Grafting of Amines on Ethanol-Extracted SBA-15 for CO2 Adsorption
Abstract
:1. Introduction
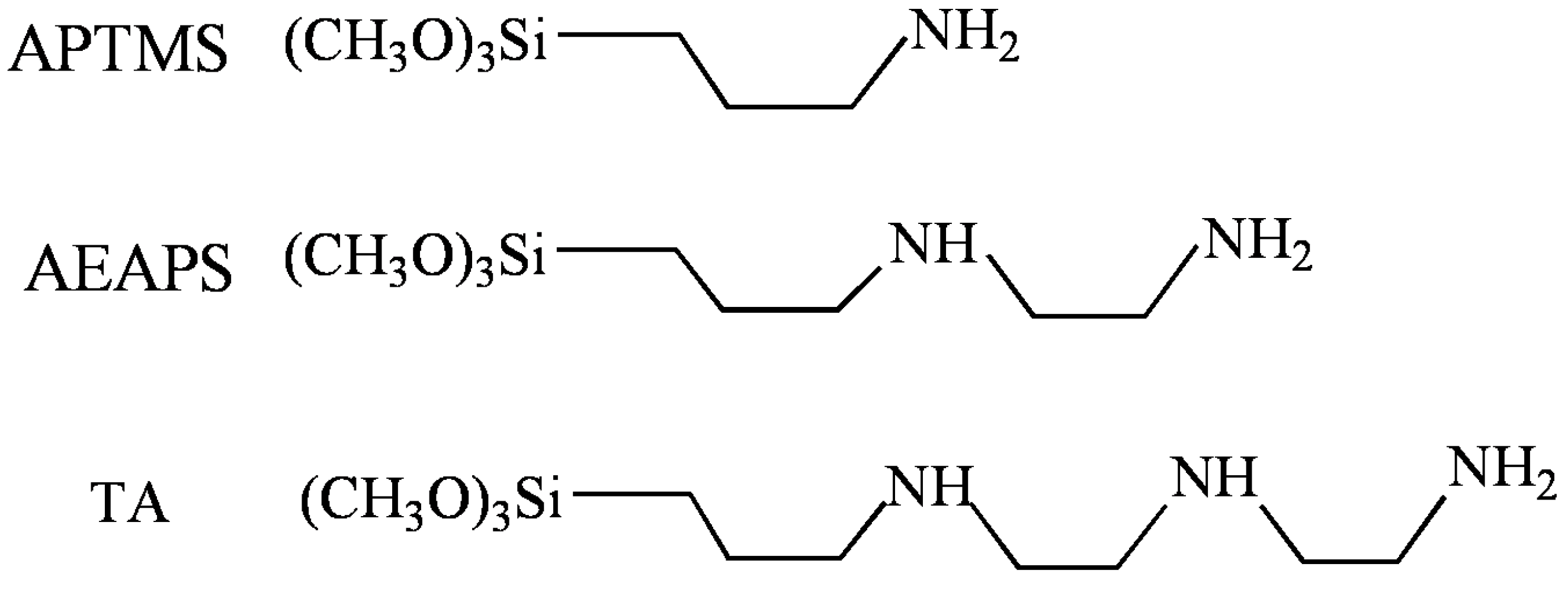
2. Results and Discussion
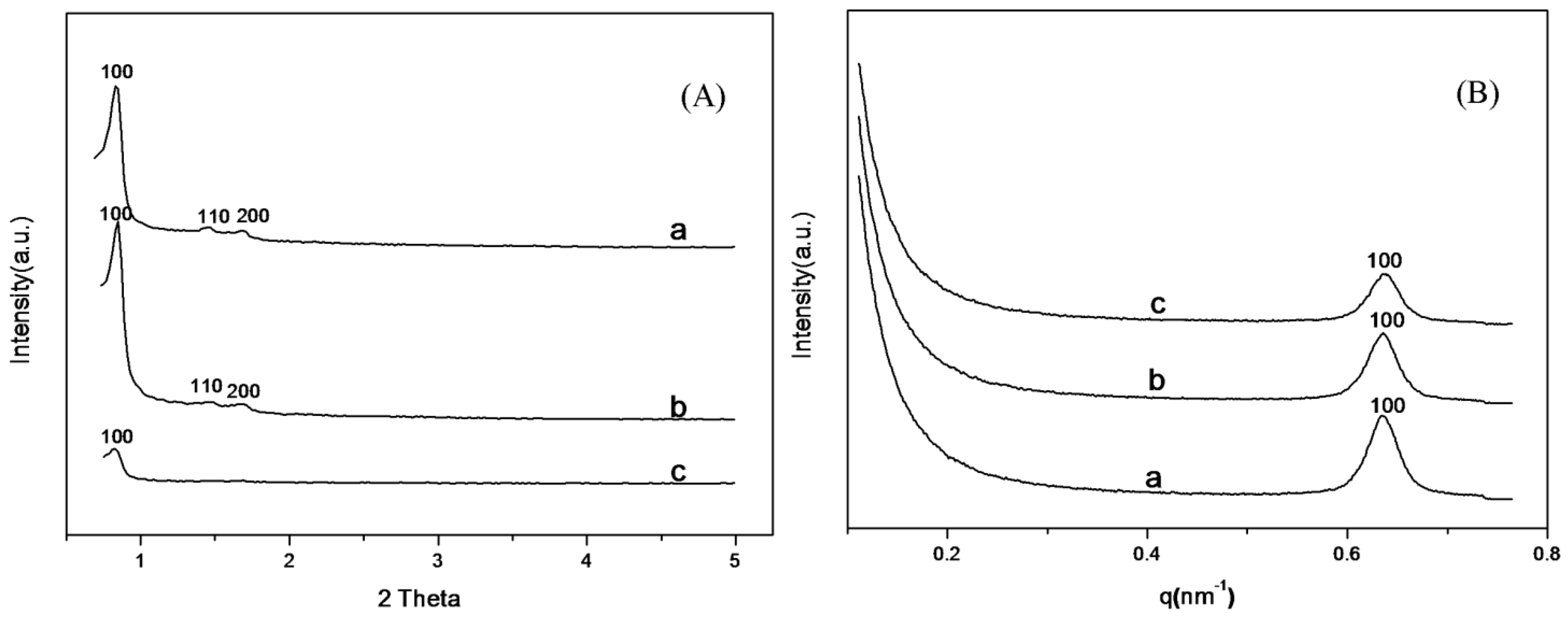
2.1. Characterization of SBA-15-c and SBA-15-ex Support
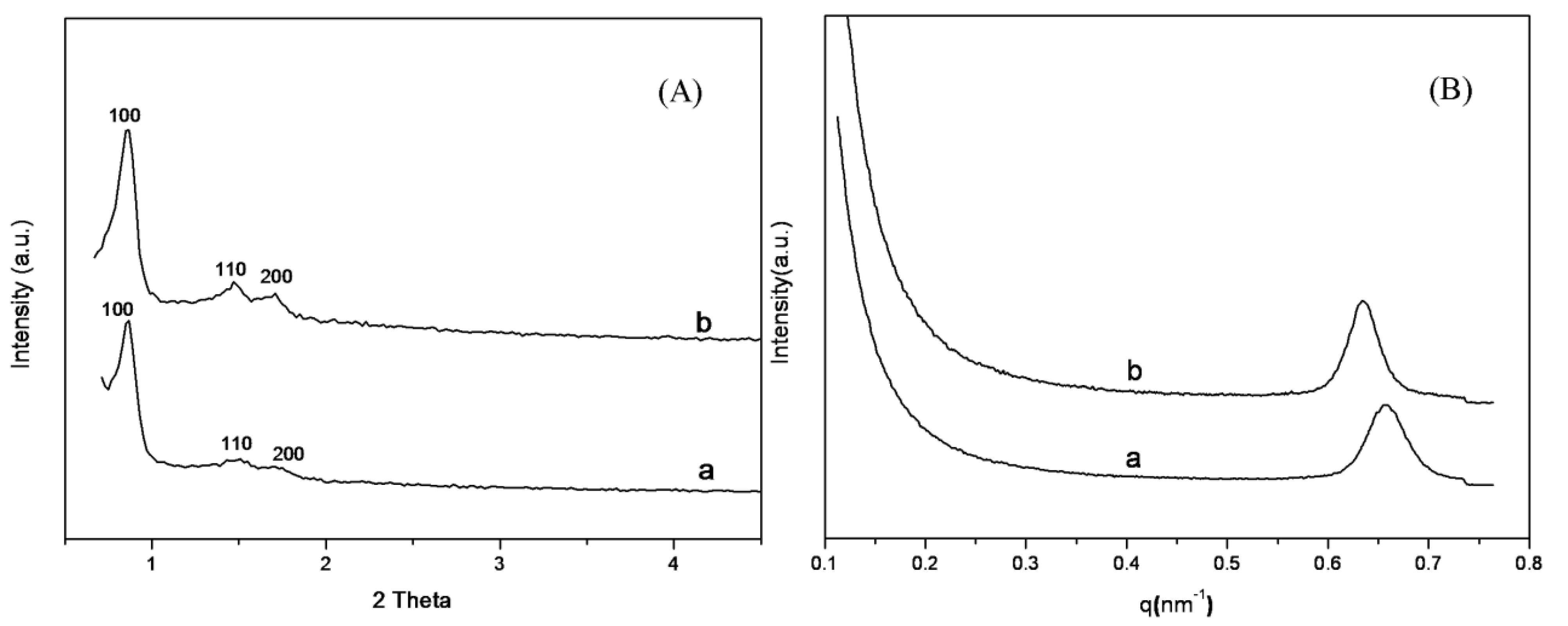
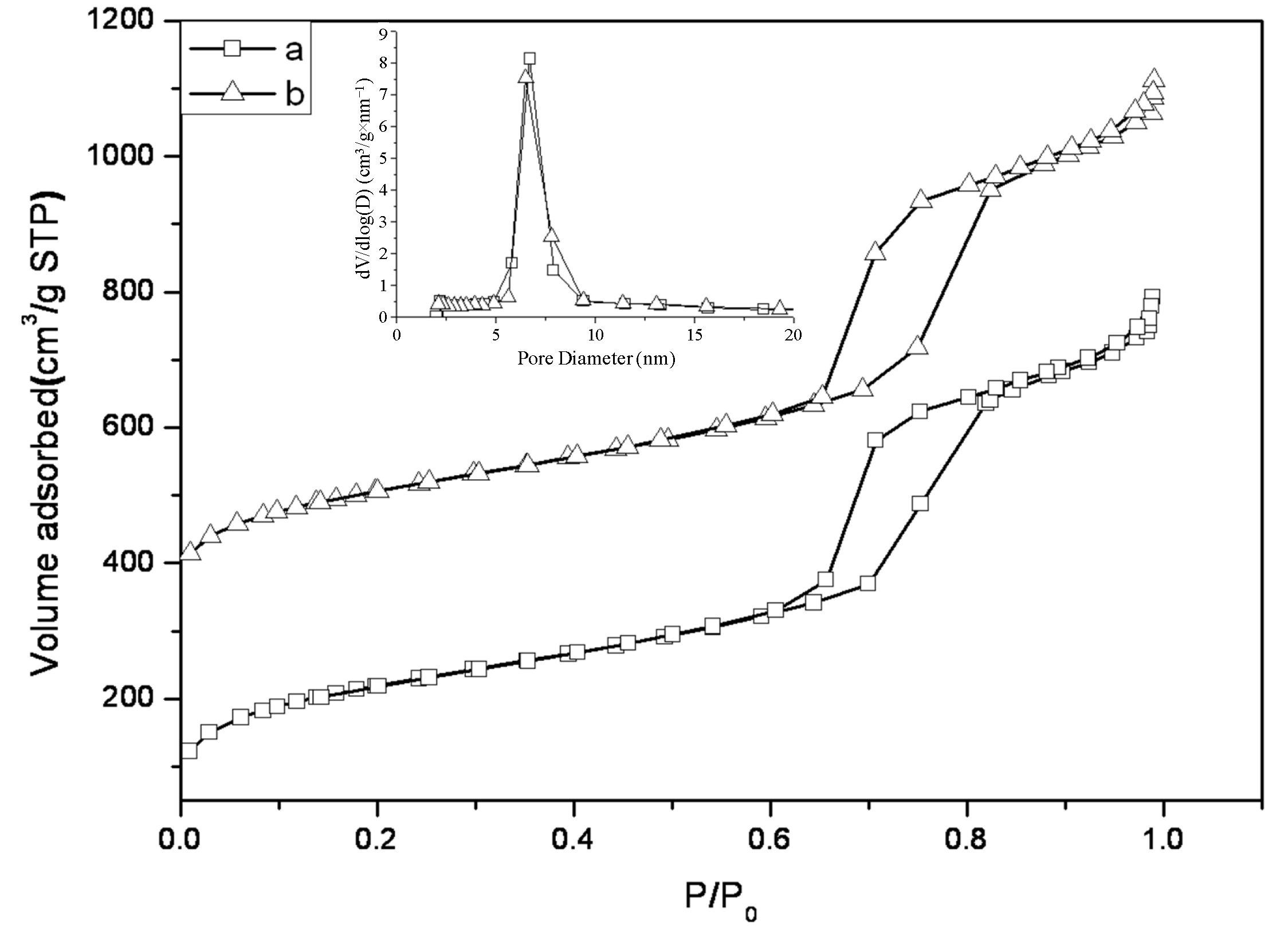
| Sample | SBET (m2/g) | VTotal (cm3/g) | Dpore (nm) | d spacing (nm) | a0 (nm) | W (nm) |
|---|---|---|---|---|---|---|
| SBA-15-c | 791 | 1.23 | 6.7 | 9.54 | 11.01 | 4.3 |
| SBA-15-ex | 750 | 1.25 | 6.5 | 9.92 | 11.46 | 5.0 |
| APTMS-SBA-15-ex | 372 | 0.69 | 6.2 | 9.89 | 11.41 | 5.2 |
| AEAPS-SBA-15-ex | 410 | 0.67 | 6.0 | 9.88 | 11.41 | 5.4 |
| TA-SBA-15-ex | 286 | 0.50 | 5.7 | 9.89 | 11.41 | 5.7 |
| APTMS-SBA-15-c | 470 | 0.76 | 6.5 | 9.53 | 11.00 | 4.5 |
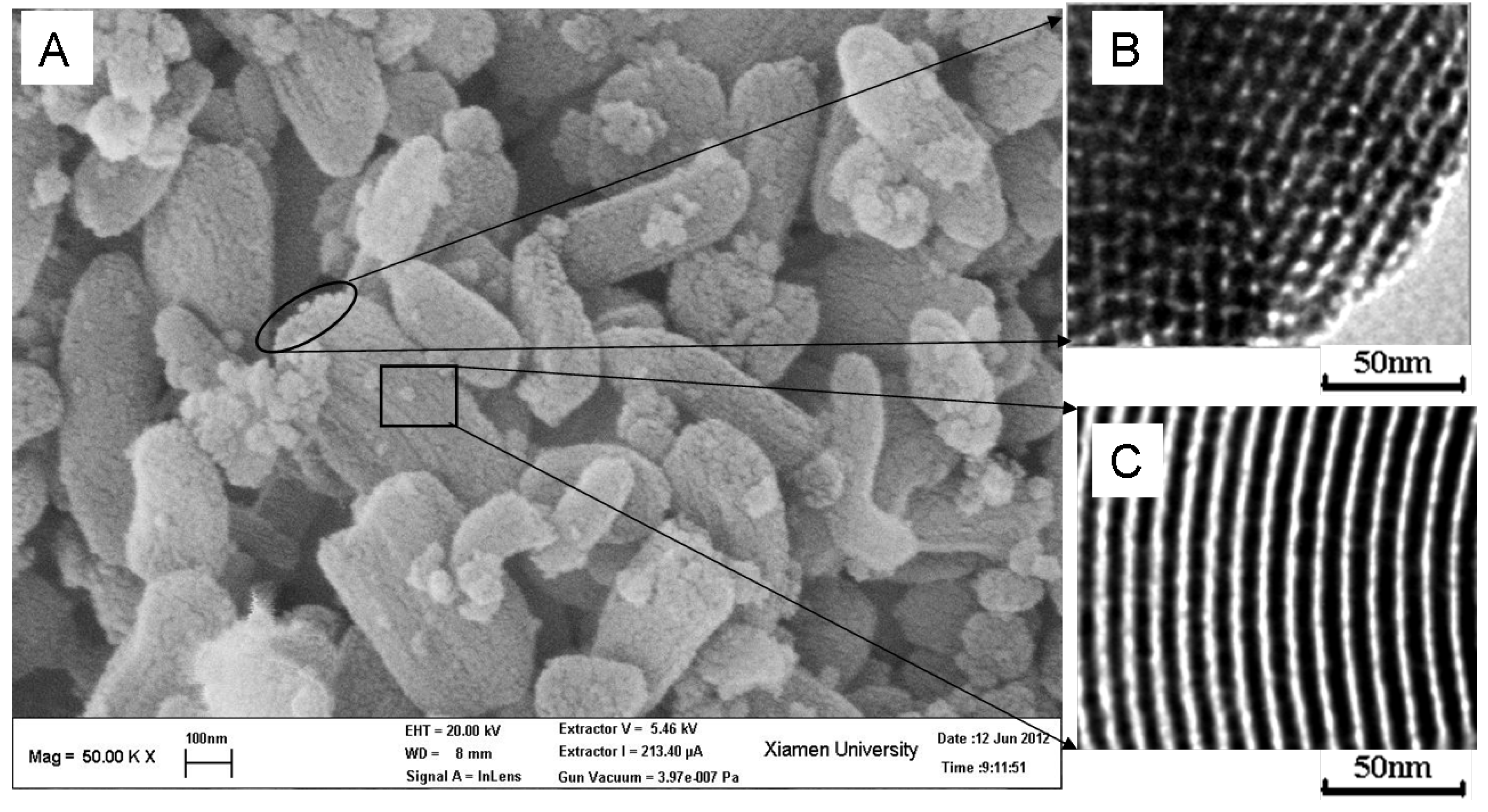

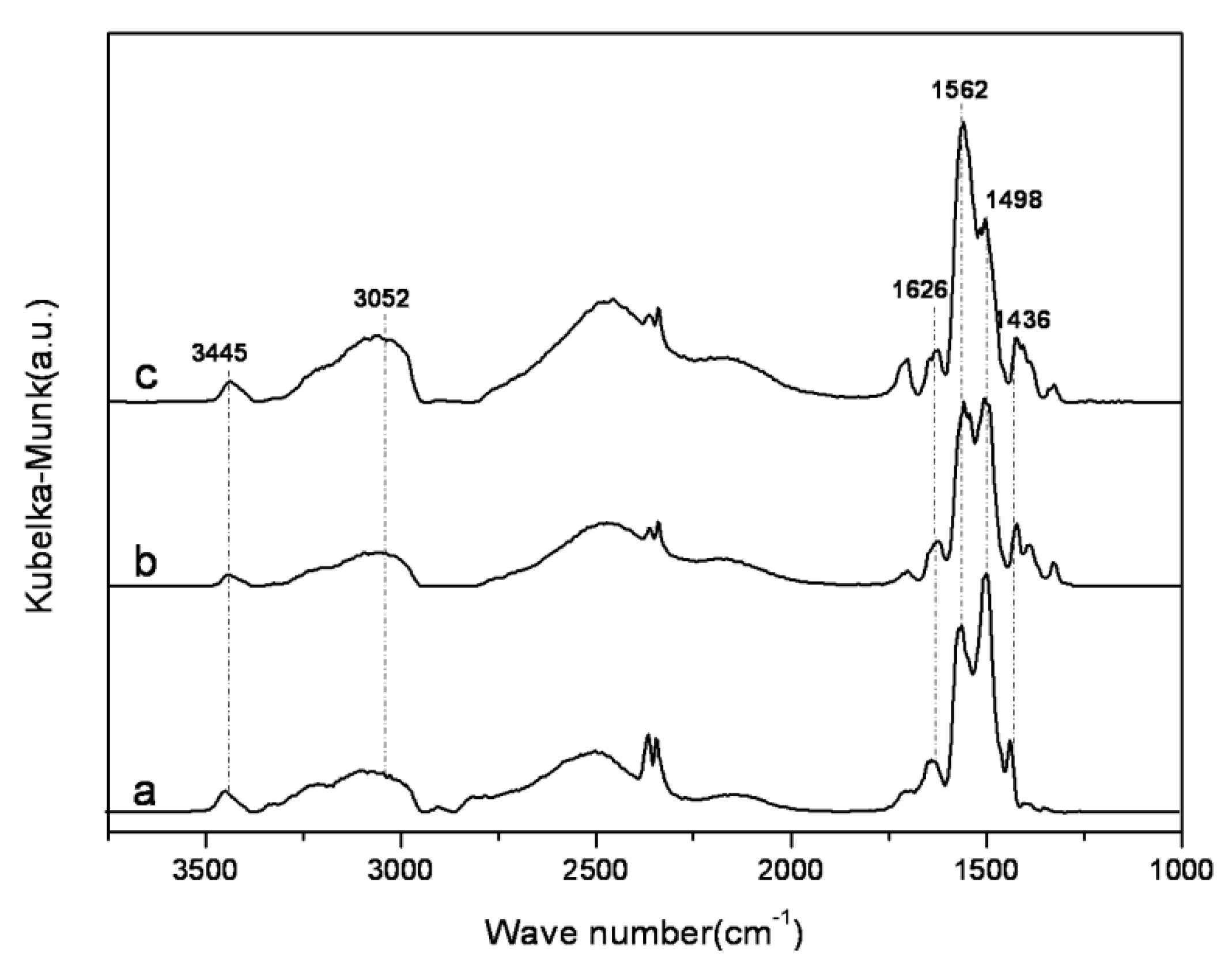
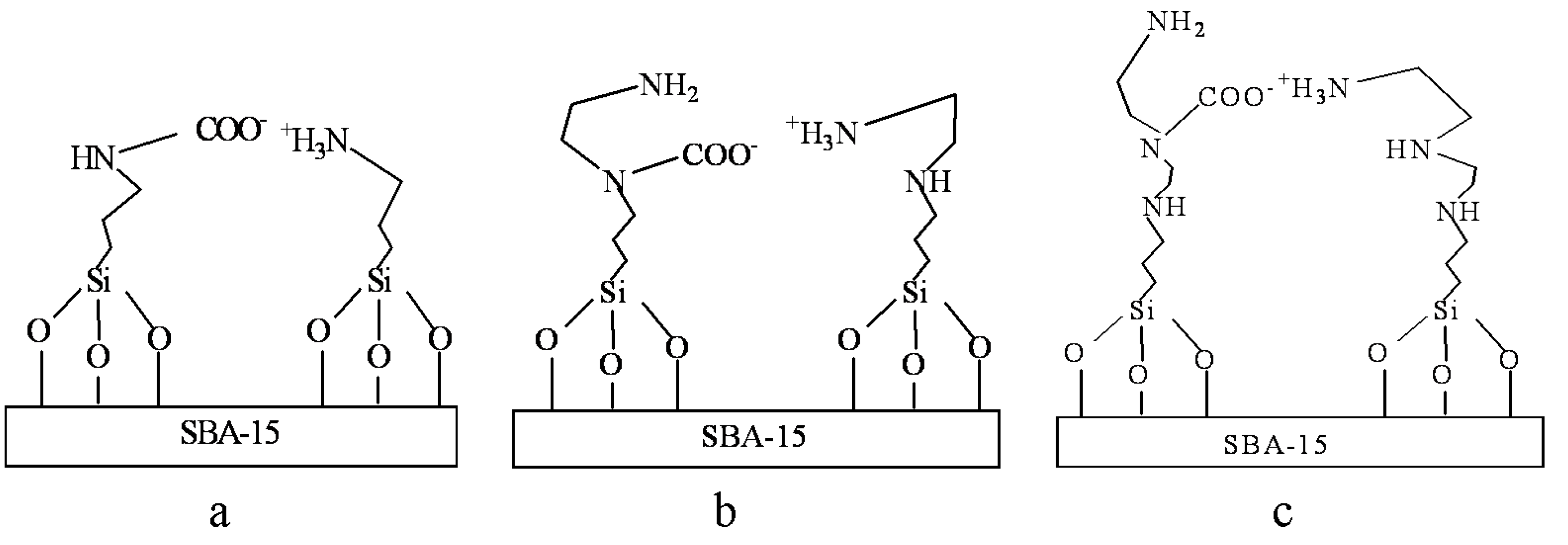
2.2. Characterization of Amine-Grafted SBA-15-ex
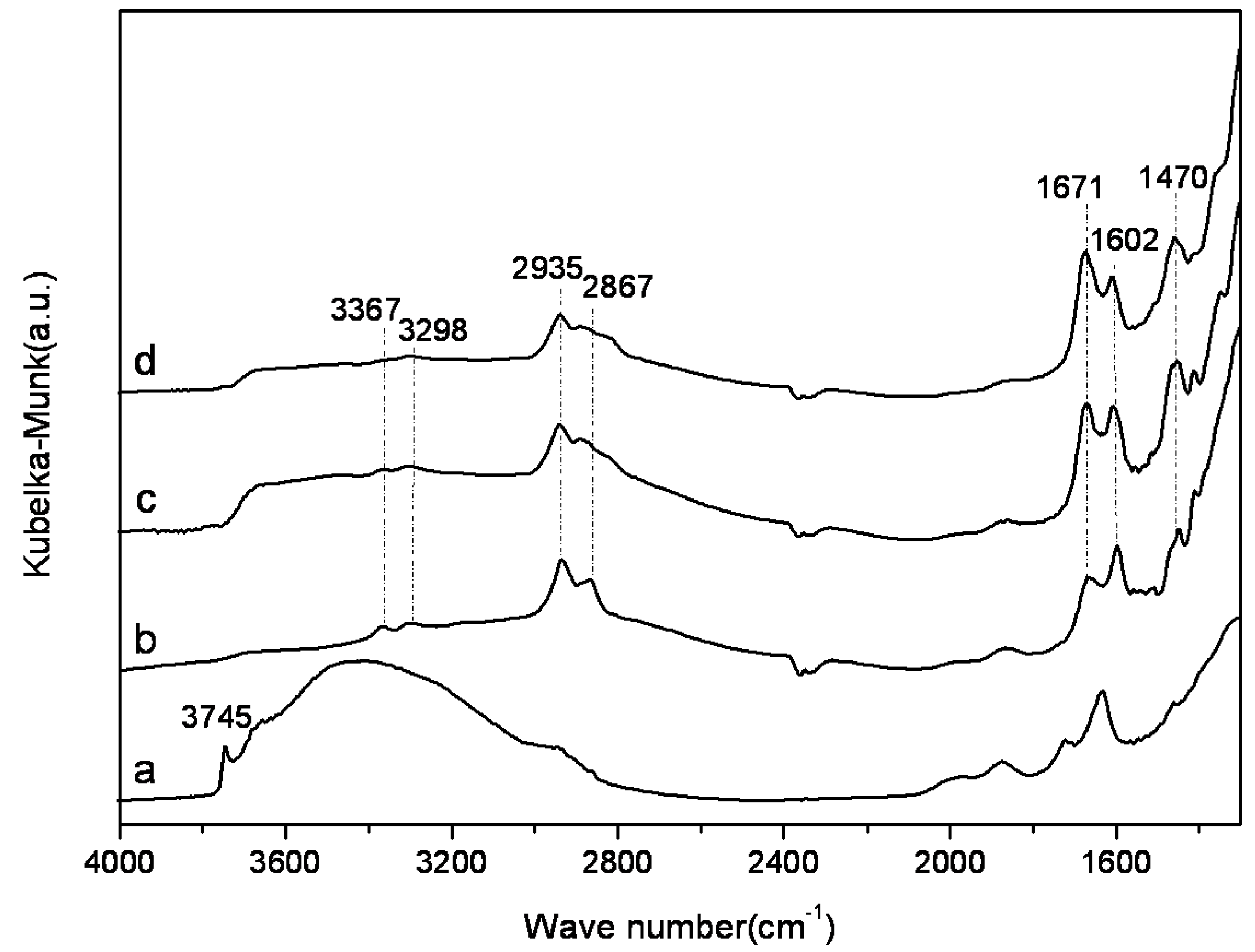
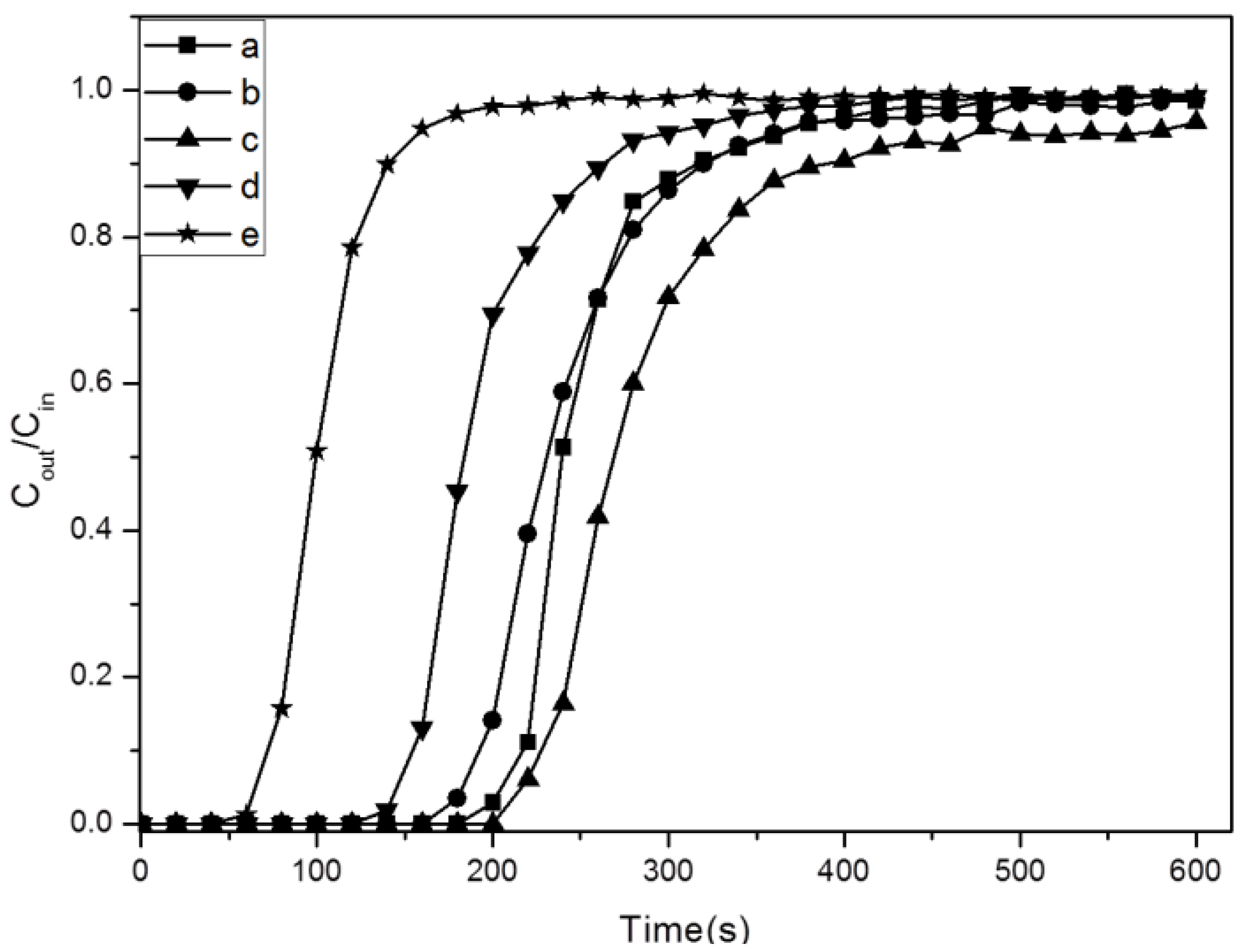
2.3. Adsorption Properties of Amine-Grafted SBA-15
| Sample | Amine content
(mmol/g) | N content
(mmol/g) | Surface density
(N atom nm−2) | Adsorption capacity
(mmol/g) | Amine efficiency
(mol(CO2)/mol(N)) | |
|---|---|---|---|---|---|---|
| SBA-15-ex | ~0 | ~0 | ~0 | 0.21 | - | |
| APTMS-SBA-15-ex | 2.44 | 2.44 | 1.94 | 1.07 | 0.44 | |
| AEAPS-SBA-15-ex | 1.72 | 3.43 | 2.15 | 0.99 | 0.29 | |
| TA-SBA-15-ex | 1.61 | 4.83 | 3.02 | 1.19 | 0.25 | |
| APTMS-SBA-15-c | 1.62 | 1.62 | 1.31 | 0.75 | 0.46 | |
| Adsorbents | Adsorption capacity (mmol/g) | Experimental conditions temperature/CO2 partial pressure | Evaluation method | Reference |
|---|---|---|---|---|
| APTMS -SBA-15 | 0.66 | 333 K/15 kPa | Flow method | [26] |
| APTES-SBA-15 | 1.5 | 298 K/10 kPa | TGA | [43] |
| APTMS-SBA-15 | 1.6 | 298 K/15 kPa | TGA | [32] |
| APTES-SBA-12 | 1.02 | 298 K/10 kPa | TGA | [43] |
| AEAPS-SBA-16 | 0.73 | 333 K/15 kPa | TGA | [28] |
| TRI-PE-MCM-41 | 2.05 | 298 K/5 kPa | Flow method | [42] |
| APTES-MCM-48 | 1.68 | 298 K/101 kPa | Static method | [52] |
| HA-SBA-15 | 1.00 | 298 K/10 kPa | Flow method | [53] |
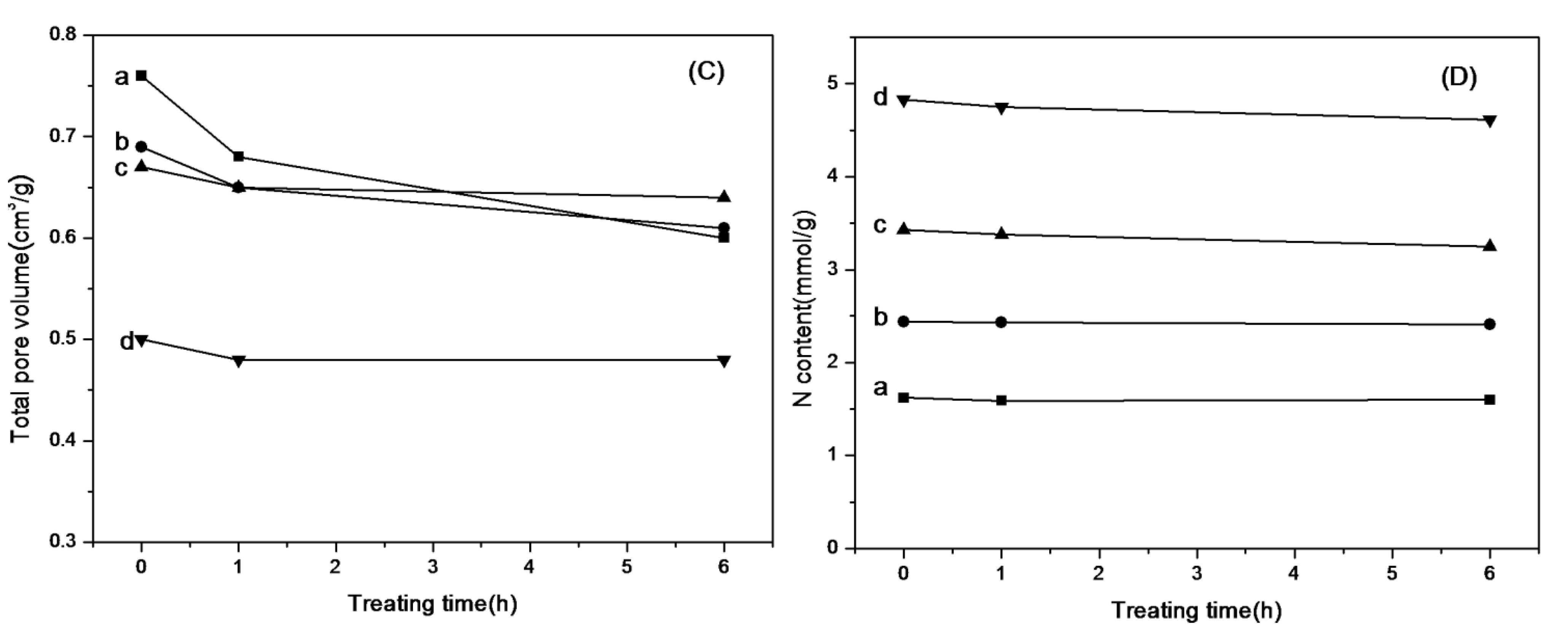
2.4. The Stability of Amine-Grafted SBA-15 after Steam Treatment

3. Experimental Section
3.1. Preparation of SBA-15 Support
3.2. Removal of Template (P123) from As-Synthesized SBA-15
3.3. Grafting of Aminosilanes on SBA-15
3.4. Characterizations
3.5. CO2 Capture Behavior
3.6. Steam Treatment of the Adsorbents
4. Conclusions
Acknowledgments
References
- Haszeldine, R.S. Carbon capture and storage: How green can black be? Science 2009, 325, 1647–1652. [Google Scholar]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. Chemsuschem 2009, 2, 796–854. [Google Scholar]
- House, K.Z.; Harvey, C.F.; Aziz, M.J.; Schrag, D.P. The energy penalty of post-combustion CO2 capture & storage and its implications for retrofitting the U.S. installed base. Energy Environ. Sci. 2009, 2, 193–205. [Google Scholar]
- Tontiwachwuthikul, P.; Meisen, A.; Lim, C.J. Solubility of CO2 in 2-Amino-2-methyl-1-propanol solutions. J. Chem. Eng. Data 1991, 36, 130–133. [Google Scholar]
- Bonenfant, D.; Kharoune, M.; Niquette, P.; Mimeault, M.; Hausler, R. Advances in principal factors influencing carbon dioxide adsorption on zeolites. Sci. Technol. Adv. Mater. 2008, 9. [Google Scholar] [CrossRef]
- Shao, W.; Zhang, L.Z.; Li, L.X.; Lee, R.L. Adsorption of CO2 and N2 on synthesized NaY zeolite at high temperatures. Adsorpt. J. Int. Adsorpt. Soc. 2009, 15, 497–505. [Google Scholar]
- Miyamoto, M.; Fujiokax, Y.; Yogo, K. Pure silica CHA type zeolite for CO2 separation using pressure swing adsorption at high pressure. J. Mater. Chem. 2012, 22, 20186–20189. [Google Scholar]
- Dantas, T.L.P.; Amorim, S.M.; Luna, F.M.T.; Silva, I.J.; de Azevedo, D.C.S.; Rodrigues, A.E.; Moreira, R. Adsorption of carbon dioxide onto activated carbon and nitrogen-enriched activated carbon: Surface changes, equilibrium, and modeling of fixed-bed adsorption. Sep. Sci. Technol. 2010, 45, 73–84. [Google Scholar]
- Yang, H.; Gong, M.; Chen, Y. Preparation of activated carbons and their adsorption properties for greenhouse gases: CH4 and CO2. J. Nat. Gas. Chem. 2011, 20, 460–464. [Google Scholar]
- Zhao, Y.; Zhao, L.; Yao, K.; Yang, Y.; Zhang, Q.; Han, Y. Novel porous carbon materials with ultrahigh nitrogen contents for selective CO2 capture. J. Mater. Chem. 2012, 22, 19726–19731. [Google Scholar]
- Soares, J.L.; Moreira, R.; Jose, H.J.; Grande, C.A.; Rodrigues, A.E. Hydrotalcite materials for carbon dioxide adsorption at high temperatures: Characterization and diffusivity measurements. Sep. Sci. Technol. 2004, 39, 1989–2010. [Google Scholar]
- Lwin, Y.; Abdullah, F. High temperature adsorption of carbon dioxide on Cu-Al hydrotalcite-derived mixed oxides: Kinetics and equilibria by thermogravimetry. J. Therm. Anal. Calorim. 2009, 97, 885–889. [Google Scholar]
- Srivastava, H.K.; Sastry, G.N. Viability of clathrate hydrates as CO2 capturing agents: A theoretical study. J. Phys. Chem. A 2011, 115, 7633–7637. [Google Scholar]
- Hong, D.Y.; Hwang, Y.K.; Serre, C.; Ferey, G.; Chang, J.S. Porous chromium terephthalate MIL-101 with coordinatively unsaturated sites: Surface functionalization, encapsulation, sorption and catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and Zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar]
- Bao, Z.; Yu, L.; Ren, Q.; Lu, X.; Deng, S. Adsorption of CO2 and CH4 on a magnesium-based metal organic framework. J. Colloid. Interf. Sci. 2011, 353, 549–556. [Google Scholar]
- Vitillo, J.G.; Savonnet, M.; Ricchiardi, G.; Bordiga, S. Tailoring metal-organic frameworks for CO2 capture: The amino effect. Chemsuschem 2011, 4, 1281–1290. [Google Scholar]
- Wang, X.; Li, H.; Hou, X. Amine-Functionalized metal organic framework as a highly selective adsorbent for CO2 over CO. J. Phys. Chem. C 2012, 116, 19814–19821. [Google Scholar]
- Yang, D.A.; Cho, H.Y.; Kim, J.; Yang, S.T.; Ahn, W.S. CO2 capture and conversion using Mg-MOF-74 prepared by a sonochemical method. Energy Environ. Sci. 2012, 5, 6465–6473. [Google Scholar]
- Siriwardane, R.V.; Robinson, C.; Shen, M.; Simonyi, T. Novel regenerable sodium-based sorbents for CO2 capture at warm gas temperatures. Energy Fuels 2007, 21, 2088–2097. [Google Scholar]
- Martavaltzi, C.S.; Lemonidou, A.A. Development of new CaO based sorbent materials for CO2 removal at high temperature. Microporous Mesoporous Mater. 2008, 110, 119–127. [Google Scholar]
- Fu, X.; Zhao, N.; Li, J.; Xiao, F.; Wei, W.; Sun, Y. Carbon dioxide capture by MgO-modified MCM-41 materials. Adsorpt. Sci. Technol. 2009, 27, 593–601. [Google Scholar]
- Li, L.; Wen, X.; Fu, X.; Wang, F.; Zhao, N.; Xiao, F.K.; Wei, W.; Sun, Y.H. MgO/Al2O3 sorbent for CO2 capture. Energy Fuels 2010, 24, 5773–5780. [Google Scholar]
- Li, L.; Li, Y.; Wen, X.; Wang, F.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. CO2 capture over K2CO3/MgO/Al2O3 Dry sorbent in a fluidized bed. Energy Fuels 2011, 25, 3835–3842. [Google Scholar]
- Soliman, E.M. Synthesis and metal collecting properties of mono, di, tri and tetramine based on silica gel matrix. Anal. Lett. 1997, 30, 1739–1751. [Google Scholar]
- Hiyoshi, N.; Yogo, K.; Yashima, T. Adsorption characteristics of carbon dioxide on organically functionalized SBA-15. Microporous Mesoporous Mater. 2005, 84, 357–365. [Google Scholar]
- Khatri, R.A.; Chuang, S.S.C.; Soong, Y.; Gray, M. Carbon dioxide capture by diamine-grafted SBA-15: A combined Fourier transform infrared and mass spectrometry study. Ind. Eng. Chem. Res. 2005, 44, 3702–3708. [Google Scholar]
- Wei, J.; Shi, J.; Pan, H.; Zhao, W.; Ye, Q.; Shi, Y. Adsorption of carbon dioxide on organically functionalized SBA-16. Microporous Mesoporous Mater. 2008, 116, 394–399. [Google Scholar]
- Li, W.; Bollini, P.; Didas, S.A.; Choi, S.; Drese, J.H.; Jones, C.W. Structural changes of silica mesocellular foam supported amine-functionalized CO2 adsorbents upon exposure to steam. ACS Appl. Mater. Interf. 2010, 2, 3363–3372. [Google Scholar]
- Li, W.; Choi, S.; Drese, J.H.; Hornbostel, M.; Krishnan, G.; Eisenberger, P.M.; Jones, C.W. Steam-Stripping for regeneration of supported amine-based CO2 adsorbents. Chemsuschem 2010, 3, 899–903. [Google Scholar]
- Ko, Y.G.; Shin, S.S.; Choi, U.S. Primary, secondary, and tertiary amines for CO2 capture: Designing for mesoporous CO2 adsorbents. J. Colloid. Interf. Sci. 2011, 361, 594–602. [Google Scholar]
- Wang, L.; Yang, R.T. Increasing selective CO2 adsorption on amine-grafted SBA-15 by increasing silanol density. J. Phys. Chem. C 2011, 115, 21264–21272. [Google Scholar]
- Chen, C.; Yang, S.T.; Ahn, W.S.; Ryoo, R. Amine-Impregnated silica monolith with a hierarchical pore structure: Enhancement of CO2 capture capacity. Chem. Commun. 2009, 24, 3627–3629. [Google Scholar]
- Fisher, J.C.; Tanthana, J.; Chuang, S.S.C. Oxide-supported tetraethylenepentamine for CO2 capture. Environ. Prog. Sustain. Energy 2009, 28, 589–598. [Google Scholar]
- Chen, C.; Son, W.J.; You, K.S.; Ahn, J.W.; Ahn, W.S. Carbon dioxide capture using amine-impregnated HMS having textural mesoporosity. Chem. Eng. J. 2010, 161, 46–52. [Google Scholar]
- Goeppert, A.; Meth, S.; Prakash, G.K.S.; Olah, G.A. Nanostructured silica as a support for regenerable high-capacity organoamine-based CO2 sorbents. Energy Environ. Sci. 2010, 3, 1949–1960. [Google Scholar]
- Wen, J.; Gu, F.; Wei, F.; Zhou, Y.; Lin, W.; Yang, J.; Yang, J.; Wang, Y.; Zou, Z.; Zhu, J. One-Pot synthesis of the amine-modified meso-structured monolith CO2 adsorbent. J. Mater. Chem. 2010, 20, 2840–2846. [Google Scholar]
- Choi, S.; Gray, M.L.; Jones, C.W. Amine-Tethered solid adsorbents coupling high adsorption capacity and regenerability for CO2 capture from ambient air. Chemsuschem 2011, 4, 628–635. [Google Scholar]
- Heydari-Gorji, A.; Sayari, A. CO2 capture on polyethylenimine-impregnated hydrophobic mesoporous silica: Experimental and kinetic modeling. Chem. Eng. J. 2011, 173, 72–79. [Google Scholar]
- Qi, G.G.; Fu, L.L.; Choi, B.H.; Giannelis, E.P. Efficient CO2 sorbents based on silica foam with ultra-large mesopores. Energy Environ. Sci. 2012, 5, 7368–7375. [Google Scholar]
- Xu, X.C.; Song, C.S.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar]
- Serna-Guerrero, R.; Belmabkhout, Y.; Sayari, A. Further investigations of CO2 capture using triamine-grafted pore-expanded mesoporous silica. Chem. Eng. J. 2010, 158, 513–519. [Google Scholar]
- Zelenák, V.; Badanicová, M.; Halamová, D.; Cejka, J.; Zukal, A.; Murafa, N.; Goerigk, G. Amine-modified ordered mesoporous silica: Effect of pore size on carbon dioxide capture. Chem. Eng. J. 2008, 144, 336–342. [Google Scholar]
- Chang, F.; Chao, K.; Cheng, H.; Tan, C. Adsorption of CO2 onto amine-grafted mesoporous silicas. Sep. Purif. Technol. 2009, 70, 87–95. [Google Scholar]
- Bhagiyalakshmi, M.; Yun, L.J.; Anuradha, R.; Jang, H.T. Synthesis of chloropropylamine grafted mesoporous MCM-41, MCM-48 and SBA-15 from rice husk ash: their application to CO2 chemisorption. J. Porous Mater. 2010, 17, 475–484. [Google Scholar]
- Zhang, F.; Yang, H.; Yan, Y.; Yu, C.; Tu, B.; Zhao, D. Understanding effect of wall structure on the hydrothermal stability of mesostructured silica SBA-15. J. Phys. Chem. B 2005, 109, 8723–8732. [Google Scholar]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic–inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar]
- Tian, B.; Liu, X.; Yu, C.; Gao, F.; Luo, Q.; Xie, S.; Tu, B.; Zhao, D. Microwave assisted template removal of siliceous porous materials. Chem. Commun. 2002, 11, 1186–1187. [Google Scholar]
- Kosuge, K.; Sato, T.; Kikukawa, N.; Takemori, M. Morphological control of rod- and fiberlike SBA-15 type mesoporous silica using water-soluble sodium silicate. Chem. Mater. 2004, 16, 899–905. [Google Scholar]
- Wang, X.; Schwartz, V.; Clark, J.C.; Ma, X.; Overbury, S.H.; Xu, C.; Song, S. Infrared study of CO2 sorption over “Molecular Basket” sorbent consisting of polyethylenimine-modified mesoporous molecular sieve. J. Phys. Chem. C 2009, 113, 7260–7268. [Google Scholar]
- Gil, M.; Tiscornia, I.; de la Iglesia, Ó; Mallada, R.; Santamaría, J. Monoamine-grafted MCM-48: An efficient material for CO2 removal at low partial pressures. Chem. Eng. J. 2011, 175, 291–297. [Google Scholar]
- Hicks, J.C.; Drese, J.H.; Fauth, D.J.; Gray, M.L.; Qi, G.; Jones, C.W. Designing adsorbents for CO2 capture from flue gas-hyperbranched aminosilicas capable of capturing CO2 reversibly. J. Am. Chem. Soc. 2008, 130, 2902–2903. [Google Scholar]
- Bossa, J.-B.; Borget, F.; Duvernay, F.; Theulé, P.; Chiavassa, T. Formation of neutral methylcarbamic acid (CH3NHCOOH) and methylammonium methylcarbamate [CH3NH3+] [CH3NHCO2−] at low temperature. J. Phys. Chem. A 2008, 112, 5113–5120. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar]
- Chuang, S.S.C.; Guzman, F. Mechanistic investigation of heterogeneous catalysis by transient infrared methods. Top. Catal. 2009, 52, 1448–1458. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Y.; Sun, N.; Li, L.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y.; Huang, W. Grafting of Amines on Ethanol-Extracted SBA-15 for CO2 Adsorption. Materials 2013, 6, 981-999. https://doi.org/10.3390/ma6030981
Li Y, Sun N, Li L, Zhao N, Xiao F, Wei W, Sun Y, Huang W. Grafting of Amines on Ethanol-Extracted SBA-15 for CO2 Adsorption. Materials. 2013; 6(3):981-999. https://doi.org/10.3390/ma6030981
Chicago/Turabian StyleLi, Yong, Nannan Sun, Lei Li, Ning Zhao, Fukui Xiao, Wei Wei, Yuhan Sun, and Wei Huang. 2013. "Grafting of Amines on Ethanol-Extracted SBA-15 for CO2 Adsorption" Materials 6, no. 3: 981-999. https://doi.org/10.3390/ma6030981
APA StyleLi, Y., Sun, N., Li, L., Zhao, N., Xiao, F., Wei, W., Sun, Y., & Huang, W. (2013). Grafting of Amines on Ethanol-Extracted SBA-15 for CO2 Adsorption. Materials, 6(3), 981-999. https://doi.org/10.3390/ma6030981




