Liquid Foam Templates Associated with the Sol-Gel Process for Production of Zirconia Ceramic Foams
Abstract
:1. Introduction
2. Experimental Section
2.1. Zirconia Foams Preparation
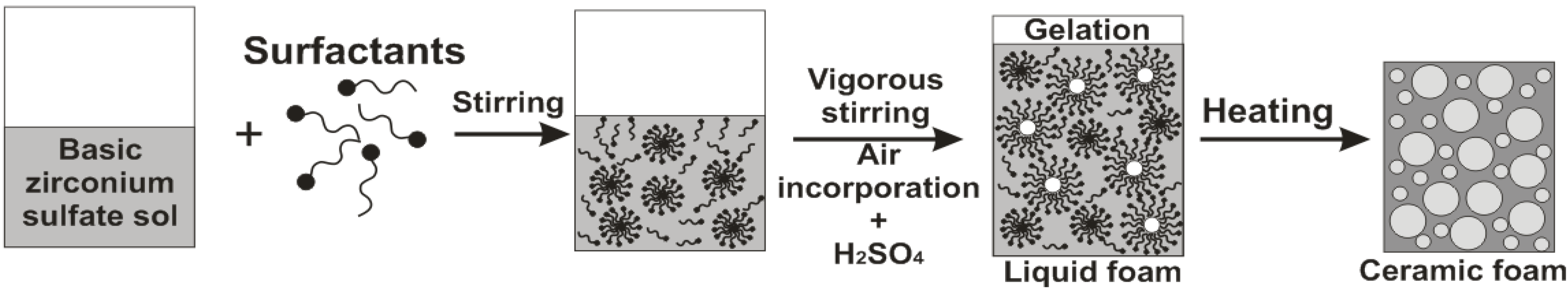
2.2. Zirconia Foams Characterization
3. Results and Discussion
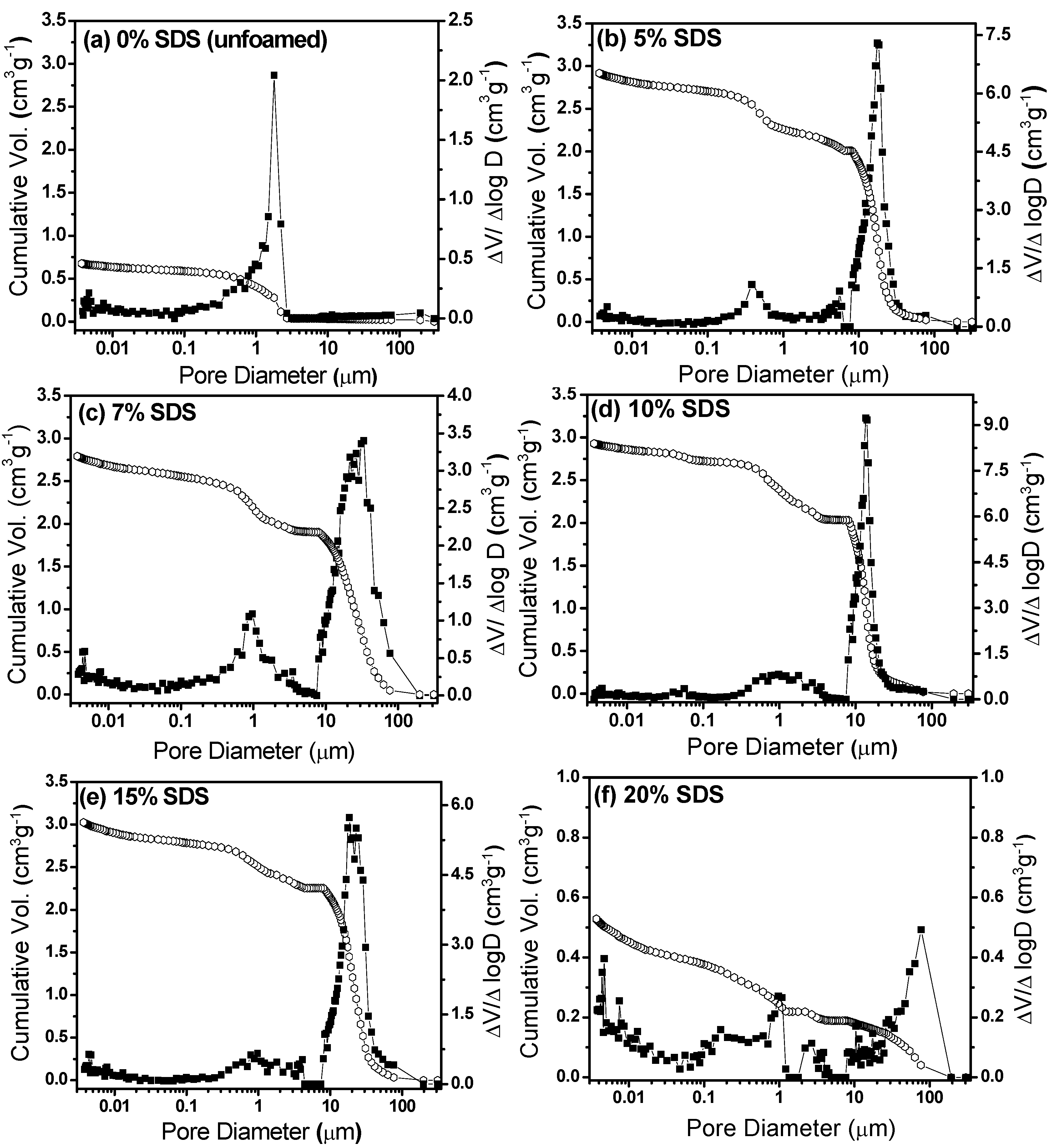
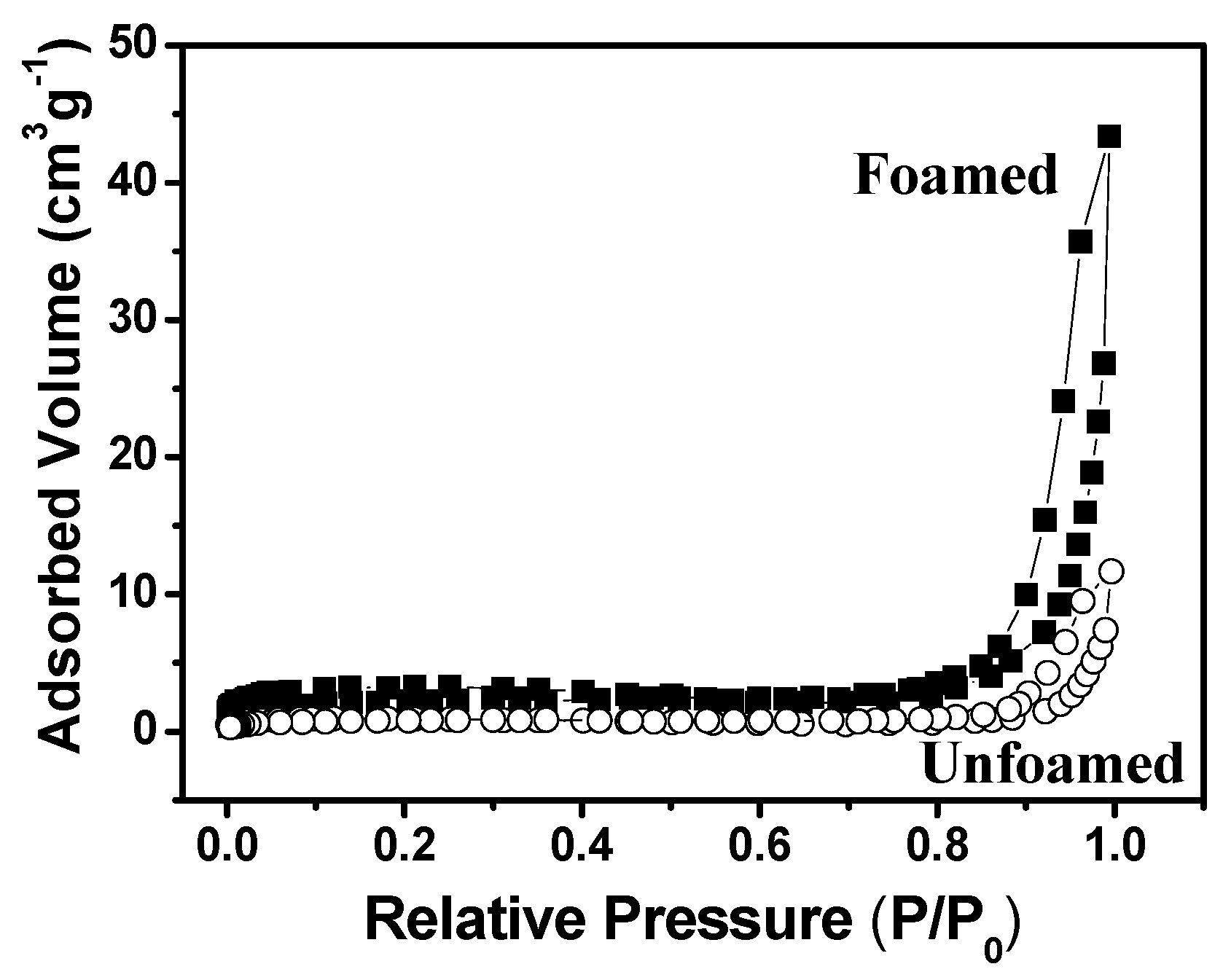
3.1. Effect of Sodium Dodecylsulfate (SDS) Amount
| SDS amount (wt %) | Specific pore volume (cm3 g−1) | Bulk density (g cm−3) | Porosity (%) | Mean macropore size (μm) | Mean supermesopore size (μm) |
|---|---|---|---|---|---|
| 0 | 0.70 ± 0.02 | 1.628 ± 0.007 | 64.5 ± 0.3 | – | 1.83 ± 0.03 |
| 5 | 3.01 ± 0.03 | 0.278 ± 0.002 | 93.7 ± 0.6 | 17.5 ± 0.1 | 0.42 ± 0.01 |
| 7 | 2.82 ± 0.03 | 0.330 ± 0.003 | 92.8 ± 0.8 | 29.5 ± 0.6 | 0.96 ± 0.02 |
| 10 | 3.19 ± 0.05 | 0.292 ± 0.004 | 94 ± 1 | 13.6 ± 0.1 | 1.3 ± 0.1 |
| 15 | 2.70 ± 0.03 | 0.338 ± 0.004 | 91 ± 1 | 22.3 ± 0.2 | 0.92 ± 0.07 |
| 20 | 0.80 ± 0.06 | 0.80 ± 0.03 | 81 ± 3 | 94 ± 7 | 0.68 ± 0.06 |
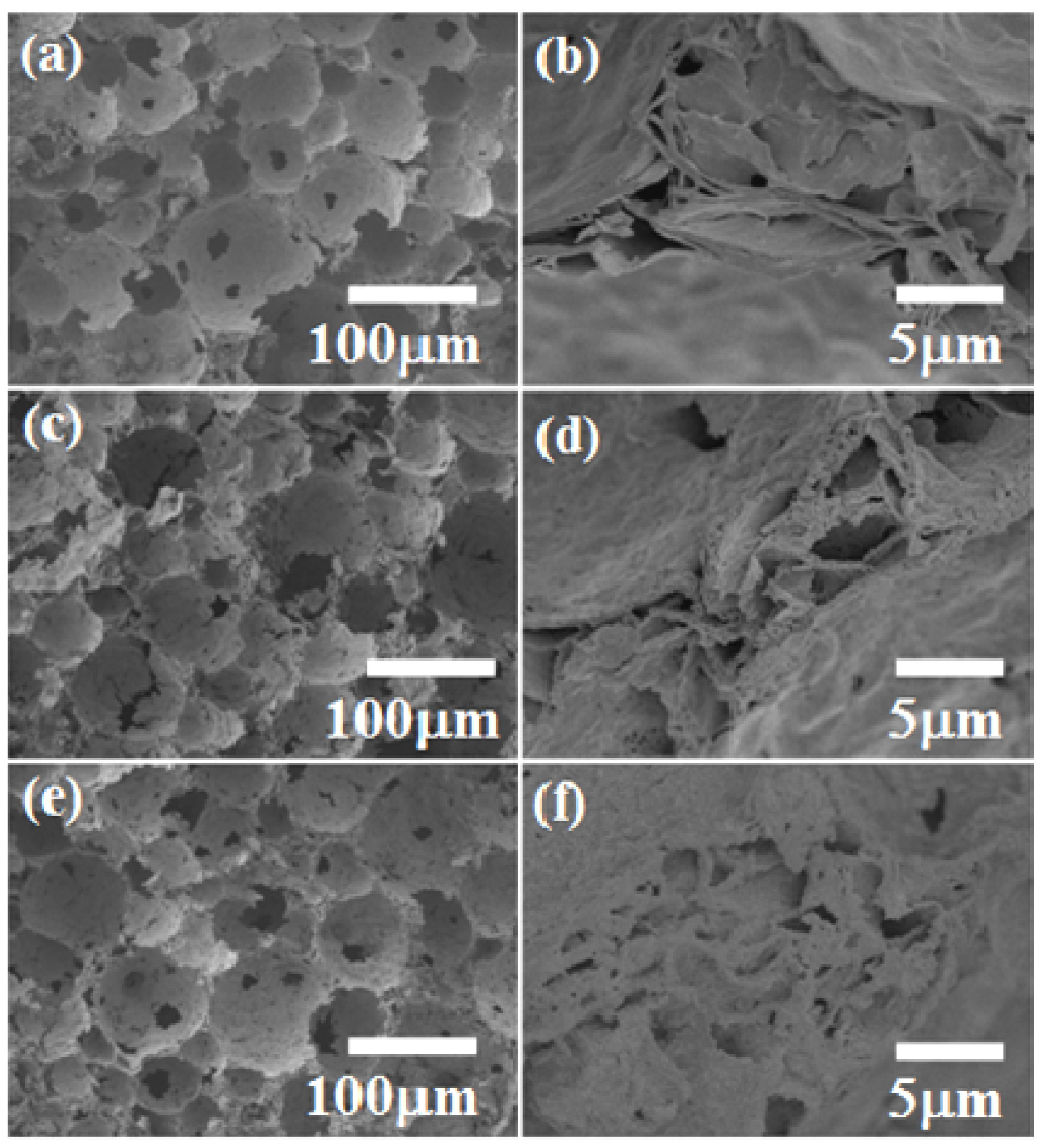
3.2. Effect of Thermal Treatment Temperature
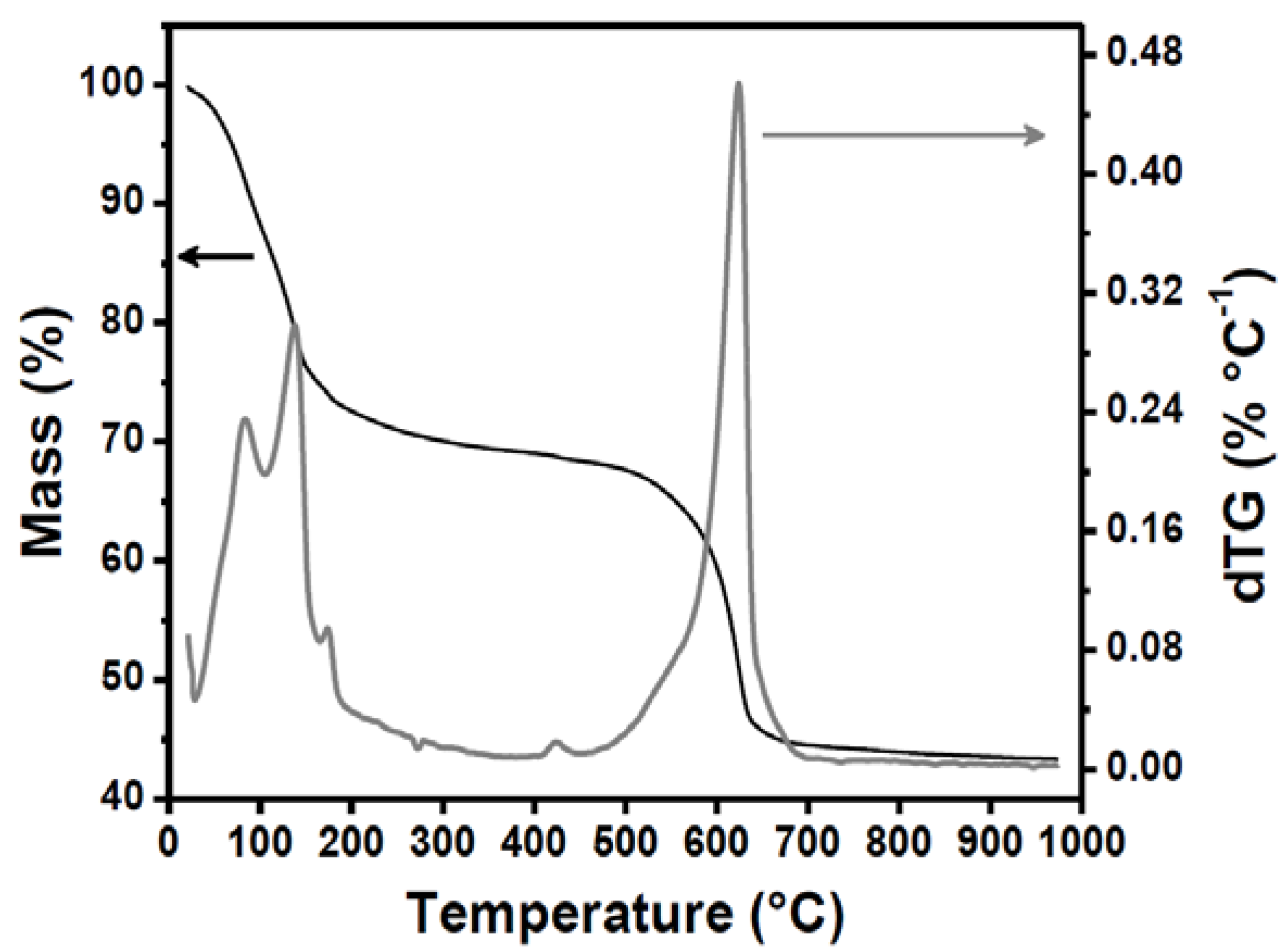
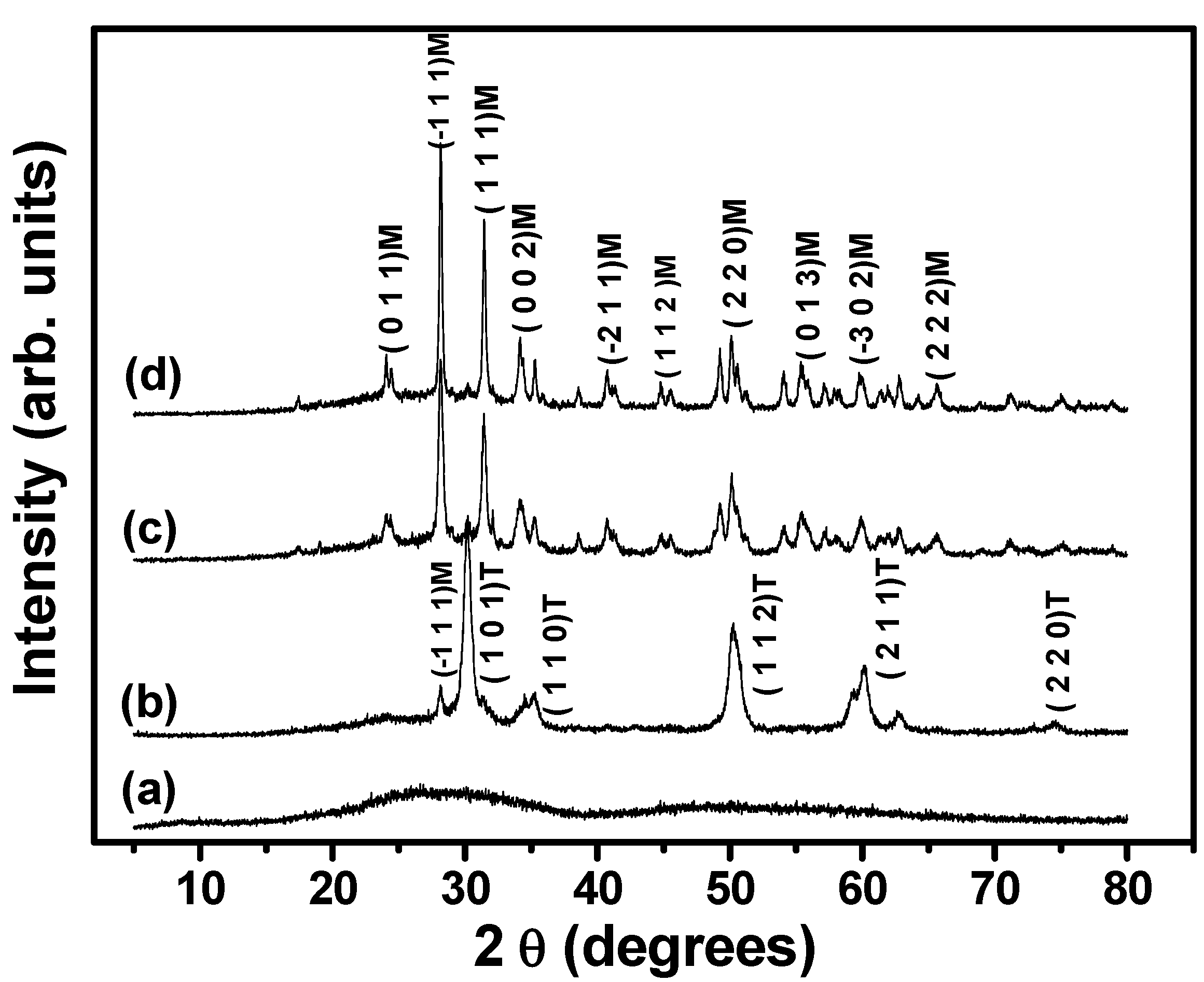
| Thermal treatment | Total pore volume (cm3 g−1) | Bulk density (g cm−3) | Porosity (%) | Mean macropore size (µm) | Mean supermesopores size (µm) |
|---|---|---|---|---|---|
| 500 °C | 2.52 ± 0.01 | 0.358 ± 0.001 | 90.1 ± 0.3 | 10.9 ± 0.2 | 2.3 ± 0.2 |
| 600 °C | 3.19 ± 0.05 | 0.293 ± 0.004 | 94 ± 1 | 13.6 ± 0.1 | 1.3 ± 0.1 |
| 800 °C | 2.71 ± 0.05 | 0.331 ± 0.006 | 93 ± 2 | 14.0 ± 0.1 | 1.25 ± 0.07 |
| 1000 °C | 2.6 ± 0.1 | 0.35 ± 0.02 | 91 ± 4 | 15.4 ± 0.1 | 1.15 ± 0.08 |
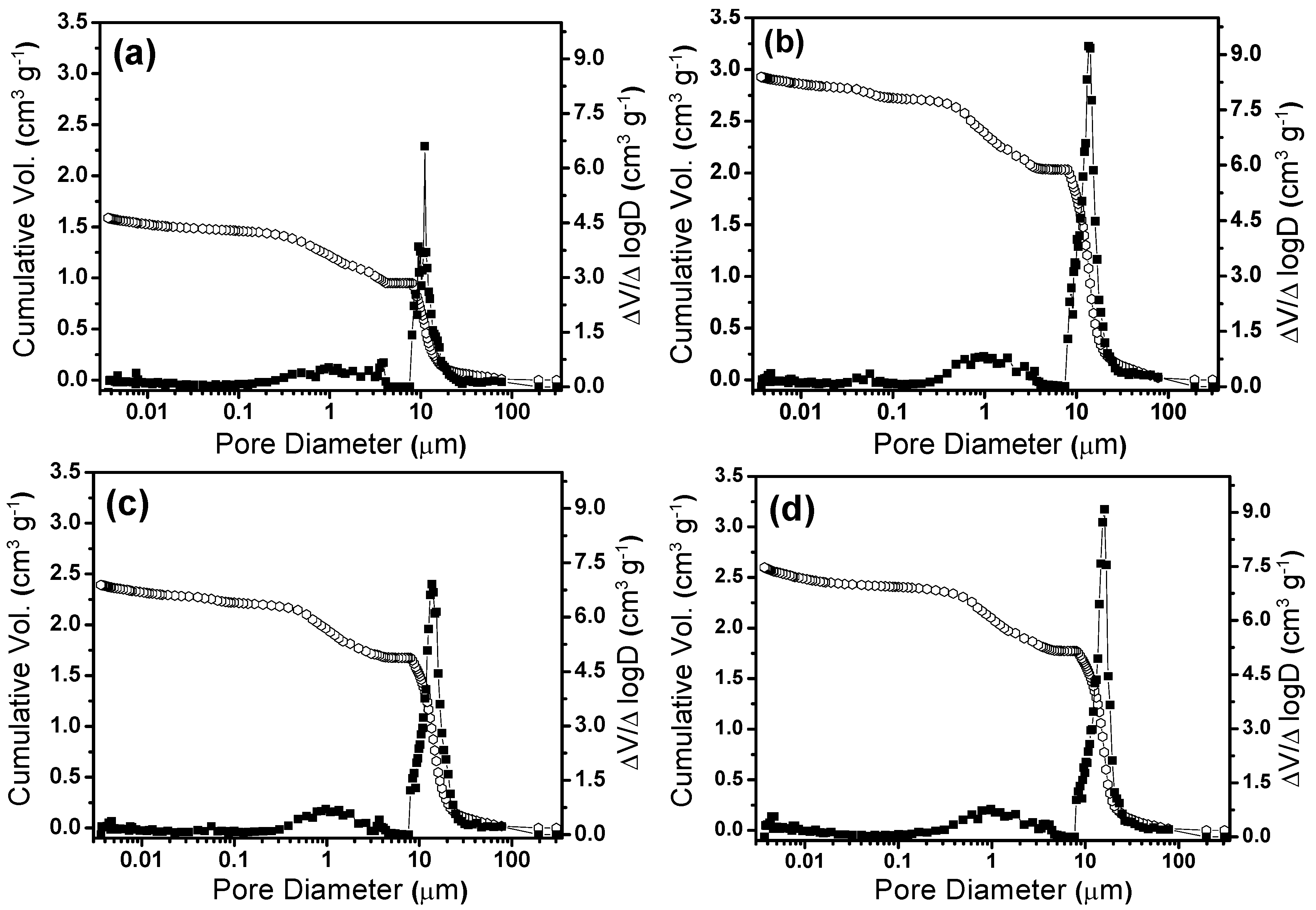
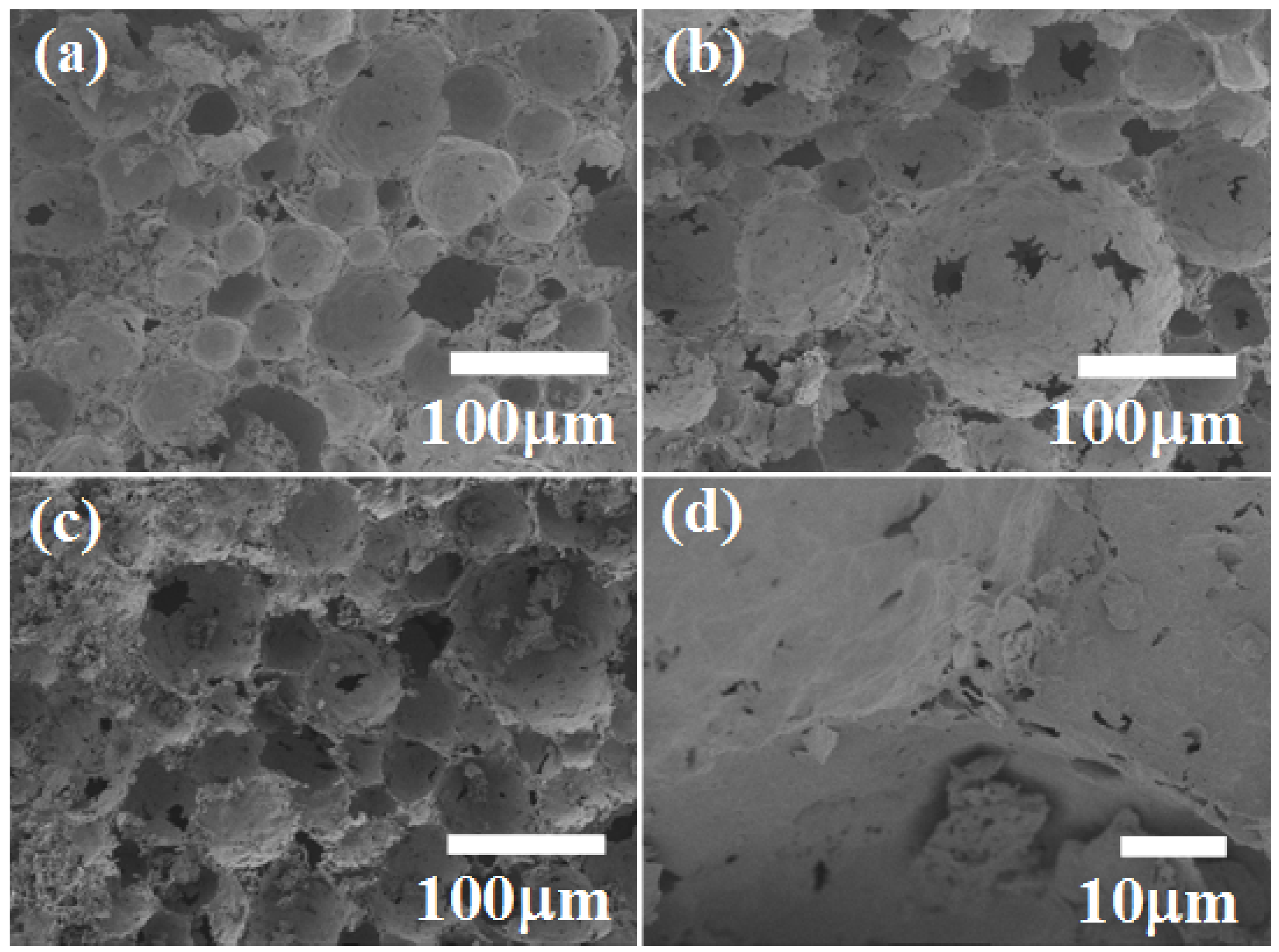
4. Conclusions
Acknowledgments
References
- Colombo, P. In praise of pores. Science 2008, 322, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Cardoso, D.; Hammer, P.; Garetto, T.; Pulcinelli, S.H.; Santilli, C.V. Efficiency of ethanol conversion induced by controlled modification of pore structure and acidic properties of alumina catalysts. Appl. Catal. A Gen. 2011, 398, 59–65. [Google Scholar] [CrossRef]
- Drisko, G.L.; Zelcer, A.; Luca, V.; Caruso, R.A.; Soler-Illia, G. One-pot synthesis of hierarchically structured ceramic monoliths with adjustable porosity. Chem. Mater. 2010, 22, 4379–4385. [Google Scholar] [CrossRef]
- Tiainen, H.; Wiedmer, D.; Haugen, H.J. Processing of highly porous TiO2 bone scaffolds with improved compressive strength. J. Eur. Ceram. Soc. 2013, 33, 15–24. [Google Scholar] [CrossRef]
- Nakanishi, K.; Tanaka, N. Sol-gel with phase separation. Hierarchically porous materials optimized for high-performance liquid chromatography separations. Acc. Chem. Res. 2007, 40, 863–873. [Google Scholar]
- Gawel, B.; Gawel, K.; Oye, G. Sol-gel synthesis of non-silica monolithic materials. Materials 2010, 3, 2815–2833. [Google Scholar] [CrossRef]
- Maekawa, H.; Esquena, J.; Bishop, S.; Solans, C.; Chmelka, B.F. Meso/macroporous inorganic oxide monoliths from polymer foams. Adv. Mater. 2003, 15, 591–596. [Google Scholar] [CrossRef]
- Backov, R. Combining soft matter and soft chemistry: Integrative chemistry towards designing novel and complex multiscale architectures. Soft Matter 2006, 2, 452–464. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J.; Huang, Y. Recent developments in gelcasting of ceramics. J. Eur. Ceram. Soc. 2011, 31, 2569–2591. [Google Scholar] [CrossRef]
- Colombo, P. Engineering porosity in polymer-derived ceramics. J. Eur. Ceram. Soc. 2008, 28, 1389–1395. [Google Scholar] [CrossRef]
- Martins, L.; Alves-Rosa, M.A.; Pulcinelli, S.H.; Santilli, C.V. Preparation of hierarchically structured porous aluminas by a dual soft template method. Microporous Mesoporous Mater. 2010, 132, 268–275. [Google Scholar] [CrossRef]
- Kanamori, K.; Nakanishi, K.; Hanada, T. Spinodal decomposition in siloxane sol-gel systems in macroporous media. Soft Matter 2009, 5, 3106–3113. [Google Scholar] [CrossRef]
- Alves-Rosa, M.A.; Martins, L.; Pulcinelli, S.H.; Santilli, C.V. Design of microstructure of zirconia foams from the emulsion template properties. Soft Matter 2013, 9, 550–558. [Google Scholar] [CrossRef]
- Alves-Rosa, M.A.; Santos, E.P.; Santilli, C.V.; Pulcinelli, S.H. Zirconia foams prepared by integration of the sol-gel method and dual soft template techniques. J. Non-Cryst. Solids 2008, 354, 4786–4789. [Google Scholar] [CrossRef]
- Carn, F.; Colin, A.; Achard, M.F.; Deleuze, H.; Sellier, E.; Birot, M.; Backov, R. Inorganic monoliths hierarchically textured via concentrated direct emulsion and micellar template. J. Mater. Chem. 2004, 14, 1370–1376. [Google Scholar] [CrossRef]
- Lins, R.F.; Alves-Rosa, M.A.; Pulcinelli, S.H.; Santilli, C.V. Formation of TiO2 ceramic foams from the integration of the sol-gel method with surfactants assembly and emulsion. J. Sol-Gel Sci. Technol. 2012, 63, 224–229. [Google Scholar] [CrossRef]
- Santos, E.P.; Santilli, C.V.; Pulcinelli, S.H. Effect of aging on the stability of ceramic foams prepared by thermostimulated sol-gel process. J. Sol-Gel Sci. Technol. 2003, 26, 165–169. [Google Scholar] [CrossRef]
- Carn, F.; Colin, A.; Achard, M.F.; Deleuze, H.; Sanchez, C.; Backov, R. Anatase and rutile TiO2 macrocellular foams: Air-liquid foaming sol-gel process towards controlling cell sizes, morphologies, and topologies. Adv. Mater. 2005, 17, 62–66. [Google Scholar] [CrossRef]
- Chiavacci, L.A.; Santilli, C.V.; Pulcinelli, S.H.; Bourgaux, C.; Briois, V. Role of the surface state and structural feature in the thermoreversible sol-gel transition of a zirconyl aqueous precursor modified by sulfuric acid. Chem. Mater. 2004, 16, 3995–4004. [Google Scholar] [CrossRef]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. The physical adsorptin of gases by nonporous solids: The type II isoterm. In Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1997; p. 303. [Google Scholar]
- Wang, M.; Du, H.Y.; Guo, A.R.; Hao, R.H.; Hou, Z.G. Microstructure control in ceramic foams via mixed cationic/anionic surfactant. Mater. Lett. 2012, 88, 97–100. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Alves-Rosa, M.A.; Sanhueza, C.S.S.; Santilli, C.V.; Pulcinelli, S.H.; Briois, V. Stimuli-responsive controlled growth of mono- and bidimensional particles from basic zirconium sulfate hydrosols. J. Phys. Chem. B 2008, 112, 9006–9012. [Google Scholar] [CrossRef] [PubMed]
- Boskovic, G.C.; Zarubica, A.R.; Kovacevic, M.N.; Putanov, P.S. Precursor memory effect determining structural properties of sulfated zirconia. J. Therm. Anal. Calorim. 2008, 91, 849–854. [Google Scholar] [CrossRef]
- Srinivasan, R.; Keogh, R.A.; Milburn, D.R.; Davis, B.H. Sulfated zirconia catalysts: Characterization by tga/dta mass spectrometry. J. Catal. 1995, 153, 123–130. [Google Scholar] [CrossRef]
- Ahmed, A.I.; El-Hakam, S.A.; Samra, S.E.; El-Khouly, A.A.; Khder, A.S. Structural characterization of sulfated zirconia and their catalytic activity in dehydration of ethanol. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 62–70. [Google Scholar] [CrossRef]
- Yadav, G.D.; Nair, J.J. Sulfated zirconia and its modified versions as promising catalysts for industrial processes. Microporous Mesoporous Mater. 1999, 33, 1–48. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Beozzo, C.C.; Alves-Rosa, M.A.; Pulcinelli, S.H.; Santilli, C.V. Liquid Foam Templates Associated with the Sol-Gel Process for Production of Zirconia Ceramic Foams. Materials 2013, 6, 1967-1979. https://doi.org/10.3390/ma6051967
Beozzo CC, Alves-Rosa MA, Pulcinelli SH, Santilli CV. Liquid Foam Templates Associated with the Sol-Gel Process for Production of Zirconia Ceramic Foams. Materials. 2013; 6(5):1967-1979. https://doi.org/10.3390/ma6051967
Chicago/Turabian StyleBeozzo, Cristiane Carolina, Marinalva Aparecida Alves-Rosa, Sandra Helena Pulcinelli, and Celso Valentim Santilli. 2013. "Liquid Foam Templates Associated with the Sol-Gel Process for Production of Zirconia Ceramic Foams" Materials 6, no. 5: 1967-1979. https://doi.org/10.3390/ma6051967
APA StyleBeozzo, C. C., Alves-Rosa, M. A., Pulcinelli, S. H., & Santilli, C. V. (2013). Liquid Foam Templates Associated with the Sol-Gel Process for Production of Zirconia Ceramic Foams. Materials, 6(5), 1967-1979. https://doi.org/10.3390/ma6051967




