Evaluation of Biological Pretreatment of Rubberwood with White Rot Fungi for Enzymatic Hydrolysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Microorganisms and Inoculation
2.2. Biomass Preparation
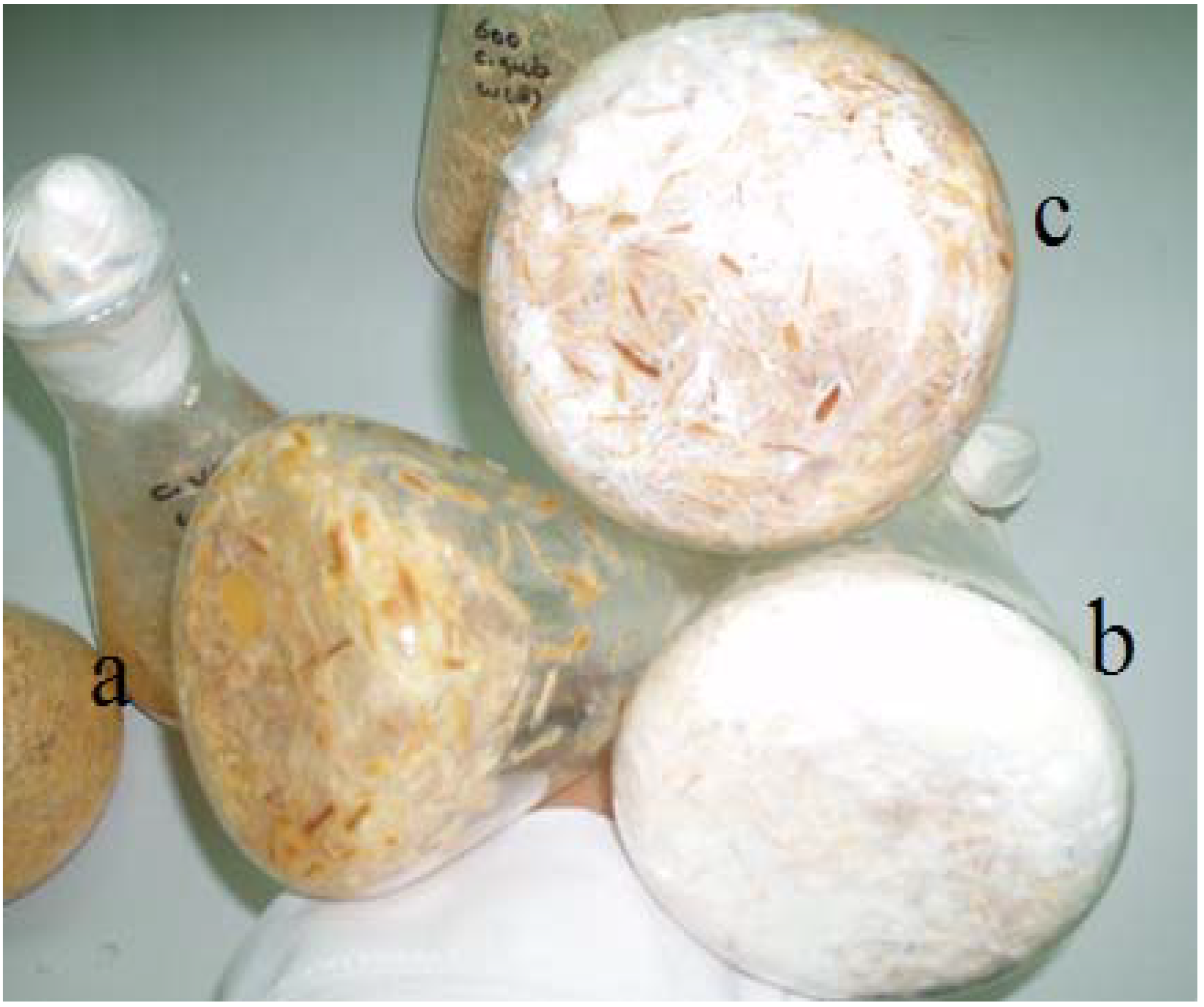
2.3. Biological Pretreatment with White Rot Fungi
2.4. Enzyme Hydrolysis
2.5. Analysis Methods
2.5.1. Analysis of Chemical Composition
2.5.2. Determination of Reducing Sugar
2.5.3. X-ray Diffraction Analysis
2.5.4. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
2.5.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Fungal Pretreatment on Chemical Composition
| Decay time (day) | Selectivity value2 | Weight loss (%) | ||
| Lignin | Hemicellulose | Cellulose | ||
| C.subvermispora | ||||
| 30 | 3.65a (0.27) | 18.80a ( 0.57) | 25.13c (0.69) | 5.17c (0.46) |
| 60 | 4.56a (0.36) | 37.30a (0.55) | 36.02a (1.48) | 8.20c (0.42) |
| 90 | 4.75a (0.31) | 45.06a (0.82) | 42.08a (1.16) | 9.50c (0.48) |
| T. versicolor | ||||
| 30 | 1.08c (0.03) | 13.34c (0.41) | 28.17a (0.54) | 12.3a (0.23) |
| 60 | 1.16c (0.03) | 26.88c (0.74) | 33.20b (1.30) | 23.12a (0.53) |
| 90 | 1.07c (0.03) | 34.40c (0.18) | 37.90b (0.58) | 32.06a (0.69) |
| Mixed culture | ||||
| 30 | 1.76b (0.15) | 15.34b (0.3) | 25.52b (0.13) | 8.70b (0.12) |
| 60 | 1.62b (0.02) | 28.75b (0.48) | 32.36b (0.79) | 17.70b (0.45) |
| 90 | 1.53b (0.03) | 37.68b (0.37) | 40.83a (0.36) | 24.53b (0.40) |
3.2. X-ray Diffraction
| Sample | CrI (%) | ||
| 30 d | 60 d | 90 d | |
| Untreated | 43.12 | 43.12 | 43.12 |
| C. subvermispora | 52.37 | 65.84 | 66.71 |
| T. versicolor | 55.48 | 61.19 | 51.68 |
| Mixed culture | 52.60 | 62.38 | 52.14 |
3.3. FT-IR Analysis
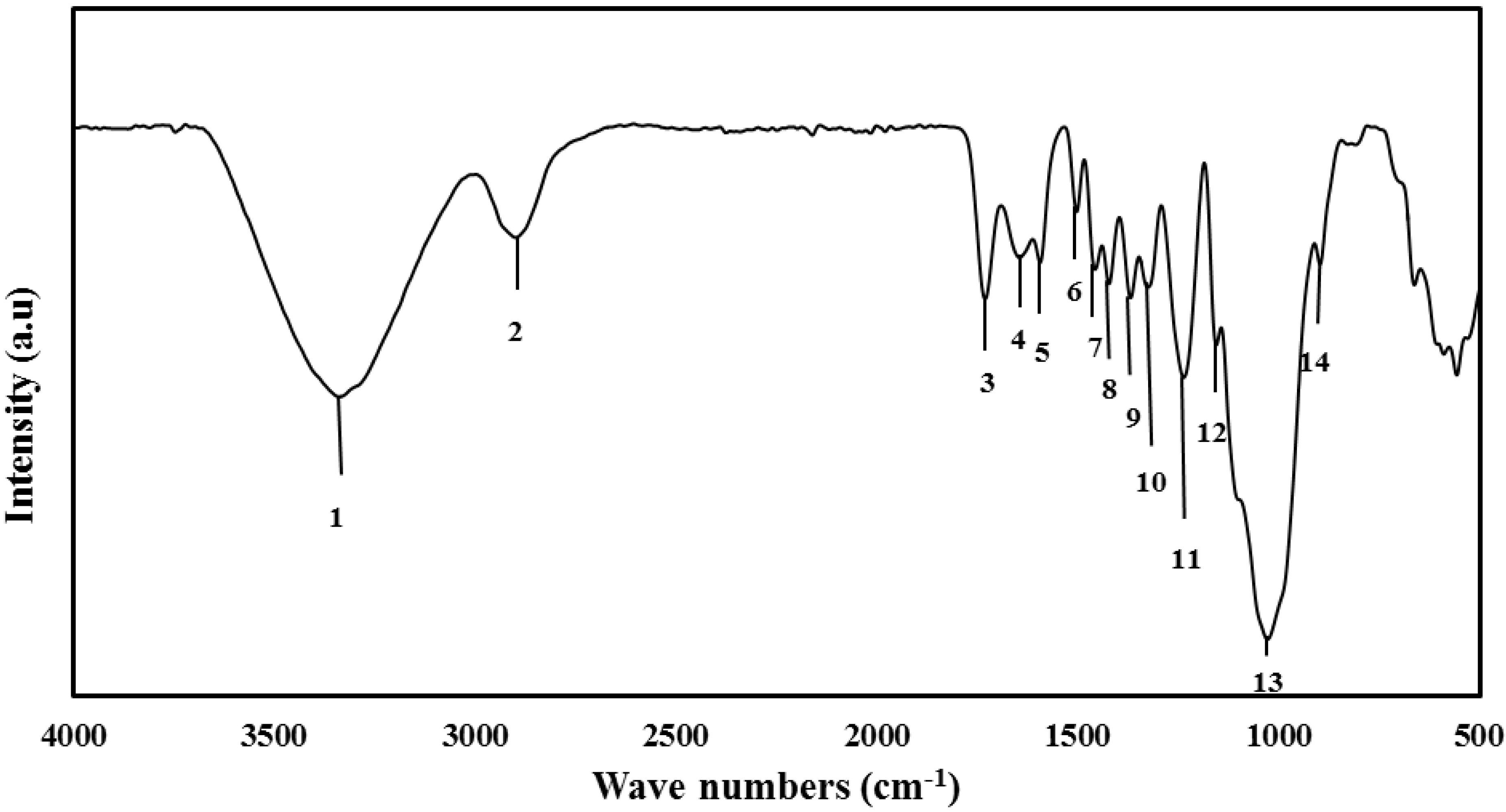
3.3.1. Undecayed Rubberwood
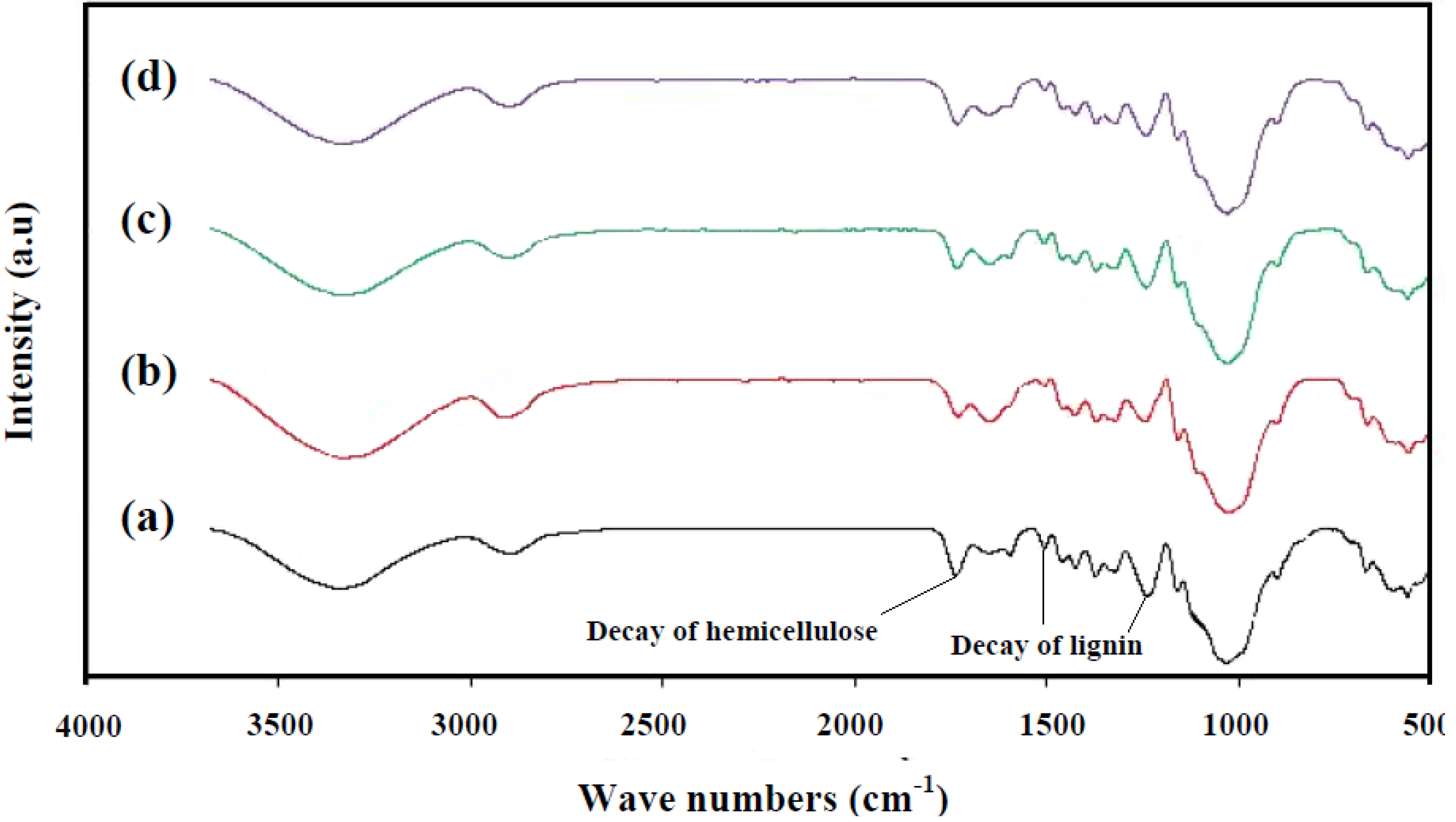
3.3.2. Comparison of Decayed Rubberwood by C. Subvermispora, T. Versicolor, and Mixed Culture
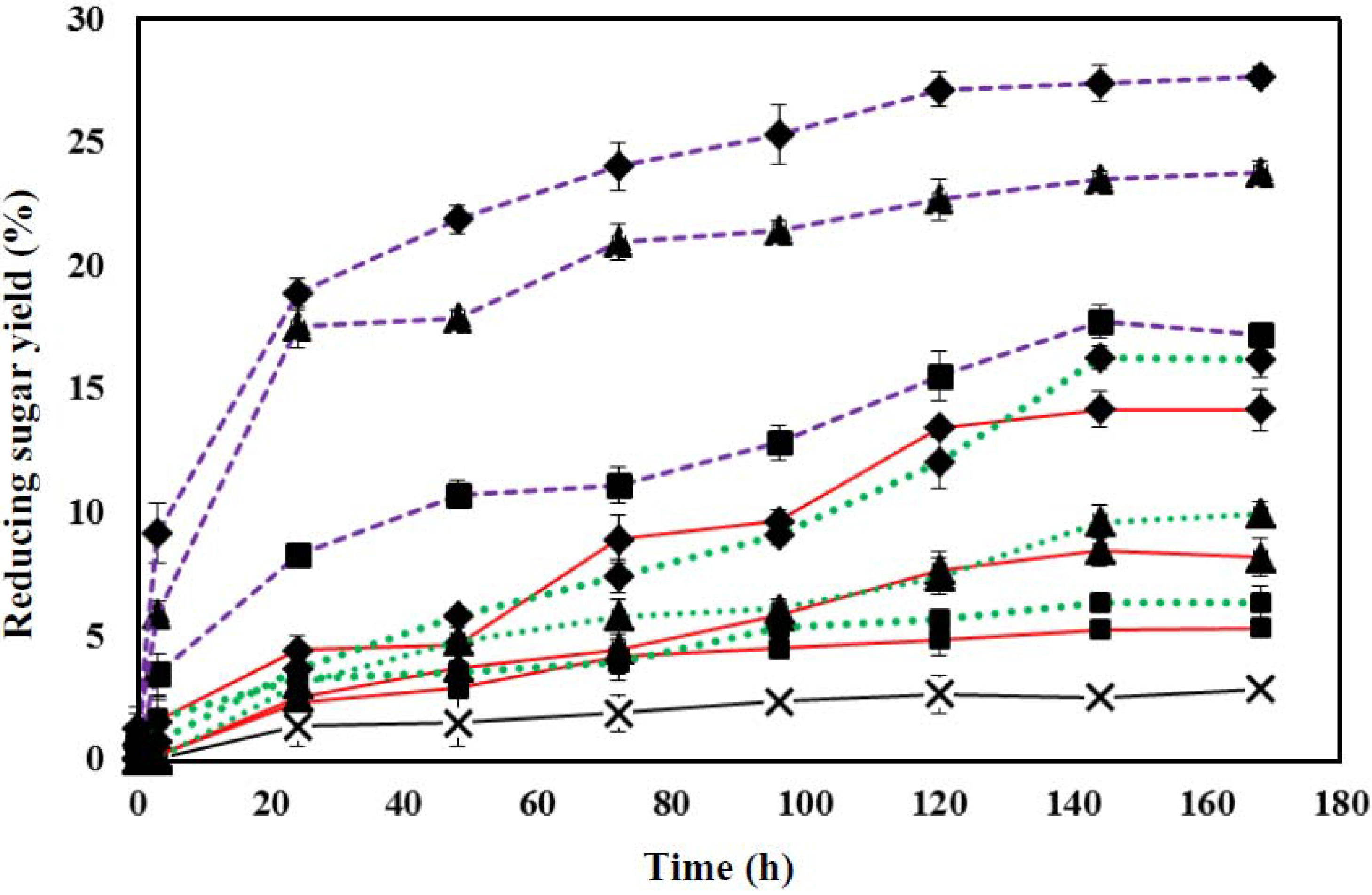
3.4. Effect of Pretreatment Time on Enzymatic Hydrolysis
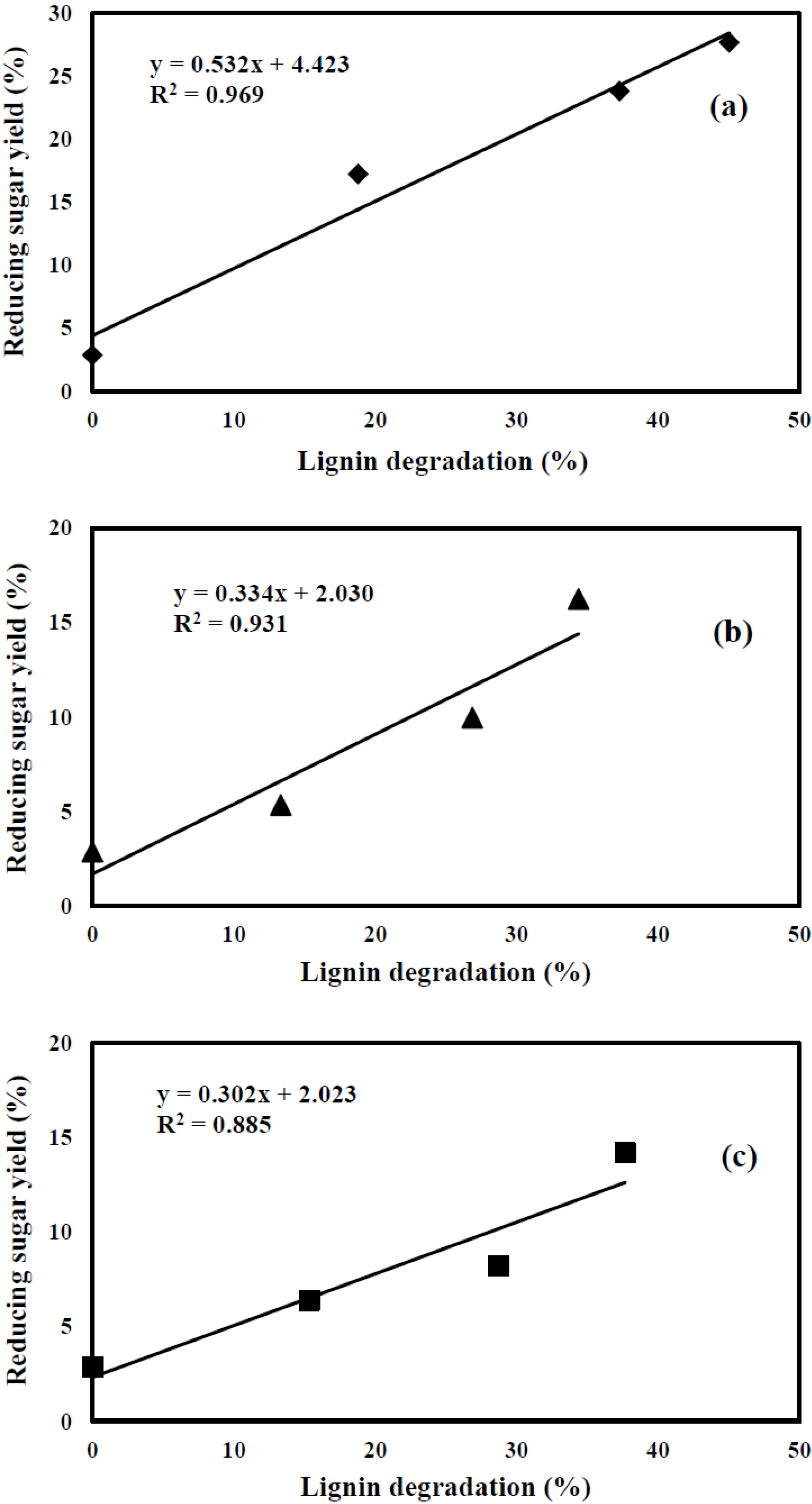
3.5. Relationship between Lignin Content and Reducing Sugar Yield
4. Conclusions
Acknowledgments
References
- Srinivasakannan, C.; Zailani Abu Bakar, M. Production of activated carbon from rubber wood sawdust. Biomass Bioenergy 2004, 27, 89–96. [Google Scholar] [CrossRef]
- Alhasan, A.M.; Kuang, D.; Mohammad, A.B.; Sharma-Shivappa, R.R. Combined effect of nitric acid and sodium hydroxide pretreatments on enzymatic saccharification of rubber wood (heavea brasiliensis). Int. J. Chem. Technol. 2010, 2, 12–20. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Ttechnol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Yu, H.; Guo, G.; Zhang, X.; Yan, K.; Xu, C. The effect of biological pretreatment with the selective white-rot fungus echinodontium taxodii on enzymatic hydrolysis of softwoods and hardwoods. Bioresour. Technol. 2009, 100, 5170–5175. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Amirta, R.; Tanabe, T.; Watanabe, T.; Honda, Y.; Kuwahara, M.; Watanabe, T. Methane fermentation of japanese cedar wood pretreated with a white rot fungus, ceriporiopsis subvermispora. J. Biotechnol. 2006, 123, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hammel, K.E.; Cullen, D. Role of fungal peroxidases in biological ligninolysis. Curr. Opin. Plant Biol. 2008, 11, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Have, R.; Teunissen, P.J.M. Oxidative mechanisms involved in lignin degradation by white-rot fungi. Chem. Rev. Columb. 2001, 101, 3397–3414. [Google Scholar] [CrossRef]
- Archibald, F.; Bourbonnais, R.; Jurasek, L.; Paice, M.; Reid, I. Kraft pulp bleaching and delignification by trametes versicolor. J. Biotechnol. 1997, 53, 215–236. [Google Scholar] [CrossRef]
- De Souza-Cruz, P.B.; Freer, J.; Siika-Aho, M.; Ferraz, A. Extraction and determination of enzymes produced by ceriporiopsis subvermispora during biopulping of pinus taeda wood chips. Enzym. Microb. Technol. 2004, 34, 228–234. [Google Scholar]
- Heidorne, F.O.; Magalhães, P.O.; Ferraz, A.L.; Milagres, A.M.F. Characterization of hemicellulases and cellulases produced by ceriporiopsis subvermispora grown on wood under biopulping conditions. Enzym. Microb. Technol. 2006, 38, 436–442. [Google Scholar] [CrossRef]
- Asiegbu, F.; Paterson, A.; Smith, J. The effects of co-fungal cultures and supplementation with carbohydrate adjuncts on lignin biodegradation and substrate digestibility. World J. Microb. Biotechnol. 1996, 12, 273–279. [Google Scholar] [CrossRef]
- Chi, Y.; Hatakka, A.; Maijala, P. Can co-culturing of two white-rot fungi increase lignin degradation and the production of lignin-degrading enzymes? Int. Biodeterior. Biodegrad. 2007, 59, 32–39. [Google Scholar] [CrossRef]
- Parani, K.; Eyini, M. Effect of co-fungal treatment on biodegradation of coffee pulp waste in solid state fermentation. Asian J. Exp. Biol. Sci. 2010, 1, 352–359. [Google Scholar]
- Gutierrez-Correa, M.; Tengerdy, R.P. Production of cellulase on sugar cane bagasse by fungal mixed culture solid substrate fermentation. Biotechnol. Lett. 1997, 19, 665–667. [Google Scholar] [CrossRef]
- Dowe, N.; McMillan, J. SSf Experimental Protocols: Lignocellulosic Biomass Hydrolysis and Fermentation; National Renewable Energy Laboratory: Golden, CO, USA, 2001. [Google Scholar]
- Adney, B.; Baker, J. Measurement of Cellulase Activities; National Renewable Energy Laboratory: Golden, CO, USA, 1996. [Google Scholar]
- Ehrman, T. Standard Method for Determination of Total Solids in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 1994. [Google Scholar]
- Ghose, T. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar]
- Segal, L.; Creely, J.; Martin, A.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Statistical Analysis Software (SAS) Program, version 6.12; SAS Institute Inc.: Cary, NC, USA, 1988.
- Fan, L.; Gharpuray, M.M.; Lee, Y.H.; Aiba, S.; Fiechter, A.; Klein, J.; Schügerl, K. Cellulose Hydrolysis; Springer-Verlag: Berlin, Germany, 1987. [Google Scholar]
- Hatakka, A.I. Pretreatment of wheat straw by white-rot fungi for enzymic saccharification of cellulose. Appl. Microb. Biotechnol. 1983, 18, 350–357. [Google Scholar] [CrossRef]
- Camarero, S.; Galletti, G.C.; Martinez, A.T. Preferential degradation of phenolic lignin units by two white rot fungi. Appl. Environ. Microb. 1994, 60, 4509–4516. [Google Scholar]
- Ferraz, A.; Parra, C.; Freer, J.; Baeza, J.; Rodríguez, J. Characterization of white zones produced on pinus radiata wood chips by ganoderma australe and ceriporiopsis subvermispora. World J. Microb. Biotechnol. 2000, 16, 641–645. [Google Scholar] [CrossRef]
- Gharpuray, M.; Lee, Y.H.; Fan, L. Structural modification of lignocellulosics by pretreatments to enhance enzymatic hydrolysis. Biotechnol. Bioeng. 1983, 25, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, A.; Sain, M. Biocomposites from wheat straw nanofibers: Morphology, thermal and mechanical properties. Compos. Sci. Technol. 2008, 68, 557–565. [Google Scholar] [CrossRef]
- Highley, T.; Kirk, T.; Ibach, R. Properties of Cellulose Degraded by the Brown-Rot Fungus Postia Placenta; International Research Group on Wood Preservation: Stockholm, Sweden, 1989. [Google Scholar]
- Kleman-Leyer, K.; Agosin, E.; Conner, A.H.; Kirk, T.K. Changes in molecular size distribution of cellulose during attack by white rot and brown rot fungi. Appl. Environ. Microb. 1992, 58, 1266–1270. [Google Scholar]
- Howell, C.; Paredes, J.; Shaler, S.; Jellison, J. Decay Resistance Properties of Hemicellulose-Extracted Oriented Strand Board; International Research Group on Wood Preservation: Stockholm, Sweden, 2008. [Google Scholar]
- Khalil, H.; Ismail, H.; Rozman, H.; Ahmad, M. The effect of acetylation on interfacial shear strength between plant fibres and various matrices. Eur. Polym. J. 2001, 37, 1037–1045. [Google Scholar] [CrossRef]
- Pandey, K.; Pitman, A. Ftir studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Le Troedec, M.; Sedan, D.; Peyratout, C.; Bonnet, J.P.; Smith, A.; Guinebretiere, R.; Gloaguen, V.; Krausz, P. Influence of various chemical treatments on the composition and structure of hemp fibres. Compos. Part A Appl. Sci. Manuf. 2008, 39, 514–522. [Google Scholar]
- Genestar, C.; Palou, J. Sem-ftir spectroscopic evaluation of deterioration in an historic coffered ceiling. Anal. Bioanal. Chem. 2006, 384, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Bodirlau, R.; Teaca, C.A.; Spiridon, I. Chemical modification of beech wood: Effect on thermal stability. Bioresources 2008, 3, 789–800. [Google Scholar]
- Sun, X.F.; Sun, R.; Tomkinson, J.; Baird, M. Degradation of wheat straw lignin and hemicellulosic polymers by a totally chlorine-free method. Poly. Degrad. Stab. 2004, 83, 47–57. [Google Scholar] [CrossRef]
- Faix, O.; Bremer, J.; Schmidt, O.; Tatjana, S.J. Monitoring of chemical changes in white-rot degraded beech wood by pyrolysis—Gas chromatography and fourier-transform infrared spectroscopy. J. Anal. Appl. Pyrolysis 1991, 21, 147–162. [Google Scholar] [CrossRef]
- Öhgren, K.; Bura, R.; Saddler, J.; Zacchi, G. Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresour. Technol. 2007, 98, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Gwak, K.; Park, J.; Park, M.; Choi, D.; Kwon, M.; Choi, I. Biological pretreatment of softwood pinus densiflora by three white rot fungi. J. Microb. 2007, 45, 485–491. [Google Scholar]
- Zhang, X.; Xu, C.; Wang, H. Pretreatment of bamboo residues with coriolus versicolor for enzymatic hydrolysis. J. Biosci. Bioeng. 2007, 104, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Mooney, C.A.; Mansfield, S.D.; Touhy, M.G.; Saddler, J.N. The effect of initial pore volume and lignin content on the enzymatic hydrolysis of softwoods. Bioresour. Technol. 1998, 64, 113–119. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Microbial pretreatment of corn stover with ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol production. Bioresour. Technol. 2010, 101, 6398–6403. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nazarpour, F.; Abdullah, D.K.; Abdullah, N.; Zamiri, R. Evaluation of Biological Pretreatment of Rubberwood with White Rot Fungi for Enzymatic Hydrolysis. Materials 2013, 6, 2059-2073. https://doi.org/10.3390/ma6052059
Nazarpour F, Abdullah DK, Abdullah N, Zamiri R. Evaluation of Biological Pretreatment of Rubberwood with White Rot Fungi for Enzymatic Hydrolysis. Materials. 2013; 6(5):2059-2073. https://doi.org/10.3390/ma6052059
Chicago/Turabian StyleNazarpour, Forough, Dzulkefly Kuang Abdullah, Norhafizah Abdullah, and Reza Zamiri. 2013. "Evaluation of Biological Pretreatment of Rubberwood with White Rot Fungi for Enzymatic Hydrolysis" Materials 6, no. 5: 2059-2073. https://doi.org/10.3390/ma6052059




