Hydrothermal Growth of Vertically Aligned ZnO Nanorods Using a Biocomposite Seed Layer of ZnO Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
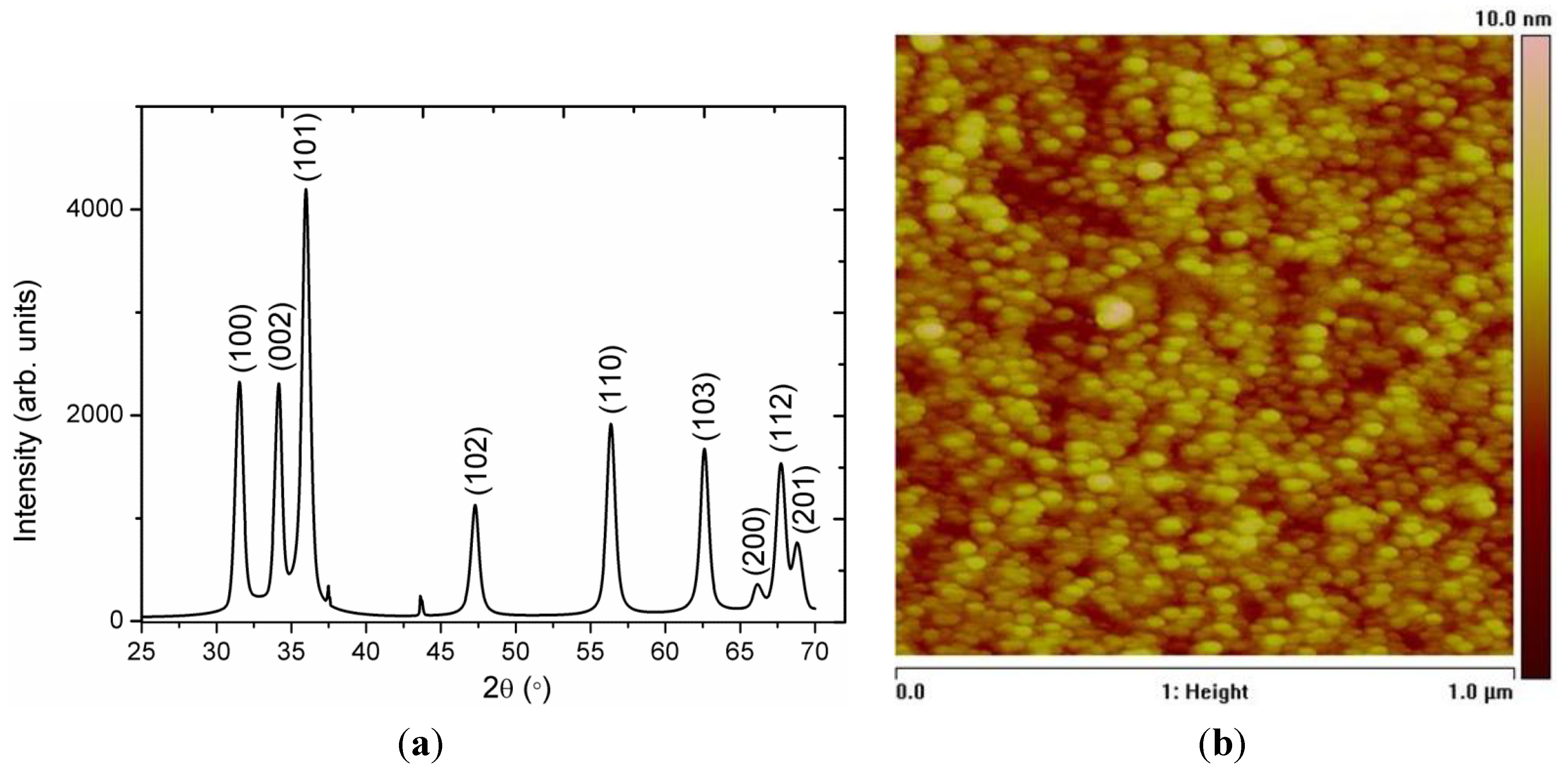
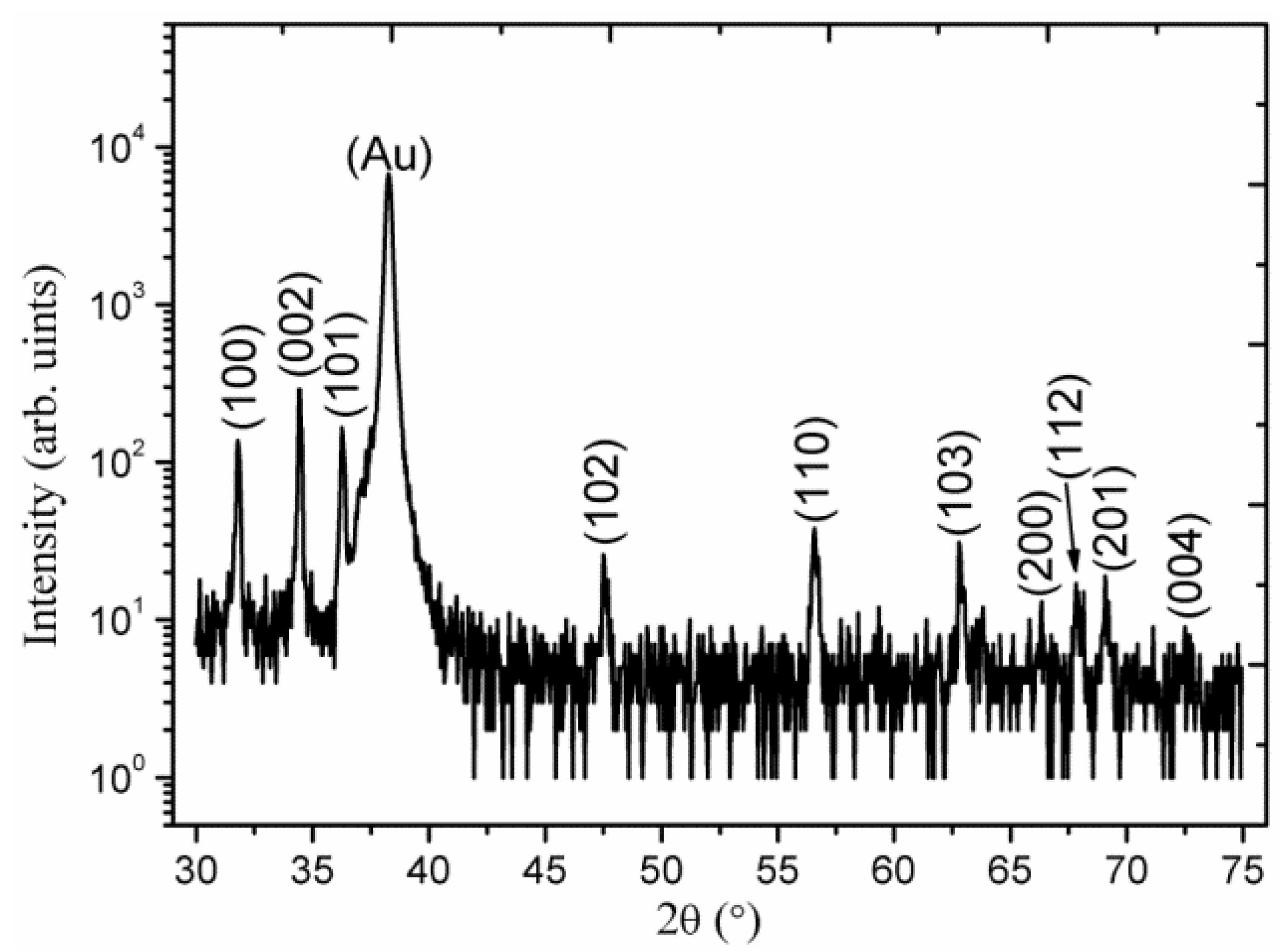
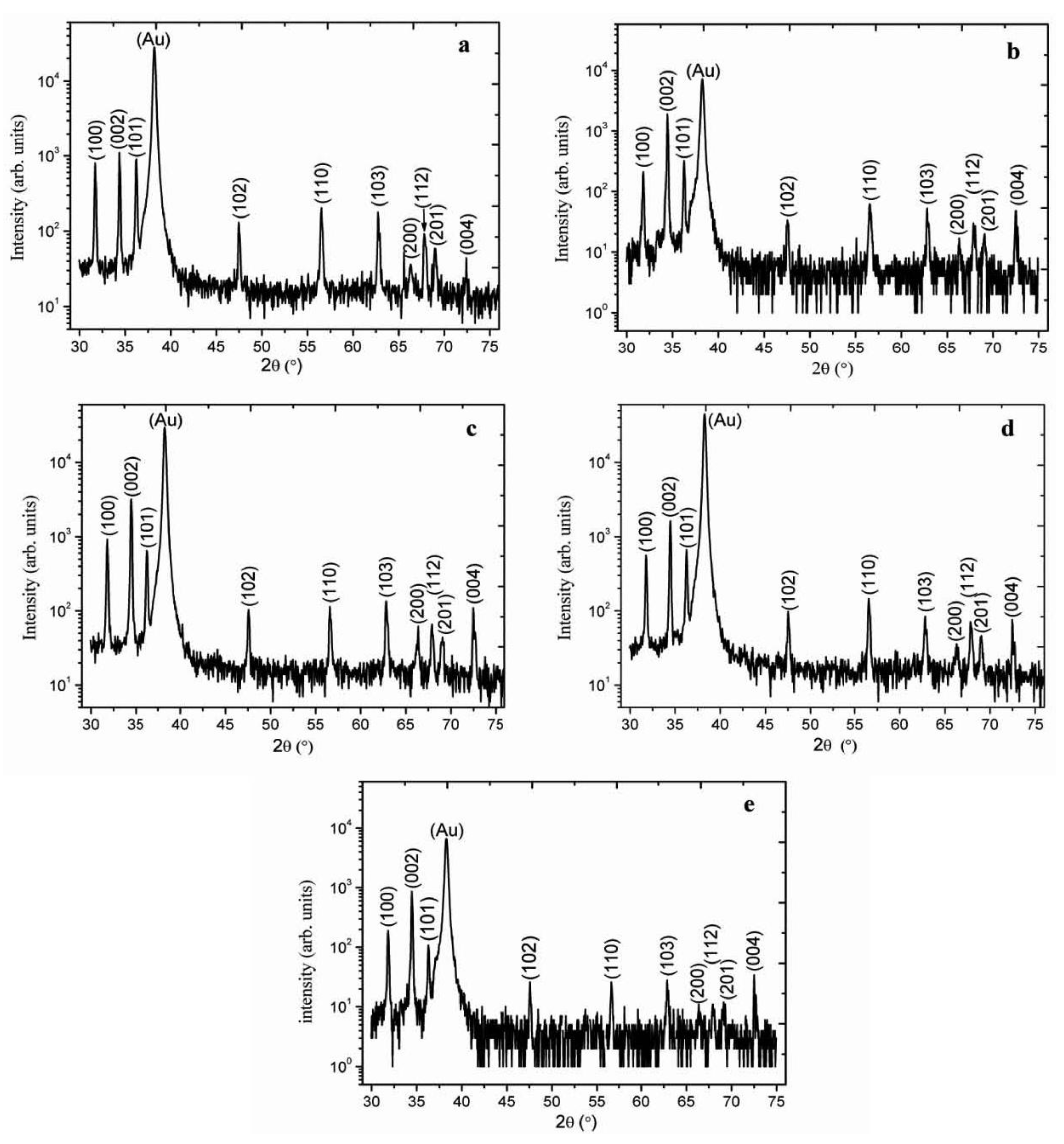
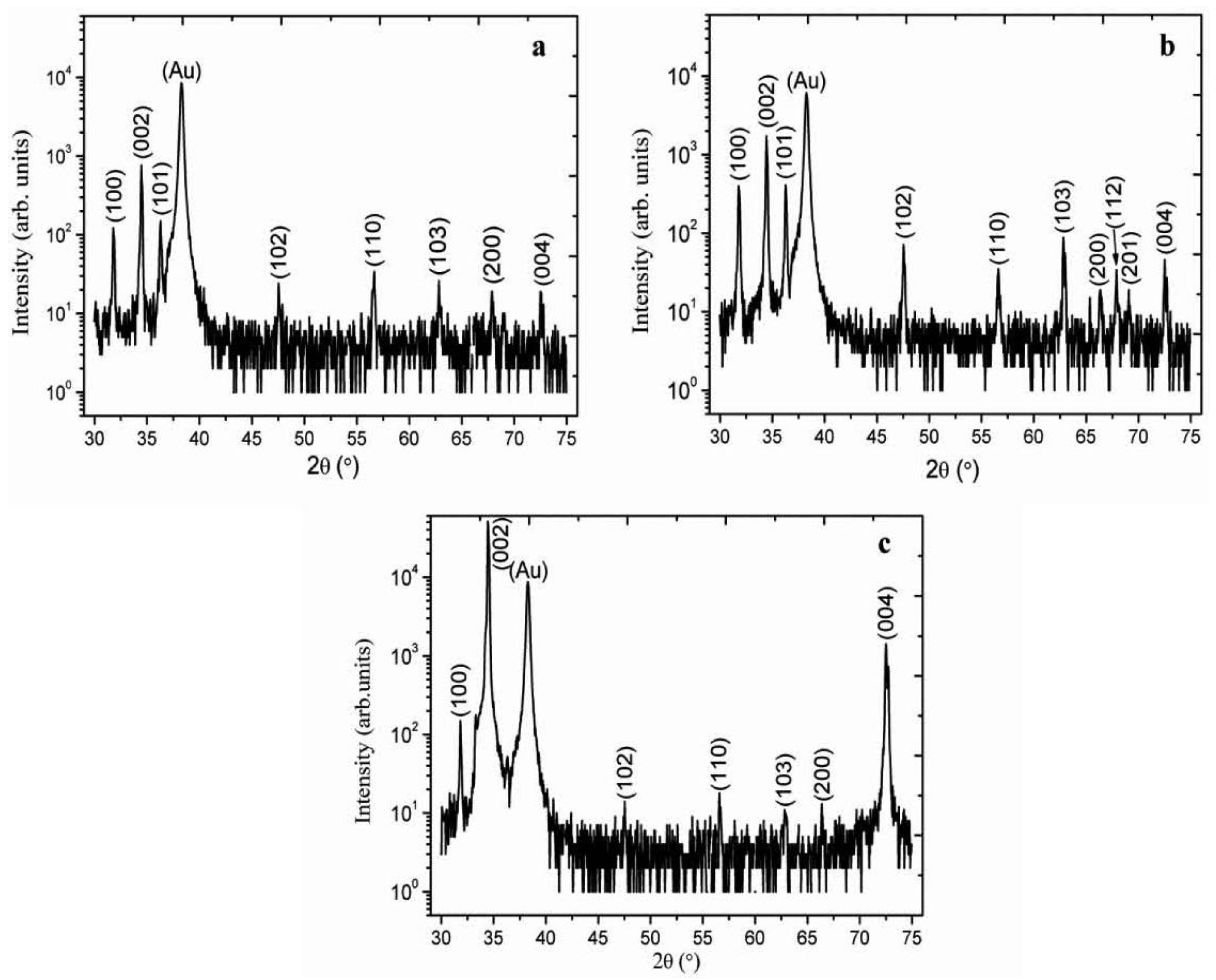
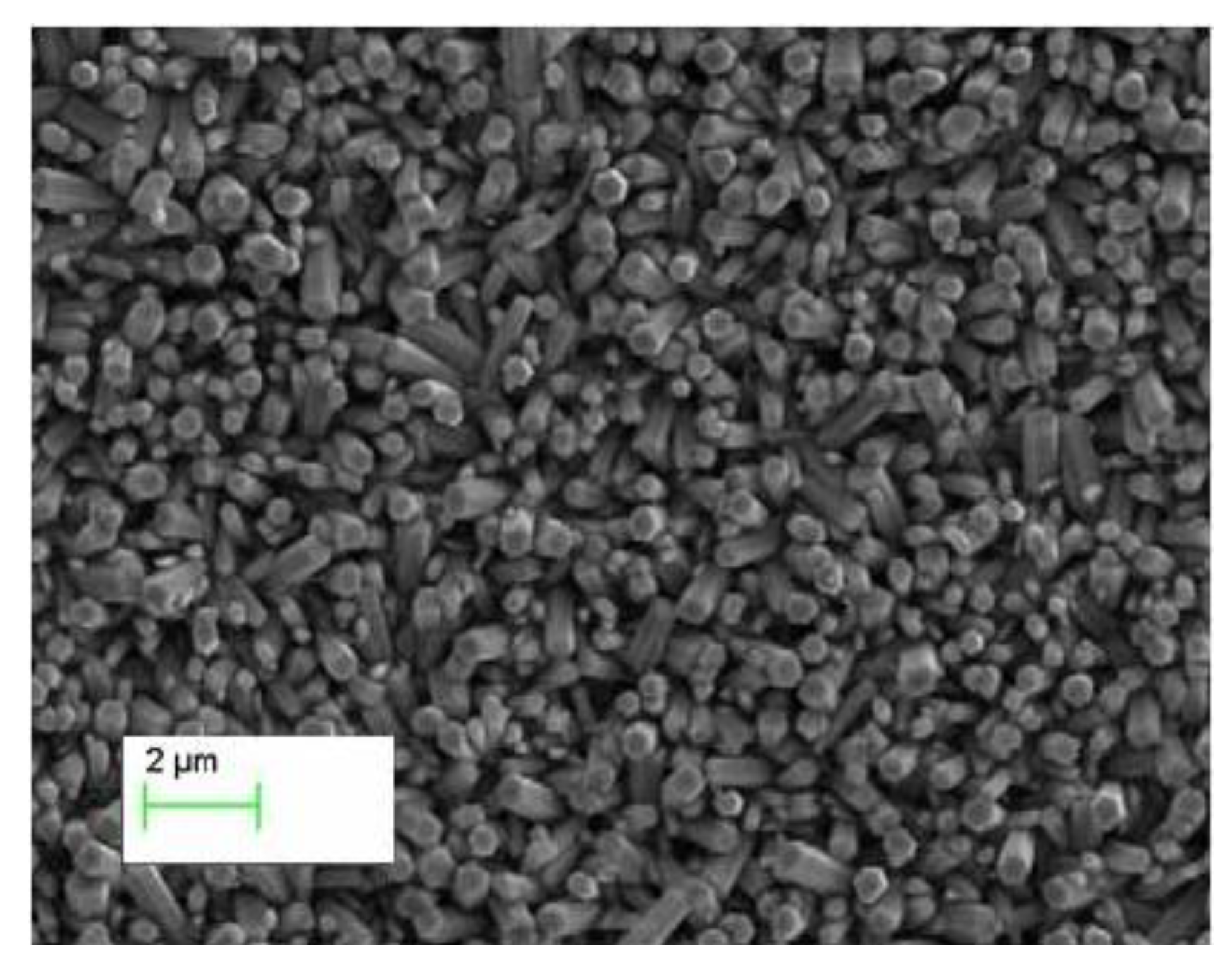
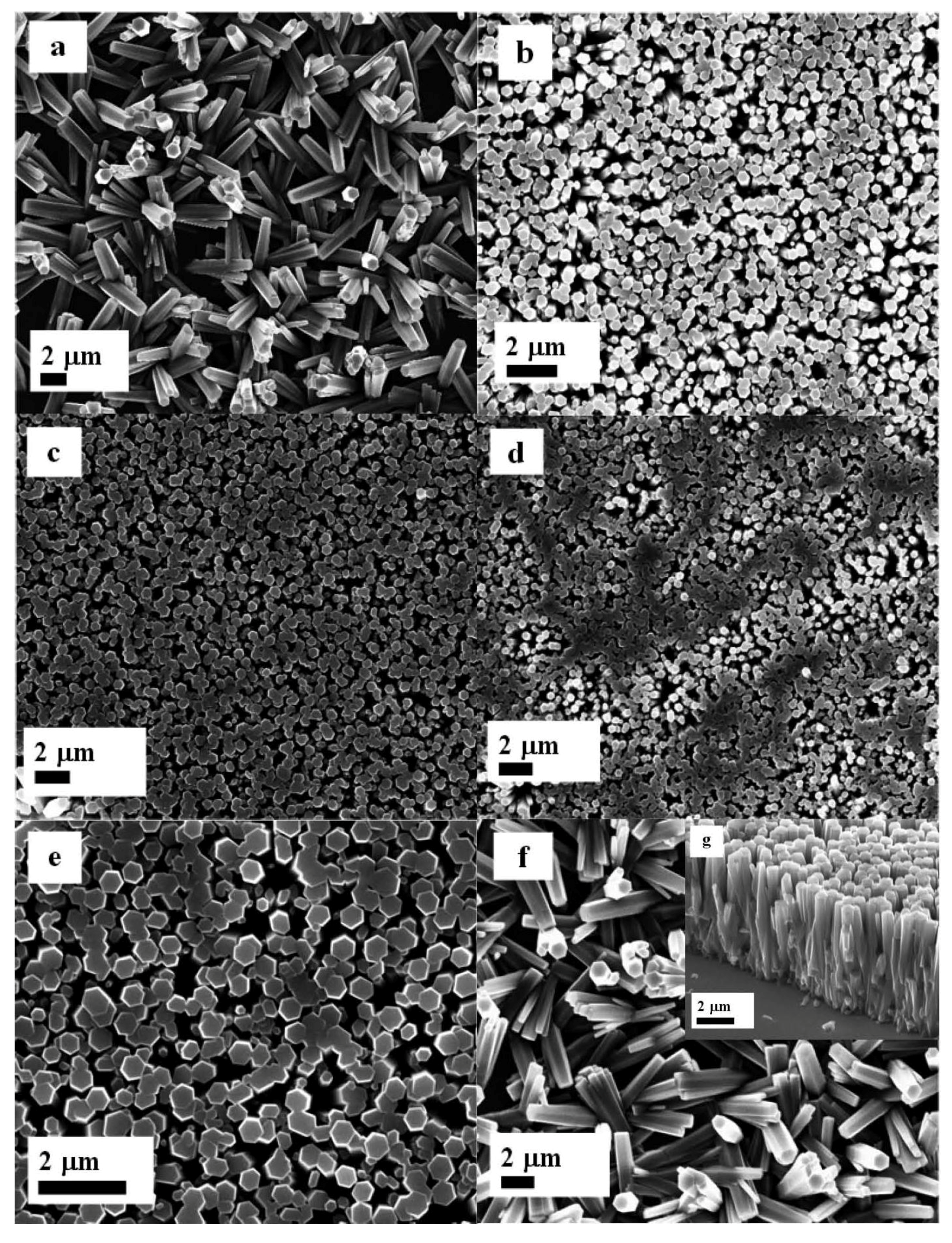
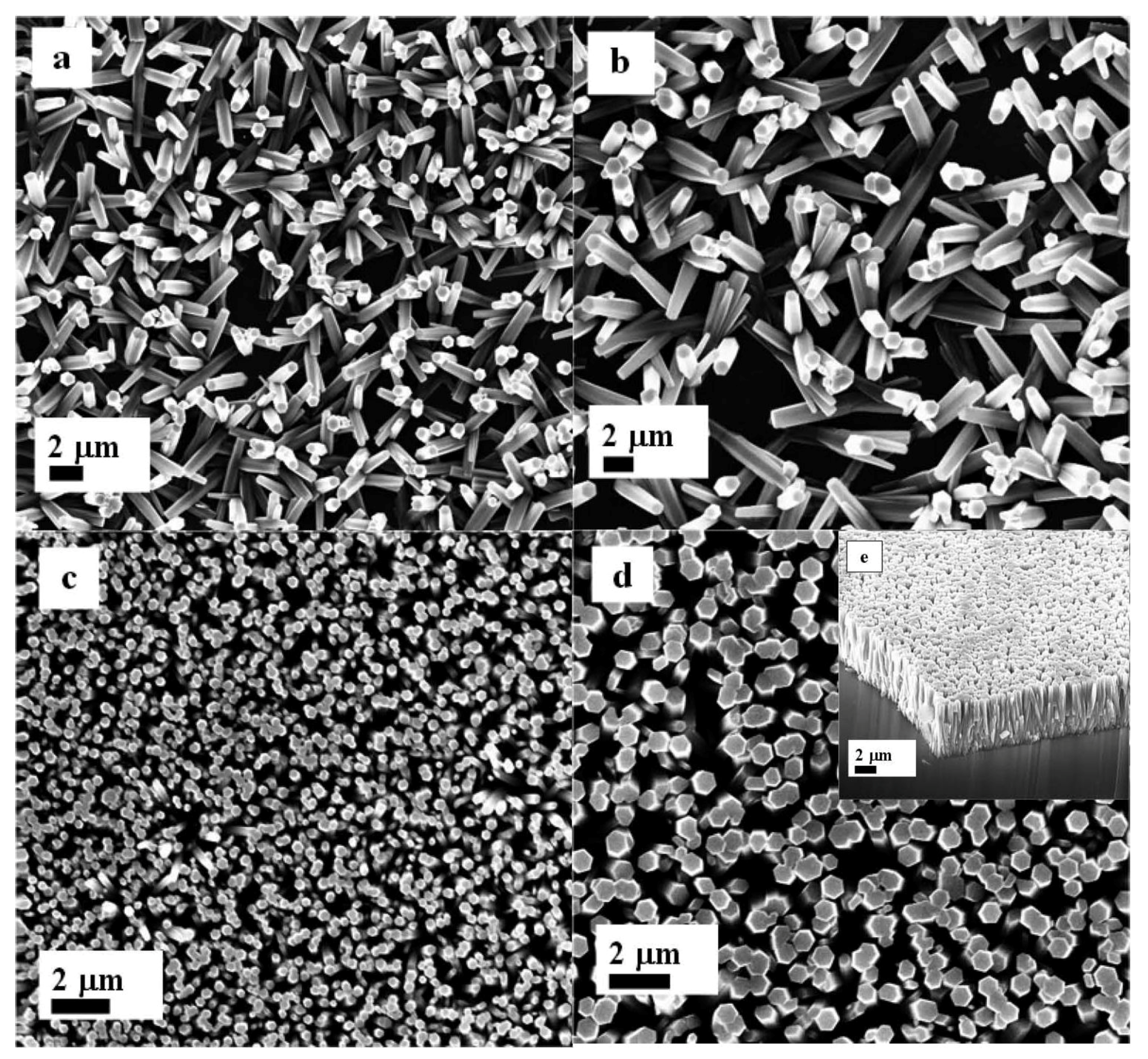

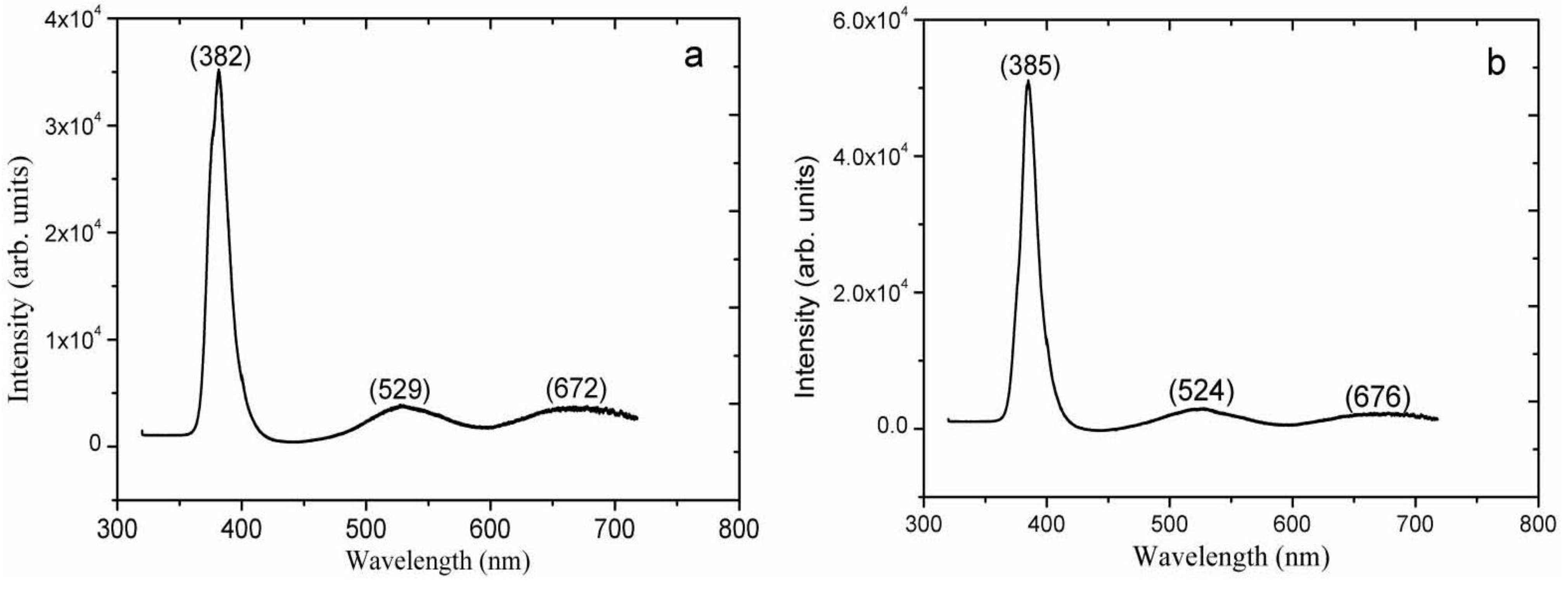
3. Experimental Section
3.1. Preparation of ZnO Nanoparticles Seed Solution in Starch and Cellulose Biopolymers
3.2. Synthesis of ZnO Nanorods on Gold-Coated Glass Substrates
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Huang, M.H.; Mao, S.; Feick, H.; Yan, H.; Wu, Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. Room-temperature ultraviolet nanowire nanolasers. Science 2001, 292, 1897–1899. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.C.; Hu, X. Elasticity and piezoelectricity of zinc oxide crystals, single layers and possible single-walled nanotubes. Phys. Rev. B 2006, 74, 035434:1–035434:6. [Google Scholar]
- Song, J.H.; Zhou, J.; Wang, Z.L. Piezoelectric and semiconducting coupled power generating process of a single ZnO Belt/Wire. A technology for harvesting electricity from the environment. Nano Lett. 2006, 6, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Summers, C.J.; Wang, Z.L. Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano-optoelectronics and nanosensor arrays. Nano Lett. 2004, 4, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Chu, X.F.; Wu, M.W. Detection of H2S down to ppb levels at room temperature using sensors based on ZnO nanorods. Sens. Actuators B Chem. 2006, 113, 320–323. [Google Scholar] [CrossRef]
- Baxter, J.B.; Walker, A.M.; Ommering, K.V.; Aydil, E.S. Synthesis and characterization of ZnO nanowires and their integration into dye-sensitized solar cells. Nanotechnology 2006, 17, S304–S312. [Google Scholar] [CrossRef]
- Liu, T.Y.; Liao, H.C.; Lin, C.C.; Hu, S.H.; Chen, S.Y. Biofunctional ZnO nanorod arrays grown on flexible substrates. Langmuir 2006, 22, 5804–5809. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.Q.; Lorenz, M.; Rahm, A.; Wenckstern, H.; Czekalla, C.; Lenzner, J.; Benndorf, G.; Grundmann, M. Phosphorus acceptor doped ZnO nanowires prepared by pulsed-laser deposition. Nanotechnology 2007, 18, 455707:1–455707:45. [Google Scholar]
- Shen, G.; Bando, Y.; Lee, C.J. Synthesis and evolution of novel hollow ZnO urchins by a simple thermal evaporation process. J. Phys. Chem. B 2005, 109, 10578–10583. [Google Scholar] [CrossRef]
- Liao, L.; Liu, D.H.; Li, J.C.; Liu, C.; Fu, Q.; Ye, M.S. Synthesis and raman analysis of 1D-ZnO nanostructure via vapor phase growth. Appl. Surf. Sci. 2005, 240, 175–179. [Google Scholar] [CrossRef]
- Heo, Y.W.; Varadarajan, V.; Kaufman, M.; Kim, K.; Norton, D.P.; Ren, F.; Fleming, P.H. Site-specific growth of Zno nanorods using catalysis-driven molecular-beam epitaxy. Appl. Phys. Lett. 2002, 81, 3046–3048. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Cao, H.; Chang, R.P.H. Growth mechanism and properties of ZnO nanorods synthesized by plasma-enhanced chemical vapor deposition. J. Appl. Phys. 2004, 95, 3141–3147. [Google Scholar] [CrossRef]
- Khranovskyy, V.; Tsiaoussis, I.; Hultman, L.; Yakimova, R. Selective homoepitaxial growth and luminescent properties of ZnO nanopillars. Nanotechnology 2011, 22, 185603:1–185603:18. [Google Scholar] [CrossRef]
- Park, W.I.; Kim, D.H.; Jung, S.W.; Brown, N.M.D. Control and mass selection of CnHm+ fragments in an inductively coupled pulsed plasma. Appl. Phys. Lett. 2002, 80, 22–24. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, M.C.; Oh, B.Y.; Lee, W.; Myoung, J.M. Fabrication of Zn/ZnO nanocables through thermal oxidation of Zn nanowires grown by RF magnetron sputtering. J. Cryst. Growth 2006, 290, 485–489. [Google Scholar] [CrossRef]
- Anthony, S.P.; Lee, J.I.; Kim, J.K. Tuning optical band gap of vertically aligned ZnO nanowire arrays grown by homoepitaxial electrodeposition. Appl. Phys. Lett. 2007, 90, 103107:1–103107:3. [Google Scholar]
- Guo, M.; Diao, P.; Wang, X.; Han, J.S.; Ma, H.; Zhao, X.H.; Zhao, X.H. A novel route to synthesize cubic ZrW2−xMoxO8 (x = 0–1.3) solid solutions and their negative thermal expansion properties. J. Solid State Chem. 2005, 178, 3166–3171. [Google Scholar]
- Li, C.; Fang, G.; Su, F.; Li, G.; Wu, X.; Zhao, X. Synthesis and photoluminescence properties of vertically aligned ZnO nanorod–nanowall junction arrays on a ZnO-coated silicon substrate. Nanotechnology 2006, 17, 3740:1–3740:15. [Google Scholar]
- Umar, A.; Karunagaran, B.; Suh, E.K.; Hahn, Y.B. Structural and optical properties of single-crystalline ZnO nanorods grown on silicon by thermal evaporation. Nanotechnology 2006, 17, 4072:1–4072:16. [Google Scholar]
- Kumar, P.S.; Raj, A.D.; Mangalaraj, D.; Nataraj, D. Growth and characterization of ZnO nanostructured thin films by a two-step chemical method. Appl. Surf. Sci. 2008, 255, 2382–2387. [Google Scholar] [CrossRef]
- Breedona, M.; Rahmani, M.B.; Keshmir, S.H.; Wlodarskia, W.; Zadeh, K.K. Aqueous synthesis of interconnected ZnO nanowires using spray pyrolysis deposited seed layers. Mater. Lett. 2010, 64, 291–294. [Google Scholar] [CrossRef]
- Zoolfakar, A.S.; Kadir, R.A.; Rani, R.A.; Balendhran, S.; Liu, X.; Kats, E.; Bhargava, S.K.; Bhaskaran, M.; Sriram, S.; Zhuiykov, S.; O’Mullane, A.P.; Zadeh, K.K. Engineering electrodeposited ZnO films and their memristive switching performance. Phys. Chem. Chem. Phys. 2013, 15, 10376–10384. [Google Scholar] [CrossRef] [PubMed]
- Zoolfakar, A.S.; Rani, R.A.; Morfa, A.J.; Balendhran, S.; O’Mullane, A.P.; Zhuiykov, S.; Zadeh, K.K. Enhancing the current density of electrodeposited ZnO–Cu2O solar cells by engineering their heterointerfaces. J. Mater. Chem. 2012, 22, 21767–21775. [Google Scholar] [CrossRef] [Green Version]
- Mann, S. The chemistry of form. Angew. Chem. Int. Ed. 2000, 39, 3392–3406. [Google Scholar] [CrossRef]
- Murphy, W.L.; Mooney, D.J. Bioinspired growth of crystalline carbonate apatite on biodegradable polymer substrata. J. Am. Chem. Soc. 2002, 124, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Landfester, K. The generation of nanoparticles in miniemulsions. Adv. Mater. 2001, 13, 765–768. [Google Scholar] [CrossRef]
- Aizenberg, J.; Black, A.J.; Whitesides, G.M. Control of crystal nucleation by patterned self-assembled monolayers. Nature 1999, 398, 495–498. [Google Scholar] [CrossRef]
- Braun, E.; Eichen, Y.; Sivan, U.; Ben-Yoseph, G. DNA-templated assembly and electrode attachment of a conducting silver wire. Nature 1998, 391, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Storhoff, J.J.; Mirken, C.A. Programmed materials synthesis with DNA. Chem. Rev. 1999, 99, 1849–1862. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, W.; Shenton, W.; Davis, S.A.; Mann, S. Template mineralization of ordered macroporous chitin-silica composites using cuttlebone-derived organic matrix. Chem. Mater. 2000, 12, 2835–2837. [Google Scholar] [CrossRef]
- Shin, Y.; Liu, J.; Chang, J.H.; Nie, Z.; Exarhos, G.J. Hierarchically ordered ceramics through surfactant-templated sol-gel mineralization of biological cellular structures. Adv. Mater. 2001, 13, 728–732. [Google Scholar] [CrossRef]
- Thomas, D.J.; Atwell, W.A. Starch Structure in Starches Practical Guide for the Food Industry; Eagan Press: St. Paul, MN, USA, 1999. [Google Scholar]
- Mishra, S.K.; Srivatava, R.K.; Prakash, S.G.; Yadav, R.S.; Panday, A.C. Photoluminescence and photoconductive characteristics of hydrothermally synthesized ZnO nanoparticles. Opto-Electron. Rev. 2000, 18, 467–473. [Google Scholar]
- Zhang, J.; Sun, L.D.; Lin, Y.J.; Su, H.; Liao, C.; Yan, C. Control of ZnO morphology via a simple solution route. Chem. Mater. 2002, 14, 4172–4177. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.D.; Pan, H.Y.; Liao, C.; Yan, C. ZnO nanowires fabricated by a convenient route. New J. Chem. 2002, 26, 33–34. [Google Scholar] [CrossRef]
- Huang, M.H.; Wu, Y.; Feick, H.; Tran, N.; Weber, E.; Yang, P. Catalytic growth of zinc oxide nanowires by vapor transport. Adv. Mater. 2001, 13, 113–116. [Google Scholar] [CrossRef]
- Wu, J.J.; Liu, S.C. Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv. Mater. 2002, 14, 215–218. [Google Scholar] [CrossRef]
- Wu, J.J.; Liu, S.C. Catalyst-free growth and characterization of ZnO nanorods. J. Phys. Chem. B 2002, 106, 9546–9551. [Google Scholar] [CrossRef]
- Li, Y.; Meng, G.W.; Zhang, L.D.; Phillipp, F. Ordered semiconductor ZnO nanowire arrays and their photoluminescence properties. Appl. Phys. Lett. 2000, 76, 2011–2013. [Google Scholar] [CrossRef]
- Wang, Y.C.; Leu, I.C.; Hon, M.H. Effect of colloid characteristics on the fabrication of ZnO nanowire arrays by electrophoretic deposition. J. Mater. Chem. 2002, 12, 2439–2444. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Goldberger, J.; Kim, F.; Johnson, J.C.; Zhang, Y.; Saykally, R.J.; Yang, P. Low-temperature wafer-scale production of ZnO nanowire arrays. Angew. Chem. Int. Ed. 2003, 42, 3031–3034. [Google Scholar] [CrossRef]
- Liu, M.; Kitai, A.H.; Mascher, P. Point defects and luminescence centres in zinc oxide and zinc oxide doped with manganese. J. Lumin. 1992, 54, 35–42. [Google Scholar] [CrossRef]
- Wu, X.L.; Siu, G.G.; Fu, C.L.; Ong, H.C. Photoluminescence and cathodoluminescence studies of stoichiometric and oxygen-deficient ZnO films. Appl. Phys. Lett. 2001, 78, 2285–2287. [Google Scholar] [CrossRef]
- Studenikin, S.A.; Golego, N.; Cocivera, M. Fabrication of green and orange photoluminescent, undoped ZnO films using spray pyrolysis. J. Appl. Phys. 1998, 84, 2287–2294. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ibupoto, Z.H.; Khun, K.; Eriksson, M.; AlSalhi, M.; Atif, M.; Ansari, A.; Willander, M. Hydrothermal Growth of Vertically Aligned ZnO Nanorods Using a Biocomposite Seed Layer of ZnO Nanoparticles. Materials 2013, 6, 3584-3597. https://doi.org/10.3390/ma6083584
Ibupoto ZH, Khun K, Eriksson M, AlSalhi M, Atif M, Ansari A, Willander M. Hydrothermal Growth of Vertically Aligned ZnO Nanorods Using a Biocomposite Seed Layer of ZnO Nanoparticles. Materials. 2013; 6(8):3584-3597. https://doi.org/10.3390/ma6083584
Chicago/Turabian StyleIbupoto, Zafar Hussain, Kimleang Khun, Martin Eriksson, Mohammad AlSalhi, Muhammad Atif, Anees Ansari, and Magnus Willander. 2013. "Hydrothermal Growth of Vertically Aligned ZnO Nanorods Using a Biocomposite Seed Layer of ZnO Nanoparticles" Materials 6, no. 8: 3584-3597. https://doi.org/10.3390/ma6083584
APA StyleIbupoto, Z. H., Khun, K., Eriksson, M., AlSalhi, M., Atif, M., Ansari, A., & Willander, M. (2013). Hydrothermal Growth of Vertically Aligned ZnO Nanorods Using a Biocomposite Seed Layer of ZnO Nanoparticles. Materials, 6(8), 3584-3597. https://doi.org/10.3390/ma6083584





