2. Results and Discussion
Fourier transform infrared (FTIR) spectra of pristine CNTs and oxidised carbon nanotubes (OCNTs) are shown in
Figure 1. The OCNTs present peaks at 1208 cm
−1, 1350 cm
−1 and 1713 cm
−1 corresponding to the characteristic C–O, O–H and C=O stretching vibrations of carboxylic groups [
13,
15]. The peak at 1560 cm
−1 is attributable to the influence of the C=O vibration of carboxilate groups produced in the oxidation process [
14].
Figure 1 also shows the typical peaks of graphene oxide (GO) for C–O stretching of epoxy and alcoxi at 1058 cm
−1 and 1240 cm
−1 respectively [
6,
7]. The peaks at 1395 cm
−1 and 1728 cm
−1 reflect the carboxylic acid groups due to the O–H in-plane deformation for the former and C=O stretching vibration for the latter [
5,
7,
8]. The peak at 1616 cm
−1 is attributable to skeletal vibrations of unoxidised graphitic domains and also to the stretching vibration of intercalated water. This is also observed in the broad peak around 3300 cm
−1 and is a feature of the O–H stretching vibration [
5,
6,
8]. Several studies have reported that complete water removal from GO is practically impossible [
8]. After reduction the peaks of oxygen functional groups vanished. The weak peaks at 1030 cm
−1, 1187 cm
−1 and 1333 cm
−1 are features of the C–N stretching [
9]. The peak at 1698 cm
−1 is ascribed to the C=O stretching [
9]. This indicates a few amine groups adhered to the graphene surface. The peak at 1570 cm
−1 is in accordance with the C=C vibrations contributed from aromatic ring mode [
10].
Figure 1.
Fourier transform infrared spectroscopy (FTIR) spectra of: (a) Carbon nanotubes (CNTs); (b) oxidised carbon nanotubes (OCNTs); (c) reduced graphene oxide (RGO) and (d) graphene oxide (GO).
Figure 1.
Fourier transform infrared spectroscopy (FTIR) spectra of: (a) Carbon nanotubes (CNTs); (b) oxidised carbon nanotubes (OCNTs); (c) reduced graphene oxide (RGO) and (d) graphene oxide (GO).
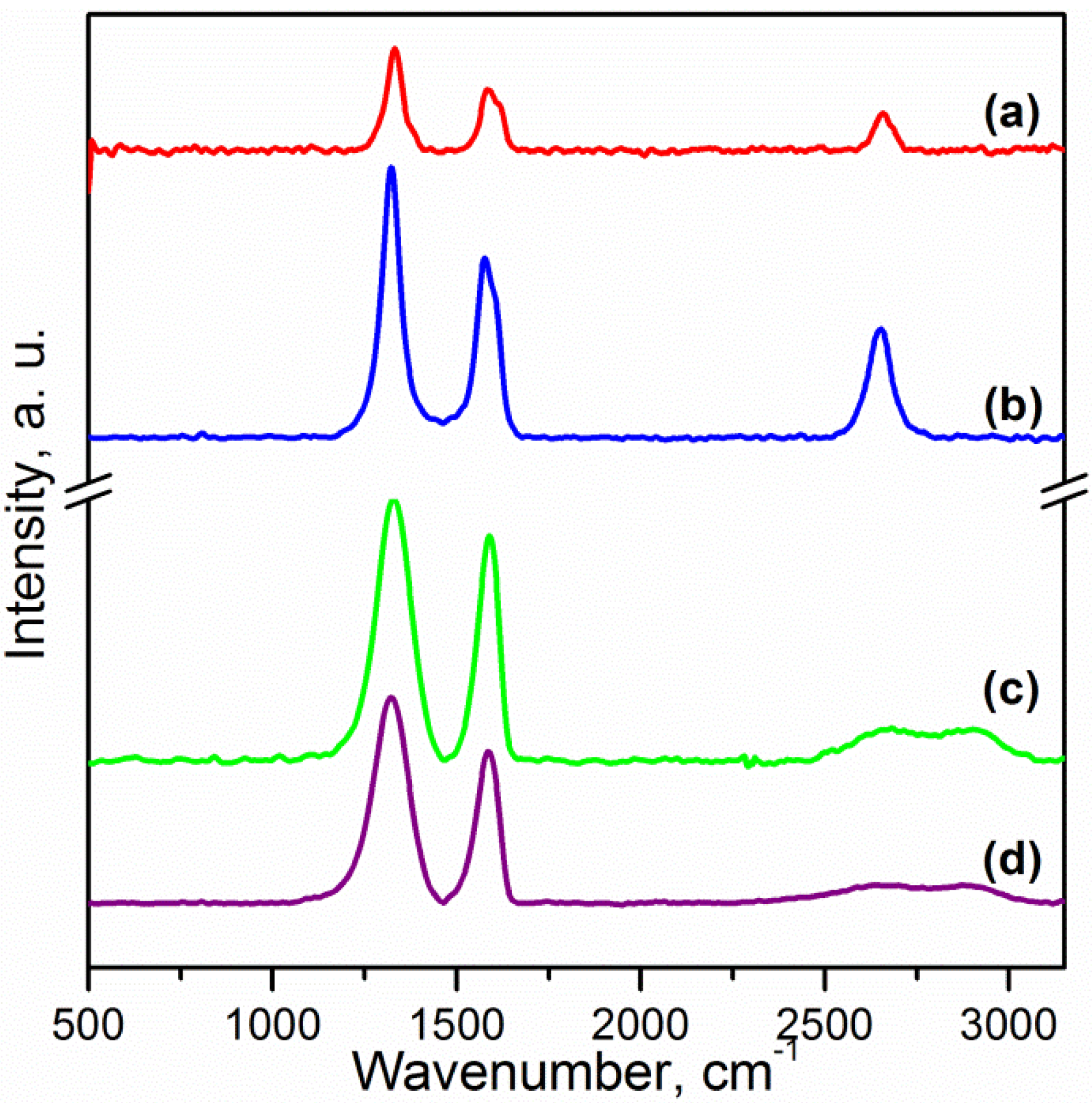
Raman spectroscopy is a suitable technique to study the ordered/disordered crystal structures of carbonaceous materials. These materials show similar features in the 800–2000 cm
−1 region, which is of interest for CNTs and graphene [
2]. The usual characteristics of carbon materials in Raman spectra are the G and the D bands. The G band (~1580 cm
−1) is usually attributed to the E
2g phonon of C sp
2 atoms and the D band (~1320 cm
−1) is due to the breathing mode of κ-point phonons of A
1g symmetry [
3]. The D band reflects the local defects and disorders particularly located at the edges of graphene. The Raman spectra of carbon nanotubes and graphene are shown in
Figure 2. All spectra display the G and D bands. The intensity ratio of the D and the G band (I
D/I
G) for CNTs and OCNTs were 1.53 and 1.40. This has been attributed to sp
2 C atoms converted to sp
3 C atoms at the surface of the CNTs after functionalisation [
12]. The values of the I
D/I
G ratio for reduced graphene oxide (RGO) and GO are 1.32 and 1.14 respectively. A larger I
D/I
G peak intensity ratio has been assigned to a decrease in the average size of the sp
2 domains upon reduction of GO, meaning that the newly created graphitic domains are smaller but more numerous in number [
27]. Moreover a 5 cm
−1 redshift in the G band of RGO was observed. This feature shows the successful reduction of graphene oxide [
7]. Another fingerprint of carbon nanomaterials is the 2D band (sometimes labelled as D′ band) at about 2650 cm
−1. The shape, position and intensity relative to the D band of this peak depend markedly on the number of layers in graphene [
28]. This band is difficult to observe in the spectra; however this weak and broad peak is in accordance with studies of chemically reduced graphene [
6].
Figure 2.
Raman spectra of: (a) CNTs; (b) OCNTs; (c) RGO and (d) GO.
Figure 2.
Raman spectra of: (a) CNTs; (b) OCNTs; (c) RGO and (d) GO.
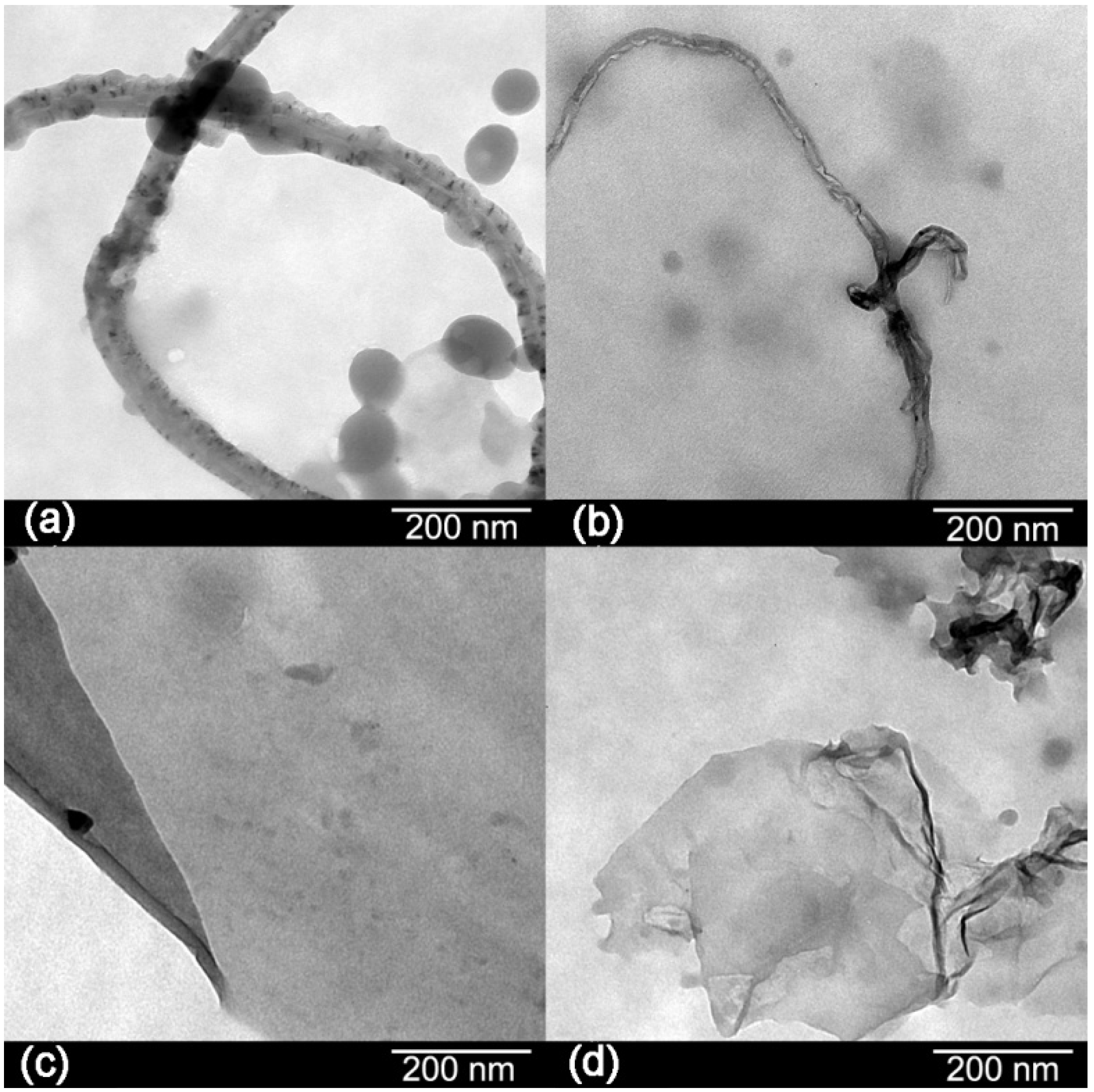
The morphologies of the carbon materials are shown in
Figure 3. Transmission electron microscopy (TEM) images showed black spots in the structure of the CNTs indicating impurities. After oxidation, there are few black spots in their structure which means that most of the impurities have been removed [
8]. Graphene sheets appear as a transparent thin paper structure with some folds. Their resemblance of crumpled silk veil waves that were corrugated and scrolled is intrinsic to graphene sheets [
6]. Wrinkling in GO is due to defects and attraction between oxygen groups formed on the surface of the sheet [
10].
Figure 3.
Transmission electron microscopy (TEM) images of: (a) CNTs; (b) OCNTs; (c) RGO and (d) GO.
Figure 3.
Transmission electron microscopy (TEM) images of: (a) CNTs; (b) OCNTs; (c) RGO and (d) GO.
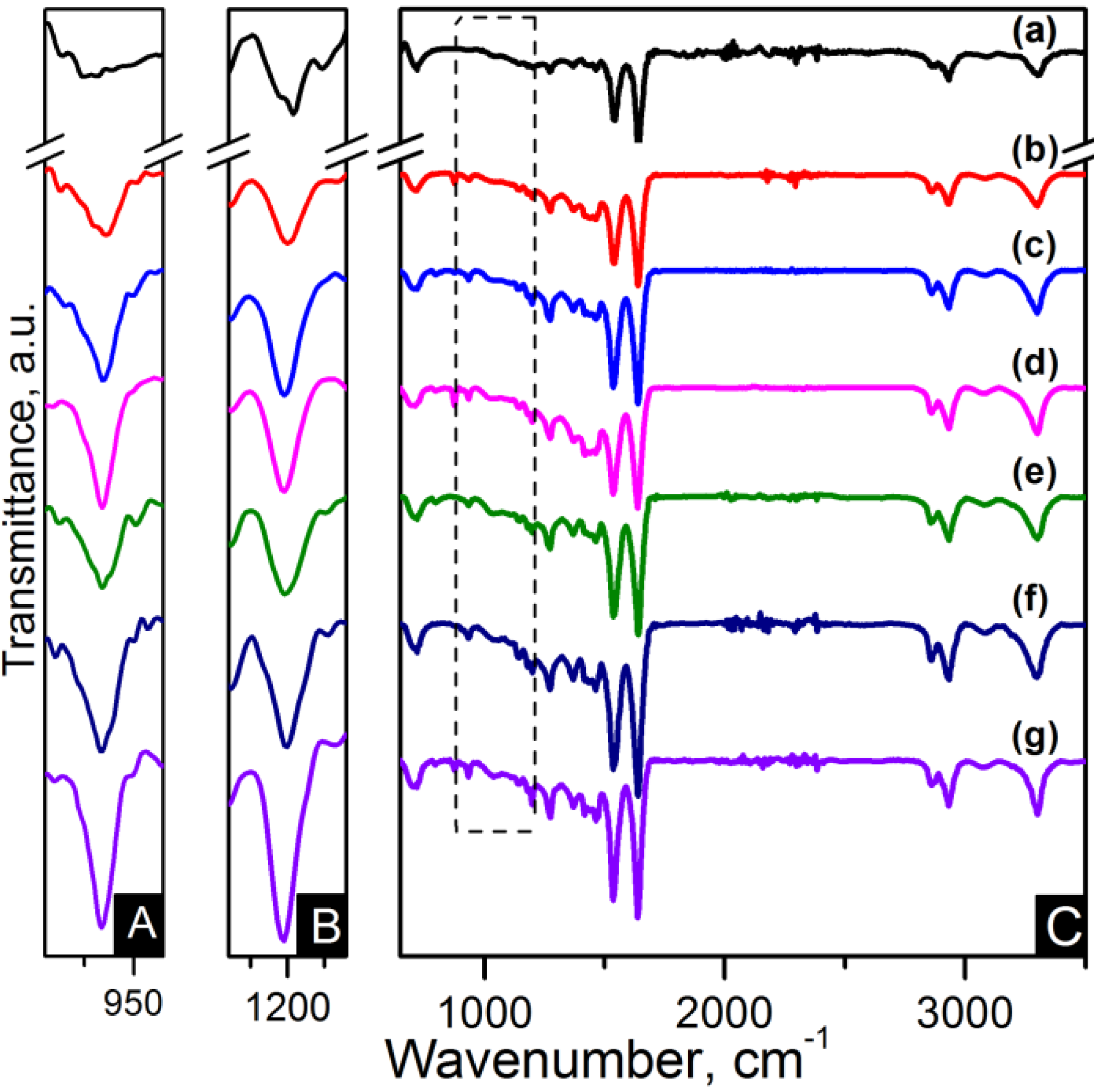
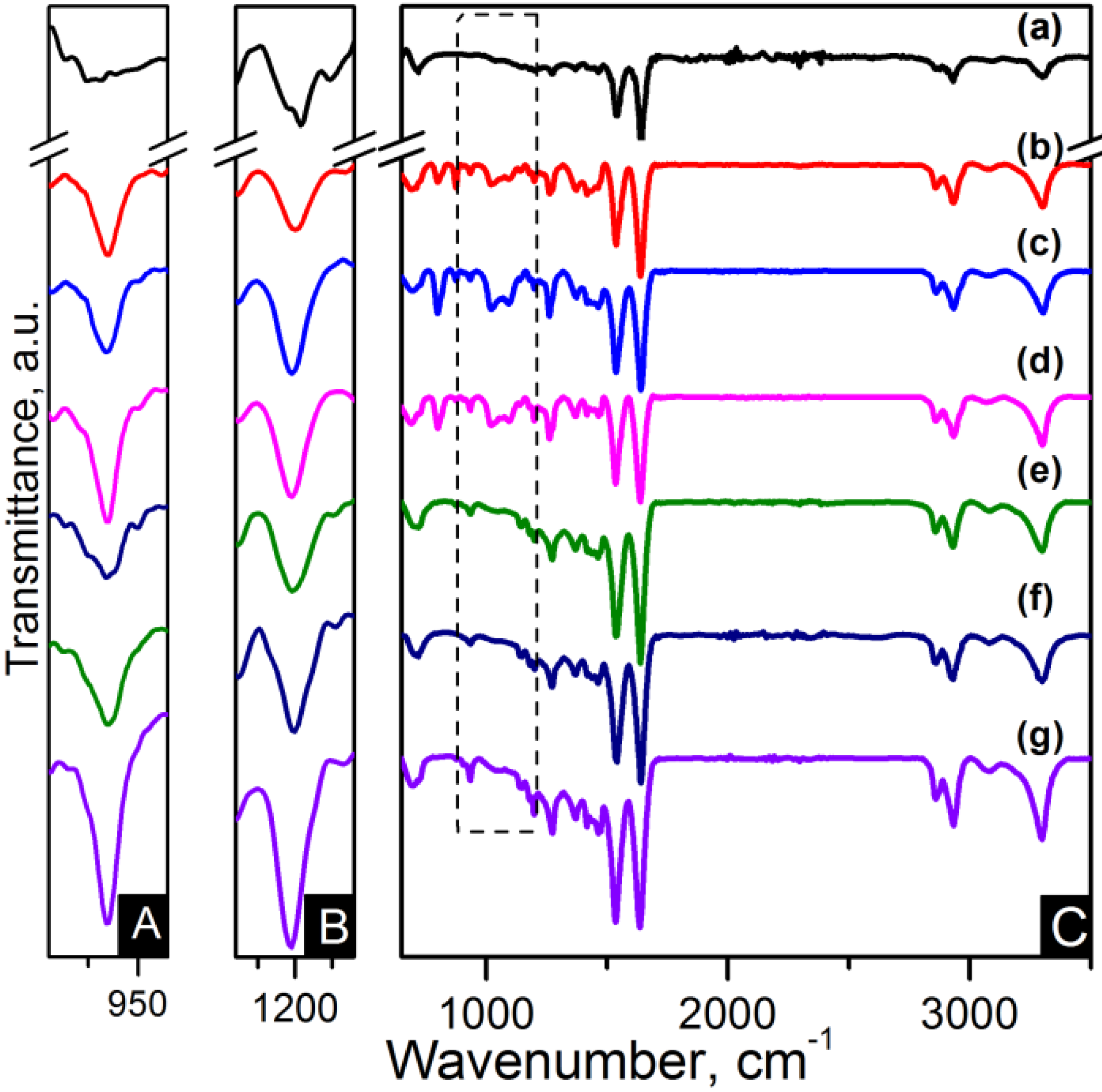
FTIR spectroscopy has been applied for identification of the basic structural units present in nylon 6,6 electrospun fibres and carbon based nanocomposites. The nanocomposites were labelled as listed in
Table 1. The assignments of all the fundamental bands are given in
Table 2 [
29,
30,
31]. The FTIR spectra of the electrospun nanofibres are presented in
Figure 4 and
Figure 5. The FTIR spectra show the characteristic bands of nylon 6,6: N–H deformation and C–N stretching of amide II band at ~1535 cm
−1, C=O stretching, N–H stretching vibration of amide I band at ~1635 cm
−1 and N–H stretching at ~3300 cm
−1 [
29,
32]. Infrarred (IR) spectra are sensitive to the conformation and packing of chain molecules, and this sensitivity has been widely exploited to characterise semicrystalline polymers in terms of their crystallinity. Nylon 6,6 has two characteristic crystalline peaks at 935 cm
−1 and 1200 cm
−1 [
29,
32,
33]. The addition of carbon nanomaterials changed the peak intensities compared to pure polymer indicating enhanced crystallinity as seen in the zoom-in of the crystalline peaks of
Figure 4 and
Figure 5.
Table 1.
Nomenclature of the electrospun fibres.
Table 1.
Nomenclature of the electrospun fibres.
| Type of nanofillers | Nanofiller content |
|---|
| 0.1 wt % | 0.5 wt % | 1.0 wt % |
|---|
| Carbon nanotubes (CNTs) | PA66/01CNTs | PA66/05CNTs | PA66/10CNTs |
| Oxidised carbon nanotubes (OCNTs) | PA66/01OCNTs | PA66/05OCNTs | PA66/10OCNTs |
| Reduced graphene oxide (RGO) | PA66/01RGO | PA66/05RGO | PA66/10RGO |
| Graphene oxide (GO) | PA66/01GO | PA66/05GO | PA66/10GO |
Table 2.
Fourier transform infrared spectroscopy (FTIR) band assignments in Nylon 6,6 [
26,
30,
33].
Table 2.
Fourier transform infrared spectroscopy (FTIR) band assignments in Nylon 6,6 [26,30,33].
| Band [cm−1] | Assignments |
|---|
| ~934 | Crystalline peak, amide axial deformation (C–C=O) |
| 1033–1043 and 1063–1066 | Triclinic structure, skeleton axial elongation (C–C) |
| 1140–1146 | Angular deformation out of plane of carbonyl groups |
| ~1202 | Crystalline peak: symmetrical angular deformation out of plane, amide III. |
| ~1220 | Angular deformation out of plane,(H–N–C=O) |
| 1300–1305 | Angular deformation out of plane, N–H |
| ~1370 | C–N axial deformation |
| ~1440 | CH2 deformation |
| 1535–1555 | C–N axial deformation and CO–N–H angular deformation, amide II |
| ~1640 | C=O axial deformation, amide I |
| ~2858 | CH2 axial deformation |
| ~2950 | CH2 axial deformation |
| ~3080 | N–H angular deformation in the plane |
| ~3300 | Free N–H axial deformation |
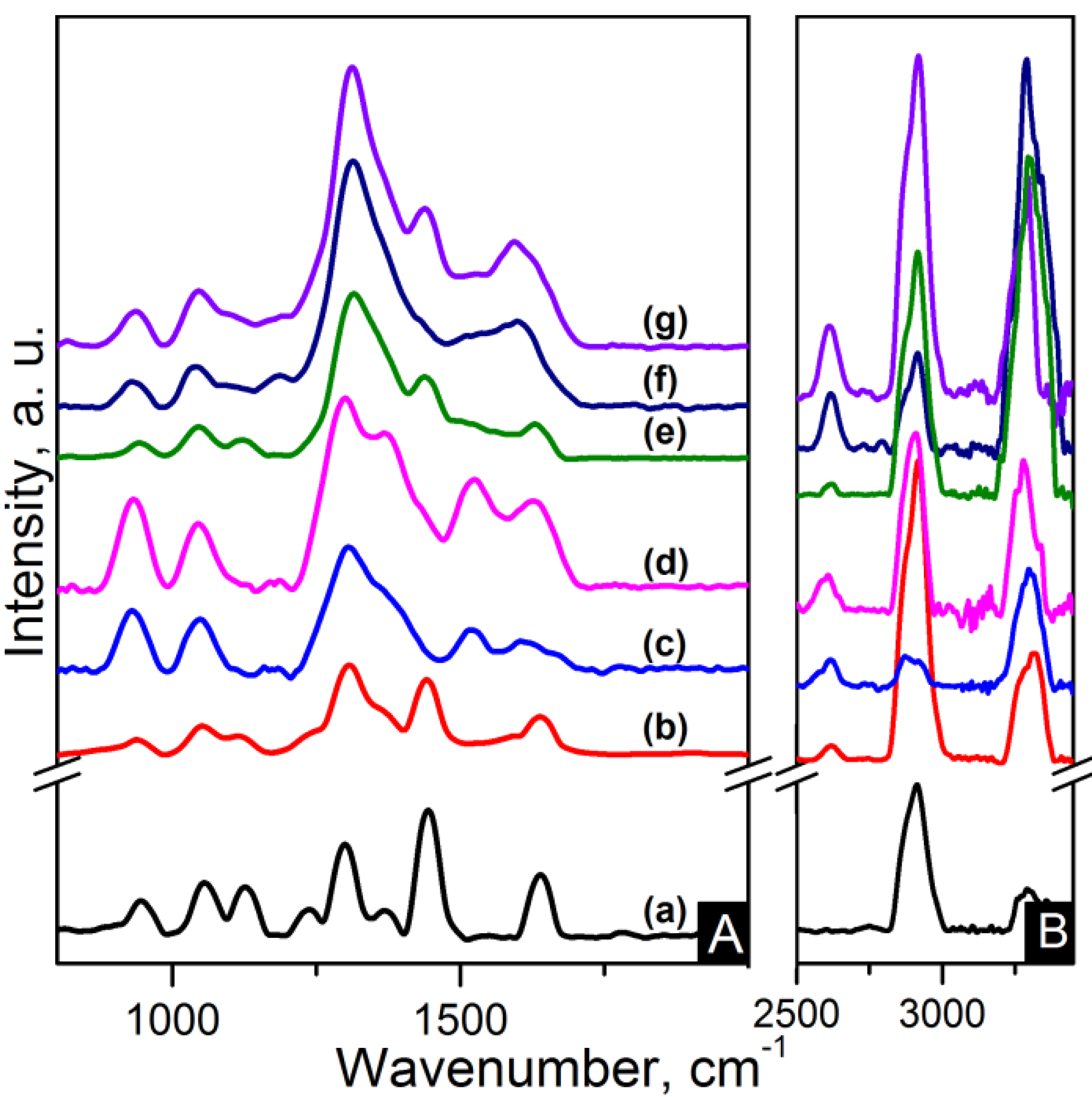
Figure 4.
FTIR spectra of (A) zoom-in of ~935 cm−1 peak; (B) zoom-in of ~1200 cm−1 peak and (C) region of 900–3500 cm−1 of the samples: (a) pure PA66; (b) PA66/01CNTs; (c) PA66/05CNTs; (d) PA66/10CNTs; (e) PA66/01OCNTs; (f) PA66/05OCNTs and (g) PA66/10OCNTs.
Figure 4.
FTIR spectra of (A) zoom-in of ~935 cm−1 peak; (B) zoom-in of ~1200 cm−1 peak and (C) region of 900–3500 cm−1 of the samples: (a) pure PA66; (b) PA66/01CNTs; (c) PA66/05CNTs; (d) PA66/10CNTs; (e) PA66/01OCNTs; (f) PA66/05OCNTs and (g) PA66/10OCNTs.
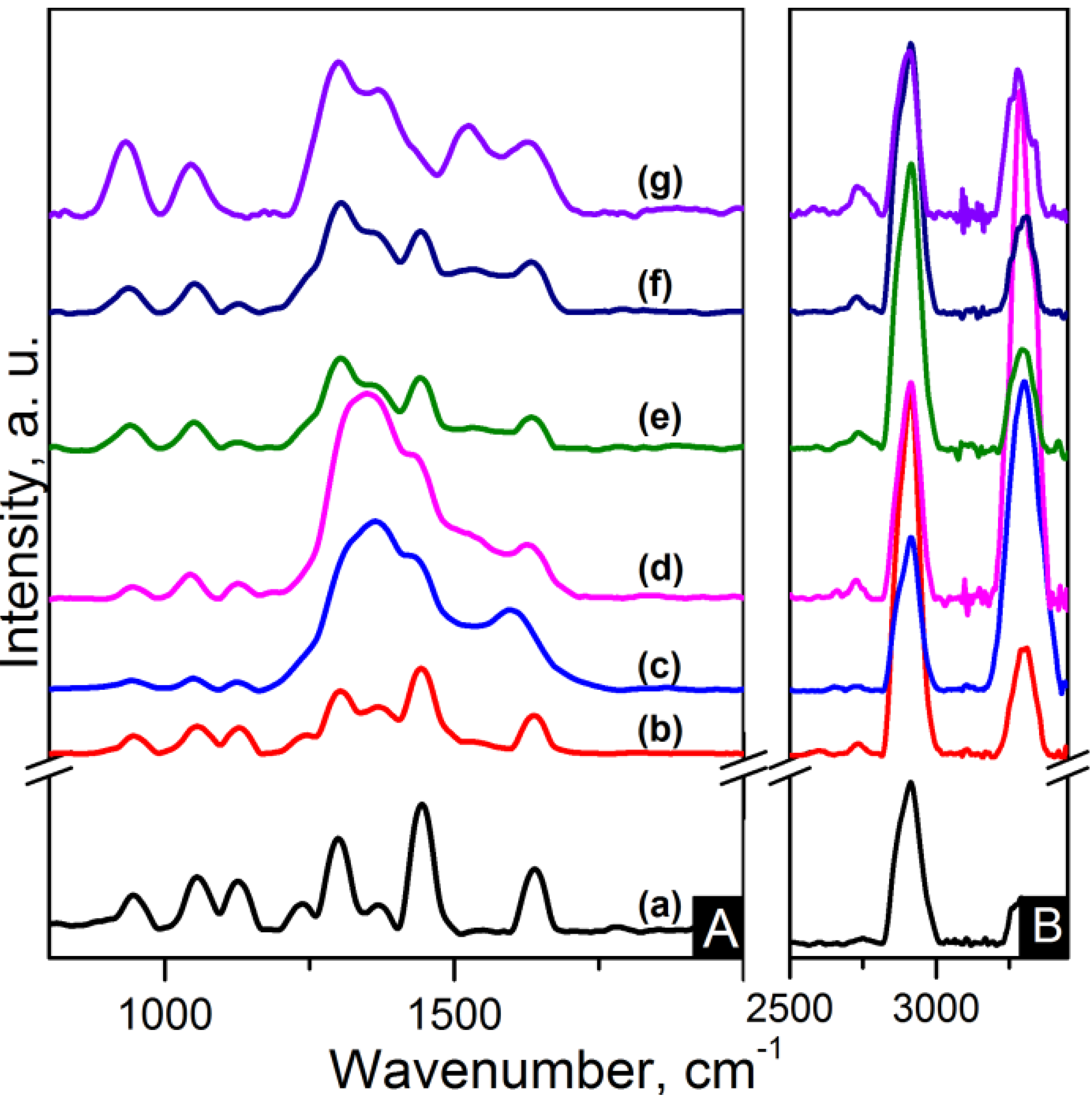
Figure 5.
FTIR spectra of (A) zoom-in of ~935 cm−1 peak; (B) zoom-in of ~1200 cm−1 peak and (C) region of 900–3500 cm−1 of the samples: (a) pure PA66; (b) PA66/01RGO; (c) PA66/05RGO; (d) PA66/10RGO; (e) PA66/01GO; (f) PA66/05GO and (g) PA66/10GO.
Figure 5.
FTIR spectra of (A) zoom-in of ~935 cm−1 peak; (B) zoom-in of ~1200 cm−1 peak and (C) region of 900–3500 cm−1 of the samples: (a) pure PA66; (b) PA66/01RGO; (c) PA66/05RGO; (d) PA66/10RGO; (e) PA66/01GO; (f) PA66/05GO and (g) PA66/10GO.
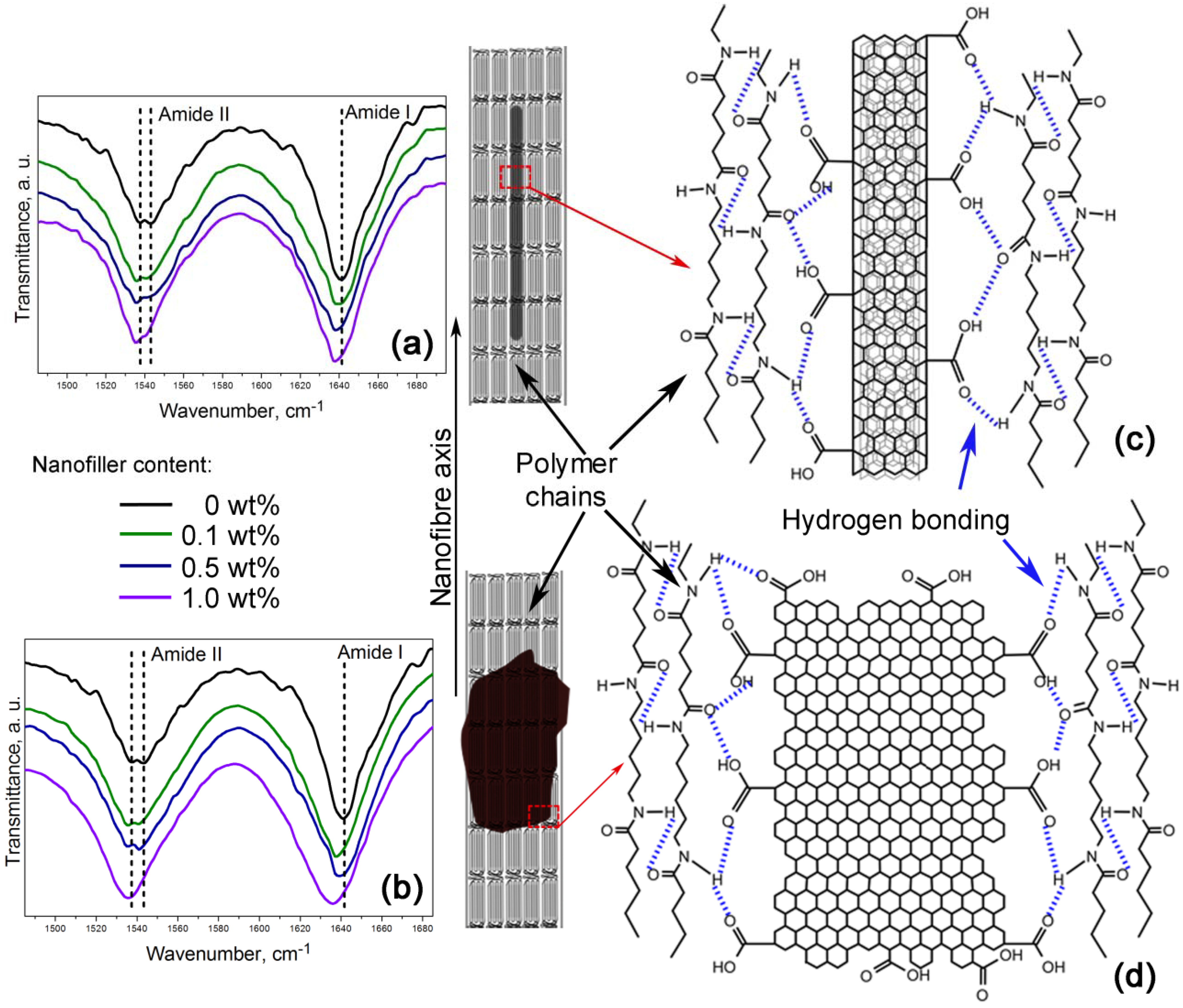
Furthermore,
Figure 6a,b shows a zoom-in of the 1485–1695 cm
−1 FTIR region indicating the amide I and the amide II bands. These bands are of particular interest because they are related to hydrogen bonding [
34,
35]. Lu
et al. studied the amide I band in nylon 6,6/clay nanocomposites, which is composed of two components, attributable to the ordered and disordered hydrogen-bonded carbonyl groups [
34]. They found the former had a higher strength and that their frequency of stretching was lower than that of the latter.
Figure 6.
Nanofibres intramolecular bonding in the (a,c) PA66/OCNTs and the (b,d) PA66/GO nanocomposites.
Figure 6.
Nanofibres intramolecular bonding in the (a,c) PA66/OCNTs and the (b,d) PA66/GO nanocomposites.
Our nanocomposites showed an increase of intensity in the amide I and the amide II bands as the nanofiller loading increased, which is also indicative of the formation of hydrogen bonding [
35]. The spectra also show that amide II band splits in two components, as evidenced by the peaks at ~1537 cm
−1 and ~1544 cm
−1. At lower frequency the peak becomes more intense as the content of OCNTs and GO is increased. The amide II band in PA66/10GO nanofibres lacks of this splitting because the component of the ordered hydrogen-bonded groups overlap the component of the ones in the disordered state. These features demonstrate the hydrogen bonding between the nanofillers and the polymer, as displayed in the schemes of
Figure 6c,d.
IR and Raman spectroscopy are complementary to each other. Strong IR bands are related to polar functional groups whereas non-polar functional groups give rise to strong Raman bands [
33]. Raman spectra of the nanocomposites are shown in
Figure 7 and
Figure 8. C–C=O stretching is found at ~945 cm
−1. The region between 1000 cm
−1 and 1170 cm
−1 is characteristic of the stretching of the C–C skeletal backbone structure. The peak at ~1235 cm
−1 is due to N–H wagging. Peaks ranging from 1265 to 1500 cm
−1 are attributable to bending vibrations of the CH
2 groups. Carbon based nanocomposites spectra show overlapping of the D band in this region. G band also is also overlapped in the zone between 1480 cm
−1 and 1700 cm
−1 where amide I band (C=O) is located at ~1640 cm
−1. The 2D band appears at ~2620 cm
−1 for CNT based nanocomposites and features as a weak wide band in the region of 2500–2800 cm
−1 for the graphene based nanocomposites. The CH
2 asymmetric and symmetric stretching and the N–H stretching appear as strong broad bands in the 2800–3000 cm
−1 region for the former and in the 3200–3400 cm
−1 region for the latter [
33].
Figure 7.
Raman spectra of the (A) 800–2000 cm−1 region and (B) zoom-out of the 2800–3350 cm−1 region of the samples: (a) pure PA66; (b) PA66/01CNTs; (c) PA66/05CNTs; (d) PA66/10CNTs; (e) PA66/01OCNTs; (f) PA66/05OCNTs and (g) PA66/10OCNTs.
Figure 7.
Raman spectra of the (A) 800–2000 cm−1 region and (B) zoom-out of the 2800–3350 cm−1 region of the samples: (a) pure PA66; (b) PA66/01CNTs; (c) PA66/05CNTs; (d) PA66/10CNTs; (e) PA66/01OCNTs; (f) PA66/05OCNTs and (g) PA66/10OCNTs.
Figure 8.
Raman spectra of the (A) 800–2000 cm−1 region and (B) zoom-out of the 2800–3350 cm−1 region of the samples: (a) pure PA66; (b) PA66/01RGO; (c) PA66/05RGO; (d) PA66/10RGO; (e) PA66/01GO; (f) PA66/05GO and (g) PA66/10GO.
Figure 8.
Raman spectra of the (A) 800–2000 cm−1 region and (B) zoom-out of the 2800–3350 cm−1 region of the samples: (a) pure PA66; (b) PA66/01RGO; (c) PA66/05RGO; (d) PA66/10RGO; (e) PA66/01GO; (f) PA66/05GO and (g) PA66/10GO.
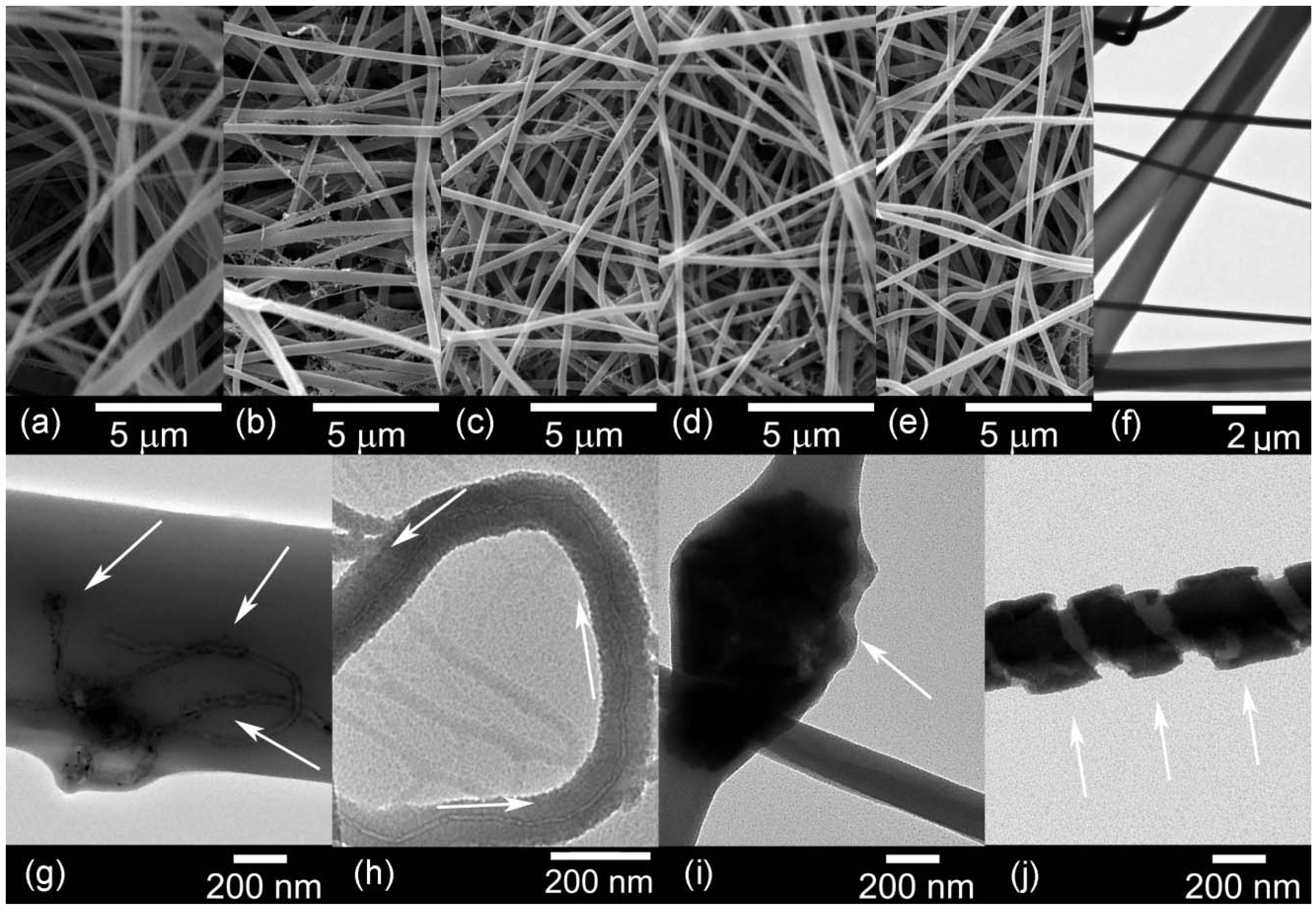
The diameters of the fibres were obtained from the scanning electron microscopy (SEM) images, as displayed in
Table 3. Nylon 6,6 nanofibres had an average diameter of 633 nm. The addition of nanometric carbon to the polymeric matrix resulted in a decrease of the nanofibre diameters. Physical properties such as viscosity and conductivity influence the morphology of the nanofibres [
31]. The incorporation of carbon nanomaterials increased the viscosity and improved the conductivity of the solution. While a more viscous solution produces thicker fibres, the increase in conductivity favours the stretching of thinner fibres [
26]. These parameters are the reason of the variable diameters in the samples.
Figure 9a–e shows the morphology of the nanofibres containing the highest nanofiller loading.
Table 3.
Average diameters of the electrospun fibres.
Table 3.
Average diameters of the electrospun fibres.
| Nanofiller wt % | PA66/CNTs [nm] | PA66/OCNTs [nm] | PA66/RGO [nm] | PA66/GO [nm] |
|---|
| 0.1 | 243 | 427 | 315 | 306 |
| 0.5 | 332 | 357 | 260 | 271 |
| 1.0 | 410 | 325 | 428 | 302 |
TEM images are included in
Figure 9g–j, the micrographs of the nanocomposites show the dispersion of the nanofillers in the matrix.
Figure 9g shows that at the highest loading pristine CNTs are agglomerated in the nanofibre, as indicated by the arrows. On the other hand,
Figure 9h shows the OCNTs are aligned along the fibre.
Figure 9i,j shows the graphene nanomaterials are embedded within the polymer and GO followed a pattern along the fibre.
Figure 9.
Scanning electron microscopy (SEM) and TEM images of: (a,f) pure PA66; (b,g) PA66/10CNTs; (c,h) PA66/10OCNTs; (d,i) PA66/10RGO and (e,j) PA66/10GO.
Figure 9.
Scanning electron microscopy (SEM) and TEM images of: (a,f) pure PA66; (b,g) PA66/10CNTs; (c,h) PA66/10OCNTs; (d,i) PA66/10RGO and (e,j) PA66/10GO.
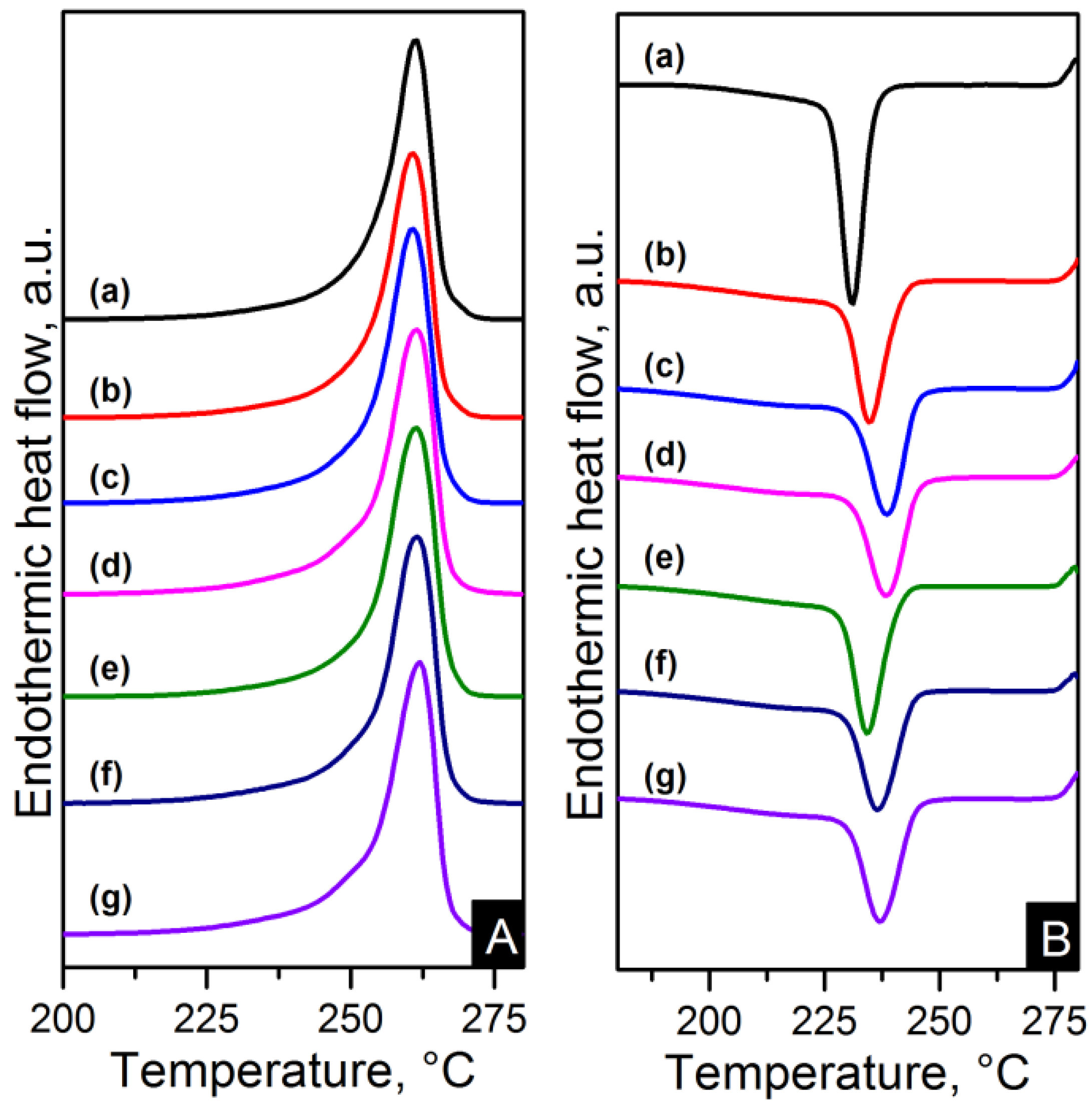
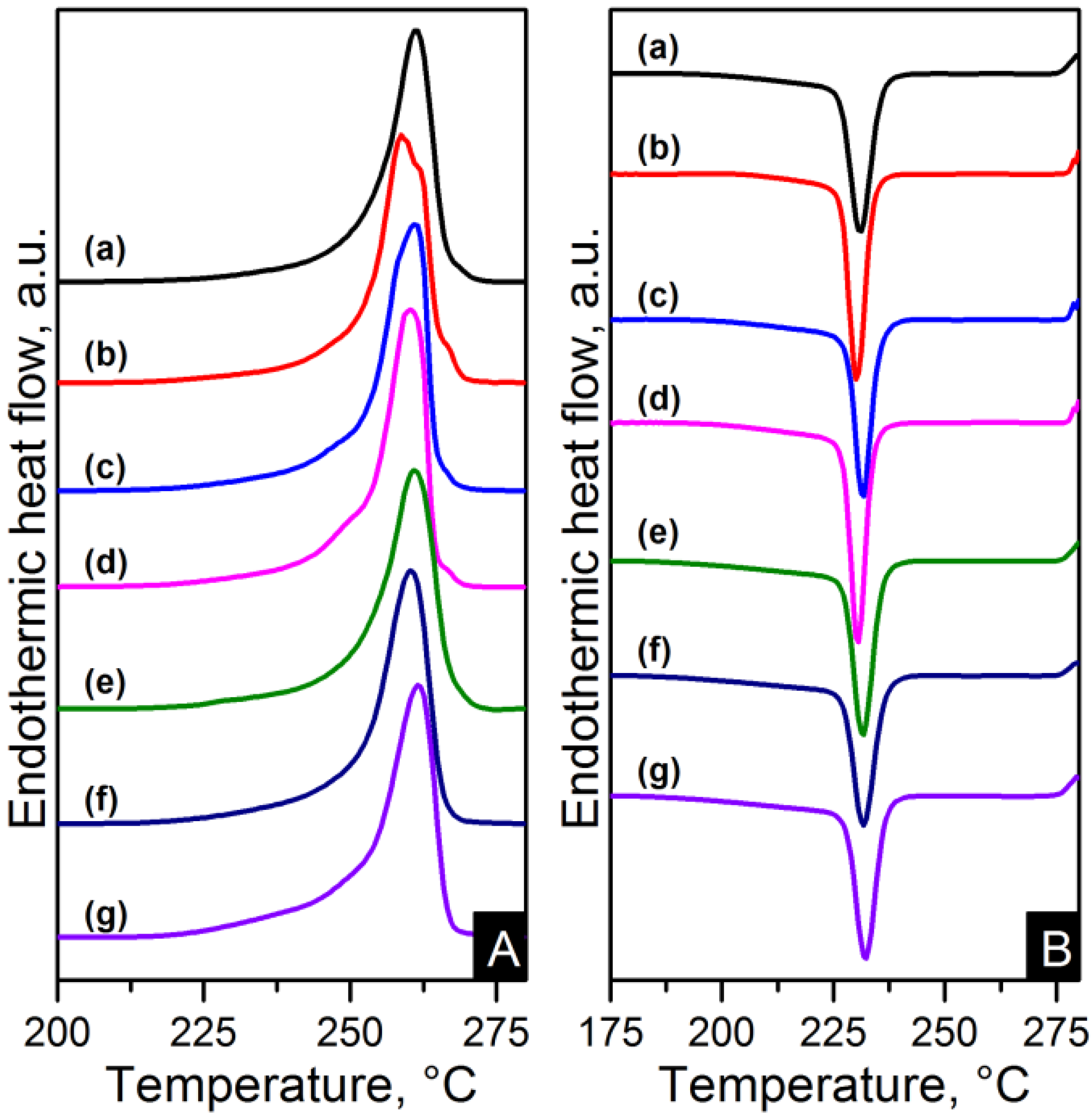
The melting and crystallisation curves obtained from differential scanning calorimetry (DSC) are shown in
Figure 10 and
Figure 11. There is a slight difference in the melting peaks as seen in
Figure 10A and
Figure 11A. Both electrospun nanocomposites and nylon 6,6 showed a broad melting peak at ~260 °C, indicating the melting of α-crystals [
22]. There is also a slight broad shoulder on the low temperature side at ~250 °C which is more noticeable in the RGO nanocomposites. This has been attributed to morphological changes in the crystallite or the melting of small and less stable crystalline units [
23,
24,
29]. The crystal characteristics found in the nanocomposites due to the different nanometric structure of CNTs and graphene will be discussed in more detail later. The degree of crystallinity (X
c) was calculated as shown in Equation (1).
where
ΔHm and
ΔHm0 (197 J/g) are the enthalpies of the nanocomposite and purely crystalline nylon 6,6 respectively [
23]. The crystallinity properties are summarised in
Table 4. The electrospun nanocomposites resulted in higher crystallinity than pure polymer. These results are consistent with earlier studies of carbon based nanocomposites [
16,
21]. The crystallinity changes from ~39% to ~44% for the PA66/10GO sample compared to nylon 6,6. The higher results in degree of crystallinity when increasing the content indicate the induced crystallisation due to the CNTs and graphene materials.
Figure 10.
Differential scanning calorimetry (DSC) (A) heating and (B) cooling thermograms of: (a) pure PA66; (b) PA66/01CNTs; (c) PA66/05CNTs; (d) PA66/10CNTs; (e) PA66/01OCNTs; (f) PA66/05OCNTs and (g) PA66/10OCNTs.
Figure 10.
Differential scanning calorimetry (DSC) (A) heating and (B) cooling thermograms of: (a) pure PA66; (b) PA66/01CNTs; (c) PA66/05CNTs; (d) PA66/10CNTs; (e) PA66/01OCNTs; (f) PA66/05OCNTs and (g) PA66/10OCNTs.
Figure 11.
DSC (A) heating and (B) cooling thermograms of: (a) pure PA66; (b) PA66/01RGO; (c) PA66/05RGO; (d) PA66/10RGO; (e) PA66/01GO; (f) PA66/05GO and (g) PA66/10GO.
Figure 11.
DSC (A) heating and (B) cooling thermograms of: (a) pure PA66; (b) PA66/01RGO; (c) PA66/05RGO; (d) PA66/10RGO; (e) PA66/01GO; (f) PA66/05GO and (g) PA66/10GO.
Table 4.
Crystallisation properties of the electrospun fibres.
Table 4.
Crystallisation properties of the electrospun fibres.
| Sample | Xc a [%] | CI b | L(100) c [nm] | L(010/110) d [nm] |
|---|
| Pure PA66 | 39.4 | 0.38 | 6.45 | 3.41 |
| PA66/01CNTs | 39.0 | 0.39 | 6.32 | 3.18 |
| PA66/05CNTs | 41.4 | 0.40 | 6.06 | 3.35 |
| PA66/10CNTs | 41.8 | 0.42 | 6.00 | 3.14 |
| PA66/01OCNTs | 40.8 | 0.39 | 6.12 | 3.09 |
| PA66/05OCNTs | 41.3 | 0.40 | 5.63 | 3.15 |
| PA66/10OCNTs | 42.1 | 0.44 | 5.61 | 3.11 |
| PA66/01RGO | 41.5 | 0.42 | 5.44 | 2.89 |
| PA66/05RGO | 42.5 | 0.43 | 5.43 | 2.98 |
| PA66/10RGO | 43.5 | 0.44 | 5.18 | 2.92 |
| PA66/01GO | 41.8 | 0.40 | 5.60 | 3.17 |
| PA66/05GO | 42.6 | 0.42 | 5.10 | 3.08 |
| PA66/10GO | 43.8 | 0.44 | 5.01 | 2.93 |
The crystallisation thermograms of the nanocomposites showed higher crystallisation temperatures (T
c) for the CNT based nanocomposites than nylon 6,6. This is explained by the increased amount of nuclei crystallising in the matrix due to addition of CNTs. The one-dimensional carbon material also blocked the nylon 6,6 chains mobility which resulted in an accelerated nucleation process [
13].
CNT thermograms displayed an average increase in T
c of 6 °C compared to pure polymer. The average T
c in OCNT nanocomposites resulted in an increase of 4.6 °C. This behaviour indicates that functionalisation of carbon nanomaterials slightly weakens the heterogeneous nucleation effect of the nanotubes as is in agreement with earlier studies [
13,
14]. On the other hand, graphene based nanocomposites showed comparable or slightly higher T
c to that of nylon 6,6. A similar nucleating effect of the graphene compared to CNTs has been found in polypropylene and poly(L-lactide) nanocomposites [
18,
19]. This behaviour has been explained by the considerable large surface area of the graphene sheets where the polymer chains need more time to adjust their conformations making the induction of crystallisation slower [
19].
The crystalline structure of the nanocomposites was also characterised using wide angle X-ray diffraction (WAXD). WAXD patterns are displayed in
Figure 12. Two peaks observed at approximately 20.5° and 23.5° are consistent with the diffraction of (100) and (010/110) crystalline planes of α-crystals [
22]. The diffraction pattern was decomposed using peak-fitting of Gaussian functions in Origin
® 8.5 in order to obtain a broad amorphous halo and sharp peaks from reflections of the crystalline peaks and to evaluate the crystallinity index (CI) as shown in Equation (2).
where
Ac is the integrated area underneath the crystalline peaks and
Aa is the integrated area of the amorphous halo. The values of CI are also shown in
Table 4. These values are in accordance with the DSC crystallinity results.
Figure 12.
(A) WAXD patterns of: (a) pure PA66; (b) PA66/01CNTs; (c) PA66/05CNTs; (d) PA66/10CNTs; (e) PA66/01OCNTs; (f) PA66/05OCNTs and (g) PA66/10OCNTs. (B) WAXD patterns of: (a) pure PA66; (b) PA66/01RGO; (c) PA66/05RGO; (d) PA66/10RGO; (e) PA66/01GO; (f) PA66/05GO and (g) PA66/10GO.
Figure 12.
(A) WAXD patterns of: (a) pure PA66; (b) PA66/01CNTs; (c) PA66/05CNTs; (d) PA66/10CNTs; (e) PA66/01OCNTs; (f) PA66/05OCNTs and (g) PA66/10OCNTs. (B) WAXD patterns of: (a) pure PA66; (b) PA66/01RGO; (c) PA66/05RGO; (d) PA66/10RGO; (e) PA66/01GO; (f) PA66/05GO and (g) PA66/10GO.
The unit cell of α-crystals in nylon 6,6 is triclinic [
22,
23,
24]. The two strong diffraction signals are a project value of interchain distance within the hydrogen-bonded sheet (100) and the (010/110) signal represents the intersheet distance [
24]. The crystallite size perpendicular to the diffraction (hkl) plane, L
hkl in nanometres, can be obtained by applying Scherrer’s equation, displayed in Equation 3.
where
is the Scherrer factor (0.9 for Gaussian function),
is the X-ray wavelength,
is the pure line breadth,
is a measured half width of the experimental peak,
is the instrumental broadening factor which is 0.17 for the diffractometer employed , and
is the Bragg angle. The crystallite sizes L
(100) and L
(010/110) of nanocomposites are smaller compared to nylon 6,6.
Table 4 reveals the crystallite size decreases with content due to the suppressed crystal growth caused by the interaction between nylon 6,6 and nanometric carbon. This means that the degree of crystal perfection decreased as the content increased [
24,
34]. Crystal size values of graphene based nanofibres are lower than crystal size of CNT based nanocomposites. Functionalisation also has an impact on this property; the effect is more noticeable in the OCNT nanofibres. RGO and GO nanocomposites showed similar results. The nanofibre crystal sizes are as expected based on studies of nylon 6,6 fibres and nylon 6,6 nanocomposites [
24,
34]. Crystallisation in nylon 6,6/graphene nanocomposites has been explained as a two factor controlled process. One factor is the nucleating effect of graphene and the other factor is the retarded migration and diffusion of polymer molecular chains to the surface of the nucleus which constrain crystal growth [
17,
19]. It has also been proposed that the growing crystals on graphene surface could show multiple orientations, which might contact the adjacent single crystals and suppress this process [
19]. These explanations serve as evidence of the different crystal size between 1D and 2D carbon based nanocomposites studied in this paper. RGO nanocomposites showed the most reduced crystal size values, confirming the presence of the smaller crystallites melting at lower temperatures in the heating thermograms. In spite of these results, the values of GO nanocomposites show a trend of decreasing crystal size with content in both directions. This is another indication of the superior dispersion of GO in the nanofibres. A decrease in crystallite size is likely to favour mechanical properties [
34].
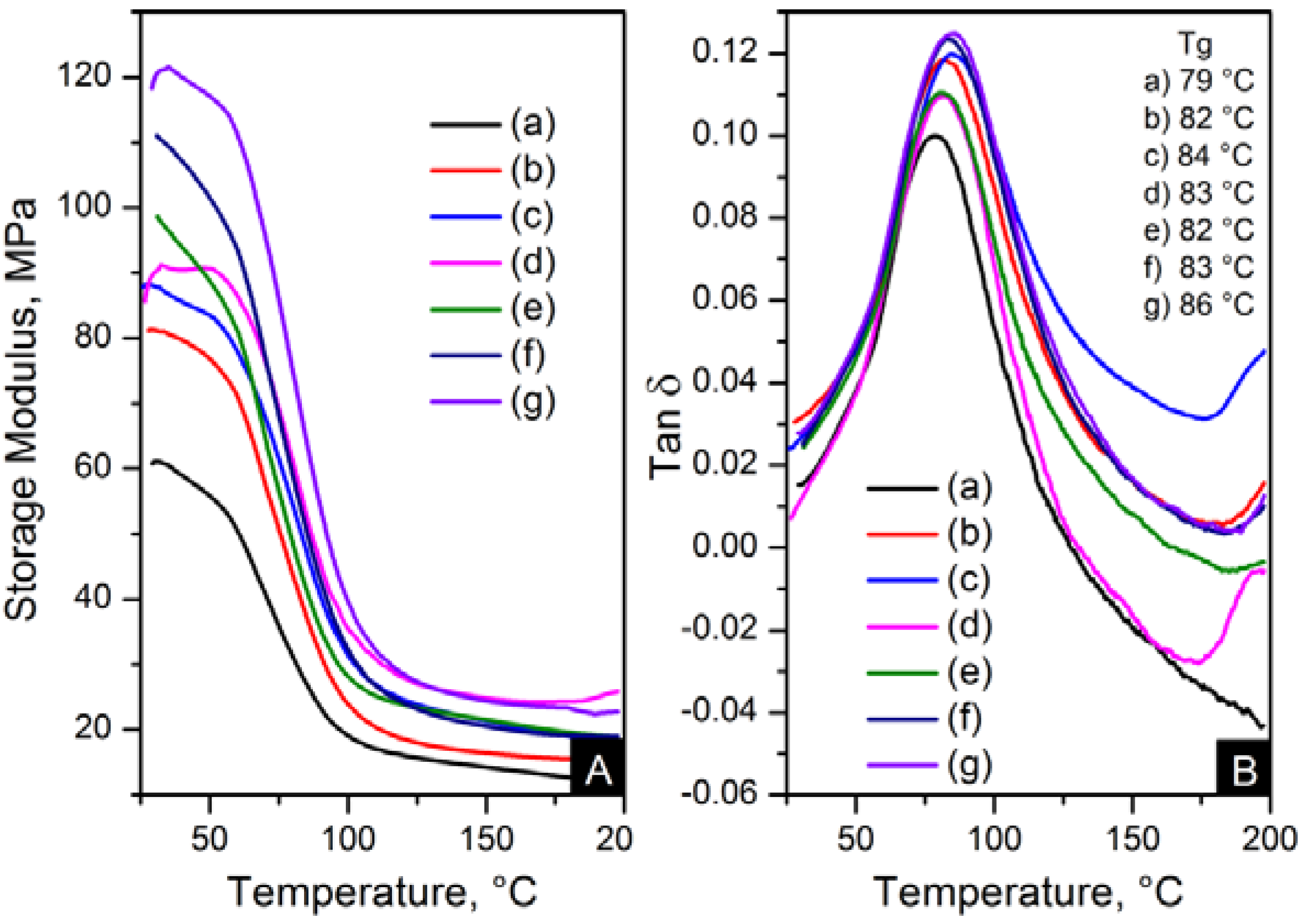
The reinforcement effect of the nanofillers in nylon 6,6 is seen in dynamic mechanical analyser (DMA) results, displayed in
Figure 13 and
Figure 14. The addition of nanomaterials affected the stiffness of the polymer. As expected, the storage modulus was enhanced compared to pure polymer. This trend is consistent with an increase in content and the decrease of crystal size, as seen in
Table 4.
Figure 13.
Dynamic mechanical analyser (DMA) results; (A) storage modulus and (B) Tanδ of: (a) pure PA66; (b) PA66/01CNTs; (c) PA66/05CNTs; (d) PA66/10CNTs; (e) PA66/01OCNTs; (f) PA66/05OCNTs and (g) PA66/10OCNTs.
Figure 13.
Dynamic mechanical analyser (DMA) results; (A) storage modulus and (B) Tanδ of: (a) pure PA66; (b) PA66/01CNTs; (c) PA66/05CNTs; (d) PA66/10CNTs; (e) PA66/01OCNTs; (f) PA66/05OCNTs and (g) PA66/10OCNTs.
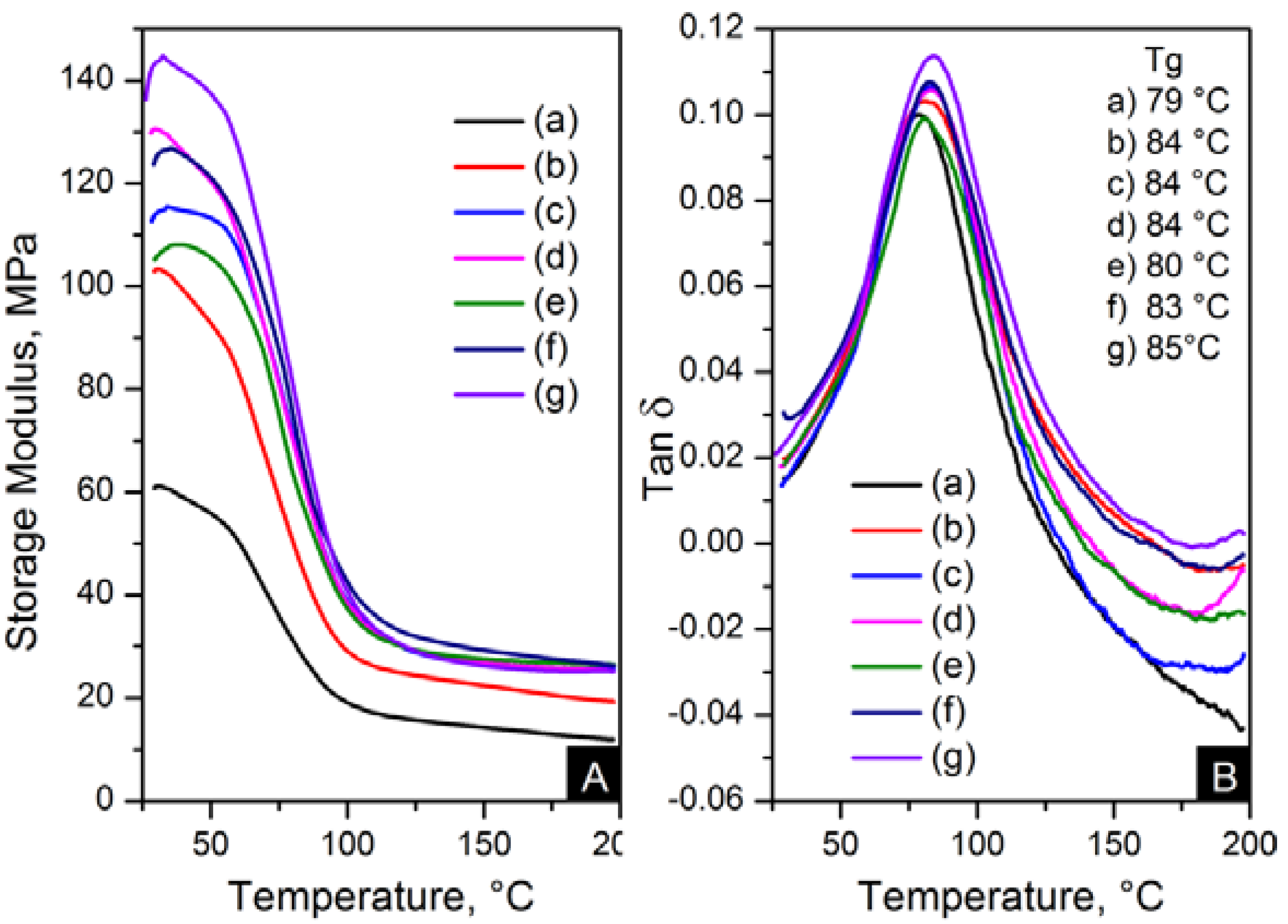
Figure 14.
DMA results; (A) storage modulus and (B) Tanδ of: (a) pure PA66, (b) PA66/01RGO; (c) PA66/05RGO; (d) PA66/10RGO; (e) PA66/01GO; (f) PA66/05GO and (g) PA66/10GO.
Figure 14.
DMA results; (A) storage modulus and (B) Tanδ of: (a) pure PA66, (b) PA66/01RGO; (c) PA66/05RGO; (d) PA66/10RGO; (e) PA66/01GO; (f) PA66/05GO and (g) PA66/10GO.
The storage modulus of the OCNT nanocomposites was almost the double than that of pristine CNT nanocomposites. The nanofibres containing 1 wt % OCNTs resulted in a 97% increase in the mechanical response. A more significant improvement in the mechanical properties resulted from the graphene based nanocomposites. Higher modulus values were obtained for GO nanocomposites, where storage modulus was improved by 139% compared to nylon 6,6. The enhanced mechanical properties can be explained on the basis of crystal features imposed by the structure of nanometric carbon and the reinforcing effect of nanomaterials. This is attributed due to the superior dispersion of the nanomaterials in the matrix provided by their functionalisation. It was evident that the improved dispersion provided by functionalisation resulted in a more homogeneous confinement of the crystals of nylon 6,6. This was demonstrated by the reduction in crystal size after incorporation of OCNTs and GO. The graphene based nanocomposites show the smallest crystal size values than the rest of the nanocomposites; this explains the enhanced mechanical response. Furthermore, it is supposed that the large surface area of graphene provided by its 2D structure play an important role to generate better interaction with the polymer favouring the stress transfer from the nanofiller to the matrix. Therefore, the graphene nanocomposites resulted in superior mechanical properties.
Figure 13B and
Figure 14B show the glass transition temperatures (T
g) obtained from the tanδ maximum. The values of T
g were higher in the nanocomposites than nylon 6,6 nanofibres. This property was increased up to 7 °C for PA66/10OCNTs. T
g in GO nanocomposites showed a 6 °C increase for the maximum filler content. This increase in T
g results from the presence of nanomaterials which restricted the molecular mobility on the matrix. The free volume of the nylon 6,6 chains is influenced by the interaction between nanofiller and the matrix [
16]. The improvement in T
g is a feature of the confinement imposed by the nanomaterials in nylon 6,6 molecular segments.