 113, 11390–11398. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, W.; Byun, D.; Lee, J.K. Electrochemical characteristics of phosphorus doped silicon for the anode material of lithium secondary batteries. Solid State Ion. 2012, 212, 43–46. [Google Scholar] [CrossRef]
- Momma, T.; Matsunaga, M.; Mukoyama, D.; Osaka, T. Ac impedance analysis of lithium ion battery under temperature control. J. Power Source 2012, 216, 304–307. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Quiroga-González, E.; Carstensen, J.; Föll, H. Structural and Electrochemical Investigation during the First Charging Cycles of Silicon Microwire Array Anodes for High Capacity Lithium Ion Batteries. Materials 2013, 6, 626-636. https://doi.org/10.3390/ma6020626
Quiroga-González E, Carstensen J, Föll H. Structural and Electrochemical Investigation during the First Charging Cycles of Silicon Microwire Array Anodes for High Capacity Lithium Ion Batteries. Materials. 2013; 6(2):626-636. https://doi.org/10.3390/ma6020626
Chicago/Turabian StyleQuiroga-González, Enrique, Jürgen Carstensen, and Helmut Föll. 2013. "Structural and Electrochemical Investigation during the First Charging Cycles of Silicon Microwire Array Anodes for High Capacity Lithium Ion Batteries" Materials 6, no. 2: 626-636. https://doi.org/10.3390/ma6020626