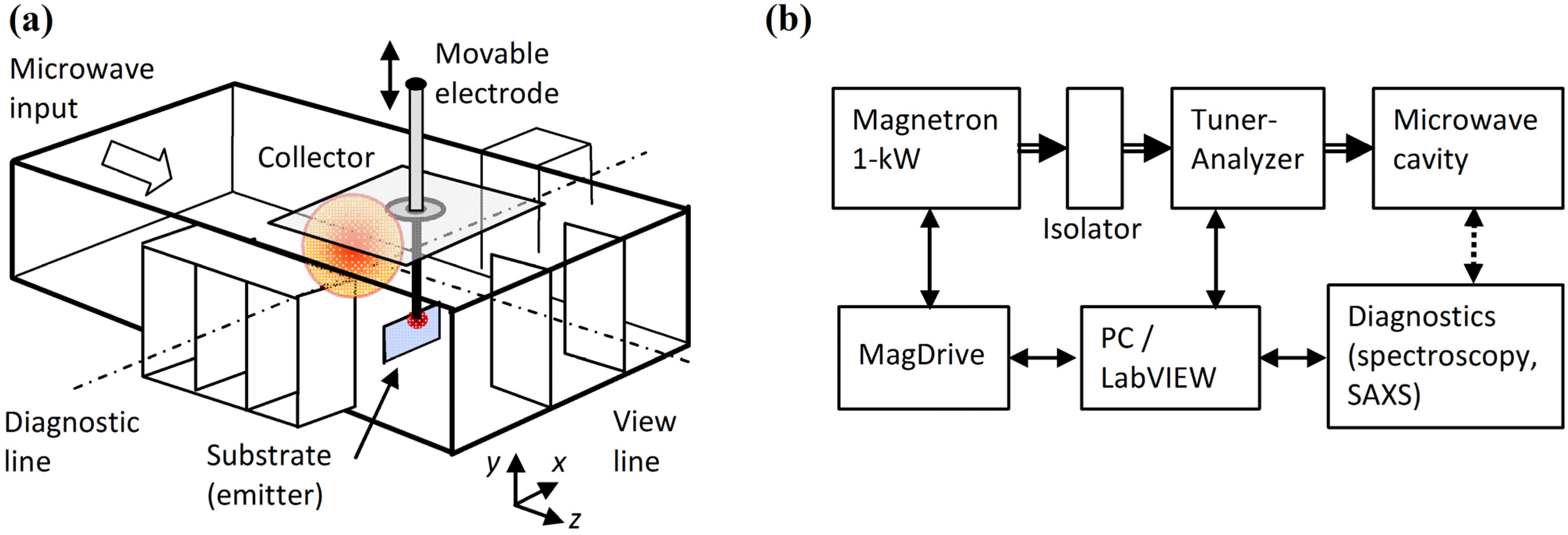
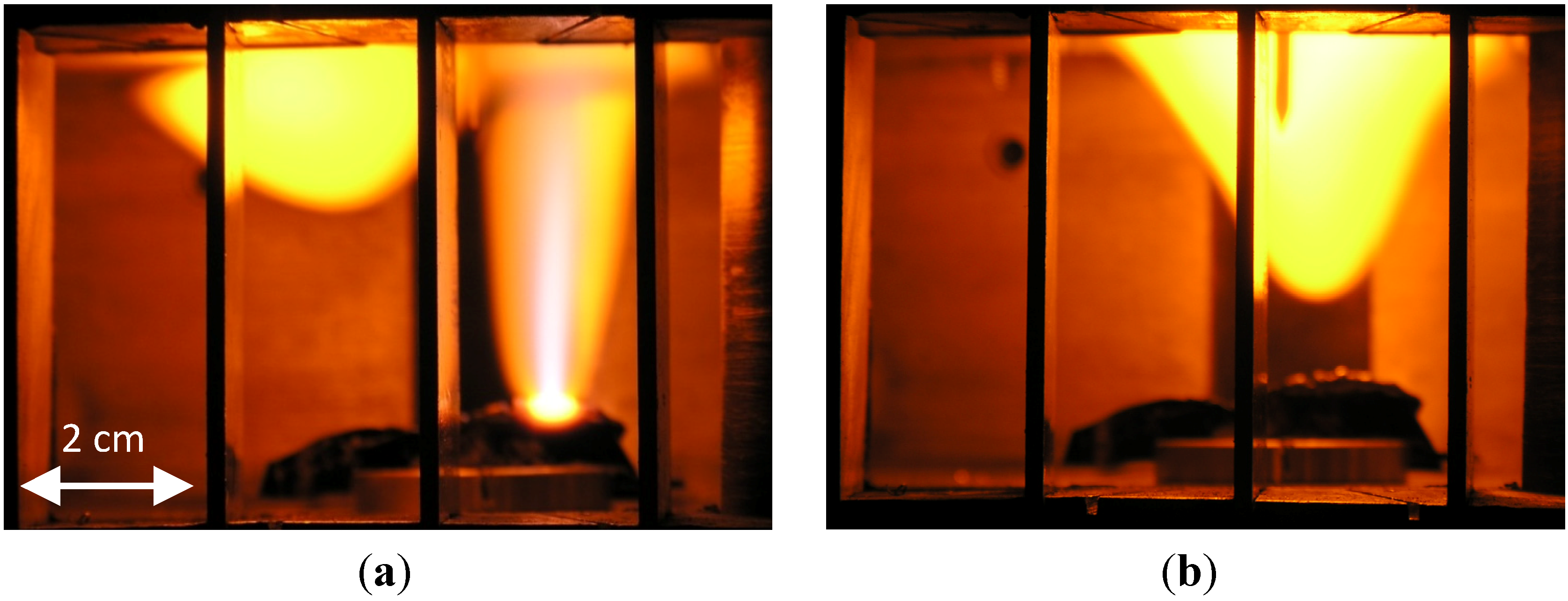
The experimental results for the plasmoid properties, obtained by microwave scattering, optical spectroscopy, SAXS, SEM and EDS measurements are presented and analyzed in the following sections. The variations in the microwave reflections during the process are analyzed using a dusty-plasma model and the other measurement results, as follows:
3.1. Microwave Scattering
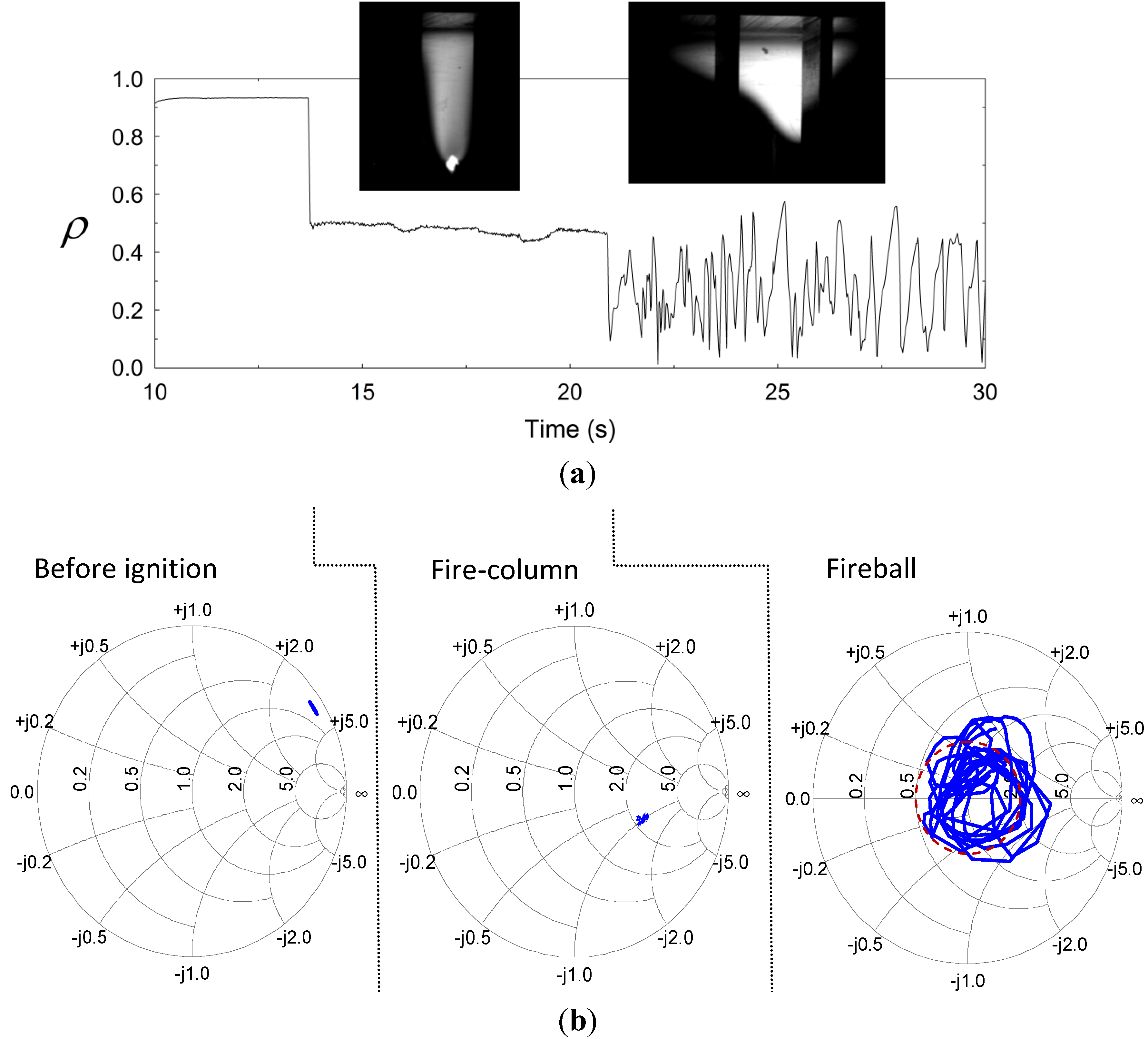
A significant increase in the microwave power absorption at 2.45 GHz was observed after fireball ignition. This effect is displayed by the reduction of the reflection coefficient
in scalar and vectorial representations in
Figure 3a,b, respectively. The microwave reflection shown in
Figure 3a (in a scalar presentation) is significantly decreased at the time of ignition of the fire-column (from ρ ~ 0.9 to ~ 0.5). The reflection is decreased further at the fireball stage, while bouncing around ρ ~ 0.3 and attracted by the Smith-chart origin as shown in
Figure 3b. The corresponding average power reflection,
, is reduced to ~10% at this stage, hence the plasmoid tends to interact with its microwave generator as a nearly perfect load.
Figure 3b presents a Smith-chart display [
25] of this effect in vectorial plots of the reflected wave, before and after ignition. These results reveal the adaptive impedance-matching effect, which dictates the plasmoid’s autonomous evolution. The fluctuations observed in the fireball stage in
Figure 3a,b are attracted by the origin of the Smith chart (at which ρ = 0) in an oscillatory manner. This self-tuning mechanism tends to adaptively maximize the microwave power absorbed by the fireball, by a self-tuning optimization of its intensity, position and size.
Figure 3.
The microwave reflections from the cavity before and after ignition: (a) The autonomous reduction in the reflected power upon ignition of the fire-column, and its fluctuation in the fireball stage, being attracted by a minimal reflection level ; and (b) Smith-chart presentations of the cavity’s input impedance, before ignition, during the fire-column stage, and in the consequent fireball stage (the latter shows the tendency of the fireball to reach a self impedance matching).
Figure 3.
The microwave reflections from the cavity before and after ignition: (a) The autonomous reduction in the reflected power upon ignition of the fire-column, and its fluctuation in the fireball stage, being attracted by a minimal reflection level ; and (b) Smith-chart presentations of the cavity’s input impedance, before ignition, during the fire-column stage, and in the consequent fireball stage (the latter shows the tendency of the fireball to reach a self impedance matching).
3.2. Optical Spectroscopy
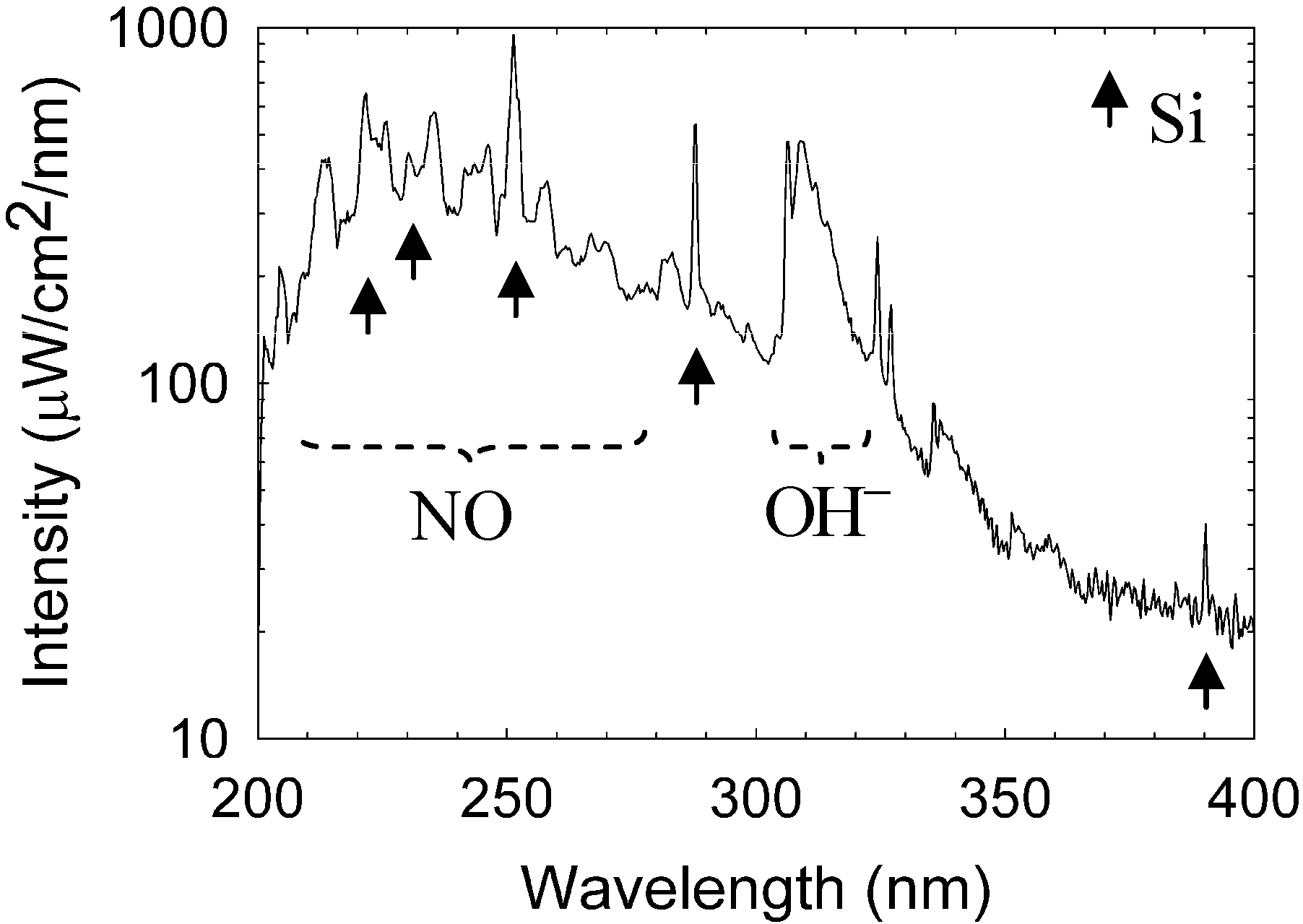
An Avaspec-3648 optical spectrometer was used to analyze the spectral emission of the plasmoid in the range of 200–1000 nm with a 0.3 nm resolution. The intensity is calibrated in this range by an AvaLight Deuterium-Halogen light source (DH-BAL-CAL UV/VIS, Avantes Ltd.). The experiment is conducted in humid air at atmospheric pressure, as describe in
Section 2 above. The optical spectrum emitted by a typical plasmoid ejected from a silicon substrate is shown in
Figure 4. Silicon lines are identified in the emission spectrum (in accordance with the substrate material from which the plasmoid has been ejected [
26]). In addition, spectral emissions of nitric oxide (NO) and hydroxyl (
) radicals are also observed in
Figure 4, hence these radicals are generated within the plasmoid (as observed also in copper fire-columns [
13]). The
band emission in the range 300–330 nm reveals the rotational temperature of the radicals, estimated by fitting the line shape to the LifBase simulation [
27]. As shown in
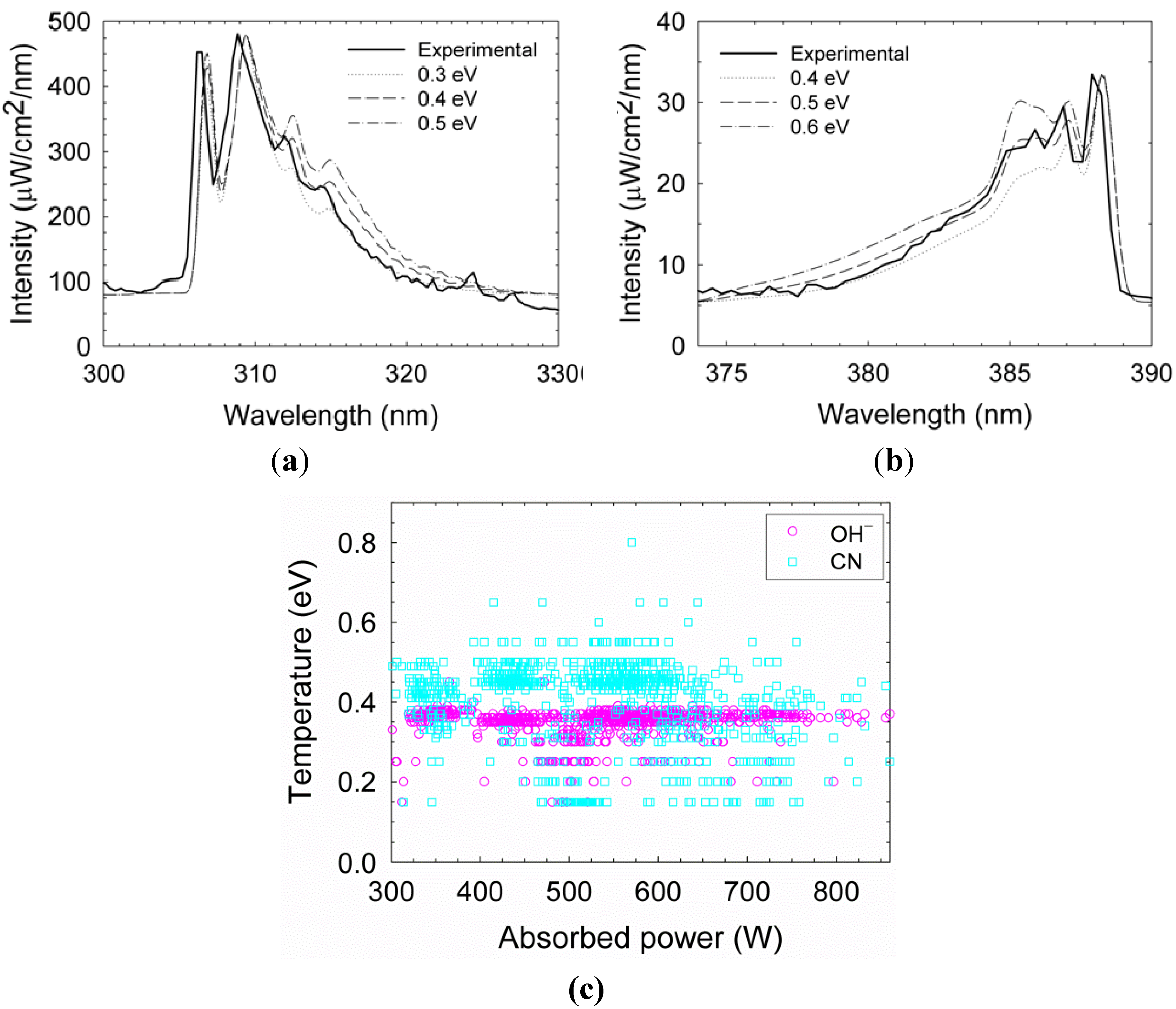
Figure 5a, the
rotational temperature is estimated as ~0.4 eV. This result can be used also to evaluate the gas temperature within the plasmoid in some conditions [
28,
29]. The
gamma band shows the dissociation of water fumes as a result of combustion [
30]. When using a graphite electrode, cyano (CN) radicals are also generated within the plasmoid. Their rotational temperature is estimated as ~0.5 eV by fitting to the LifBase simulation, as shown in
Figure 5b. The violet spectrum of the CN band
can also be used to estimate the gas temperature [
31,
32]. An accumulation of 941 fittings frames for
and 973 frames for CN in various power levels, as shown in
Figure 5c, results in mean temperatures of 0.34 ± 0.04 eV and 0.40 ± 0.11 eV for
and CN respectively. The data exhibit relatively stable temperatures, with no significant notable correlations with the absorbed microwave power.
Figure 4.
Optical spectroscopy measurements. A typical spectrum of silicon plasmoid reveals silicon excited atoms, and hydroxyl and nitric oxide radical production.
Figure 4.
Optical spectroscopy measurements. A typical spectrum of silicon plasmoid reveals silicon excited atoms, and hydroxyl and nitric oxide radical production.
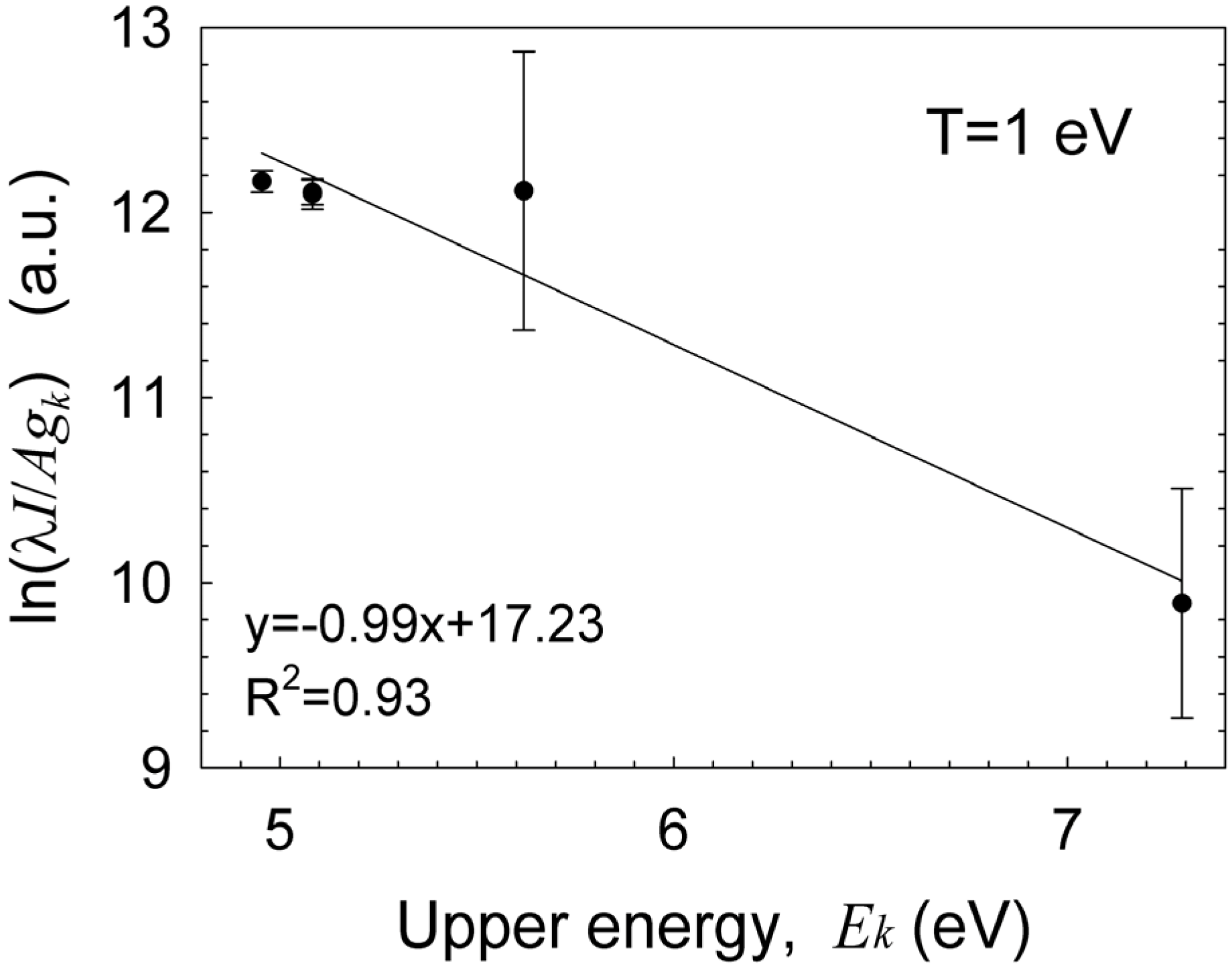
The Boltzmann plot technique [
33] is applied to the silicon neutral lines, as shown in
Figure 6, in order to estimate the excitation temperature. Assuming conditions for partial local thermal equilibrium (pLTE) as discussed below in
Section 3.5, the Boltzmann equation for the intensity of the upper
k to the lower
i energy transition level;
Iki, is given by
; where λ
ki and
Aki, are the wavelength and transition probability, respectively;
kB is the Boltzmann constant; and
Ek is the upper energy state with a degeneracy
gk. Therefore, the slope of the curve obtained is inversely proportional to the plasma excitation temperature,
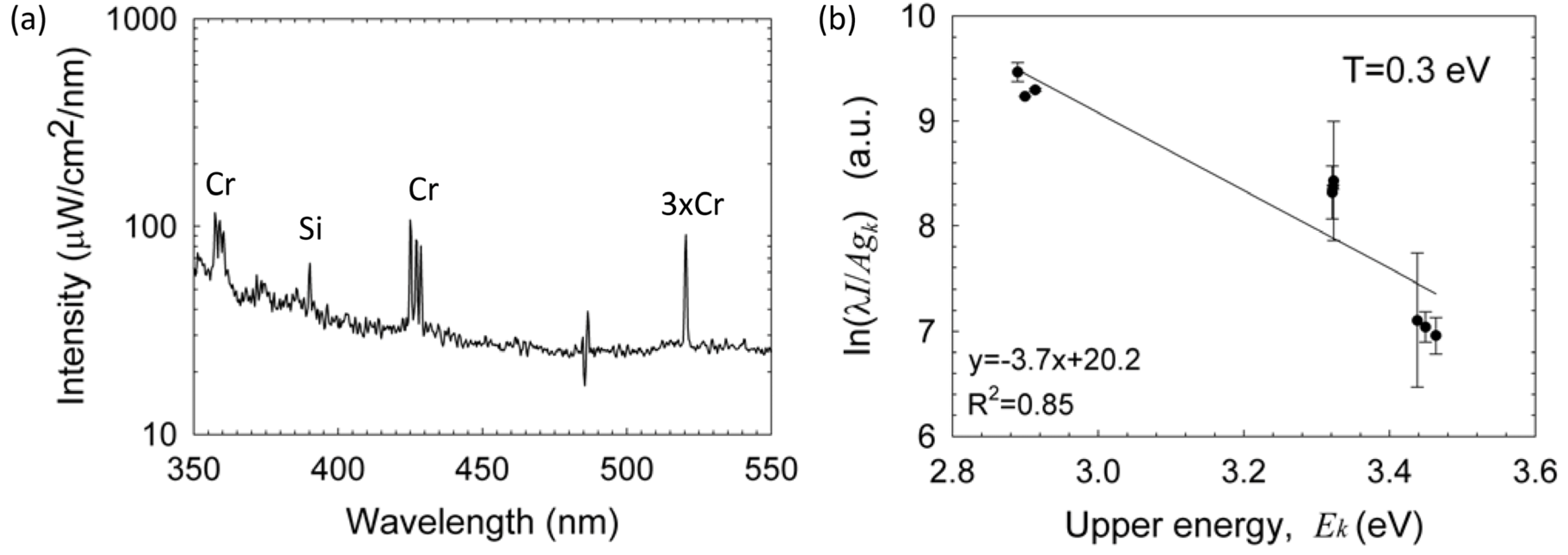
Texc. The results show an excitation temperature of ~1 eV for the detected silicon lines. Impurities originating probably from the electrode are also observed, e.g., the chromium emission lines marked in
Figure 7a. Similarly to the analysis of radical molecules presented above, the Boltzmann-plot technique applied to the chromium spectral lines results in ~0.3 eV as shown in
Figure 7b (note that this plot may be more accurate than that for silicon in
Figure 6 due to the larger number of chromium spectral lines, the partial overlap of the silicon lines and the NO radical emission, and the relatively low spectral resolution and sampling available).
Figure 5.
Temperature evaluation by fitting the radicals emission, (
a) OH
–; (
b) CN by the LifBase simulation [
27]; and (
c) an accumulation of 941 fittings frames for OH
– and 973 frames for CN with respect to the instantaneous effective microwave power. The mean temperature does not seem to vary significantly with power in this range.
Figure 5.
Temperature evaluation by fitting the radicals emission, (
a) OH
–; (
b) CN by the LifBase simulation [
27]; and (
c) an accumulation of 941 fittings frames for OH
– and 973 frames for CN with respect to the instantaneous effective microwave power. The mean temperature does not seem to vary significantly with power in this range.
Figure 6.
A Boltzmann plot of the silicon lines resulting in a ~1 eV excitation temperature.
Figure 6.
A Boltzmann plot of the silicon lines resulting in a ~1 eV excitation temperature.
Figure 7.
(
a) An emission spectrum of silicon lines with chromium impurities; and (
b) a Boltzmann-plot fit of the chromium lines resulting in ~0.3 eV excitation temperature. (Note a diode line defect at 485 nm in
Figure 7a)
Figure 7.
(
a) An emission spectrum of silicon lines with chromium impurities; and (
b) a Boltzmann-plot fit of the chromium lines resulting in ~0.3 eV excitation temperature. (Note a diode line defect at 485 nm in
Figure 7a)
3.3. SAXS Measurements
The fireball apparatus was installed at the ESRF for
in-situ SAXS measurements and the X-ray beam was aligned along the diagnostic line illustrated in
Figure 1. X-ray scattering experiments were performed by illuminating the fireball with a 12.4 keV X-ray (λ ~ 0.1 nm) and detecting the scattered X-rays using a high brilliance pinhole SAXS instrument. For comparison, the SAXS measurements were performed both in a dry air atmosphere and in damp air enriched with a micro-droplet aerosol of water solution of NaHCO
3 (decomposed above 70 °C into sodium carbonate, water and carbon dioxide. The carbonate converts at ~1000 °C to sodium oxide
[
34]). Representative SAXS profiles are shown in
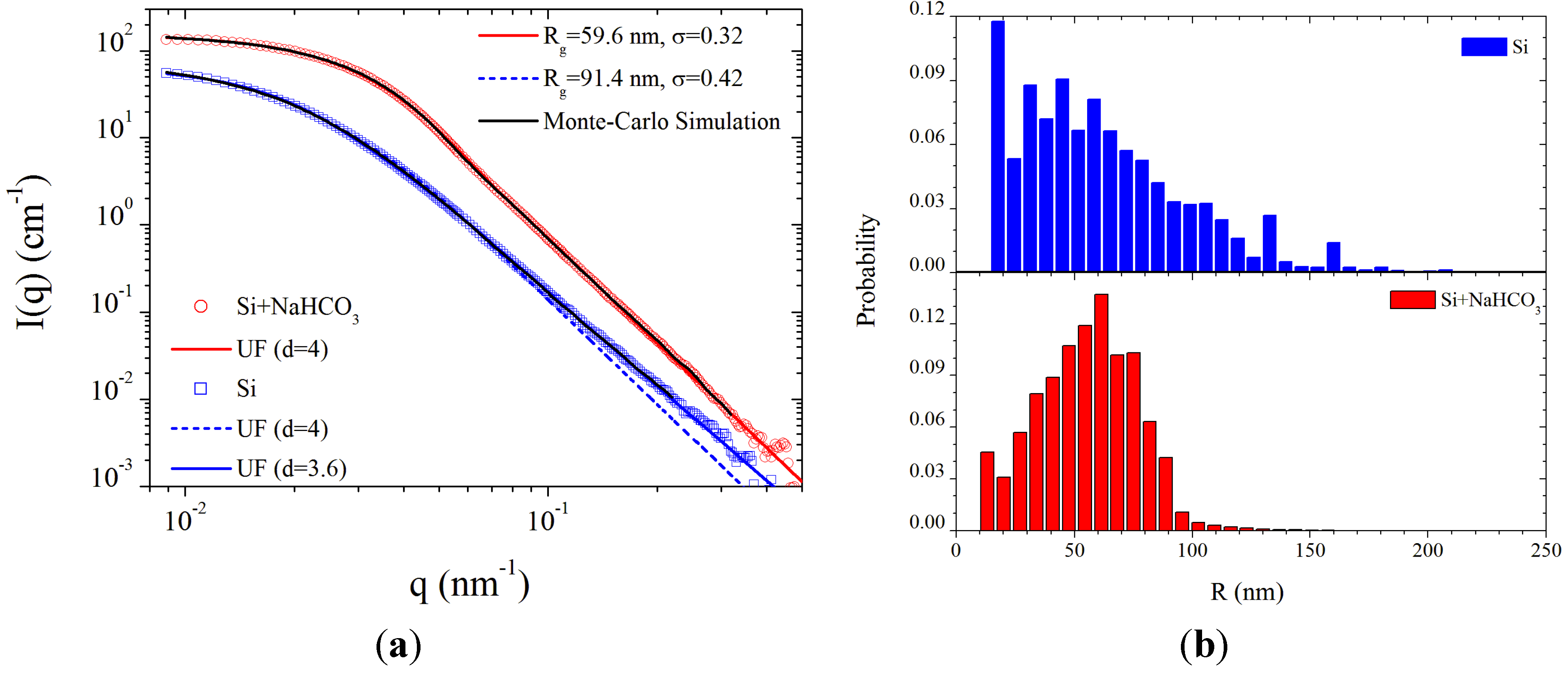
Figure 8a as a function of the scattering vector
where
θ and λ are the scattering angle and X-ray wavelength, respectively. It should be noted that these intensities correspond to their maximum level while scanning the fireball apparatus through the X-ray beam, and therefore represent the region relatively concentrated with particles. The SAXS intensities from the dry air fireball were found to be relatively stable, while some level of intermittency was observed during the damp-air fireball lifetime.
The SAXS data are modeled in terms of a single level unified scattering (UF) function [
35], in the form
as in [
13], where the whole scattering curve could be described by a single structural level with four parameters: radius of gyration (
Rg); amplitudes
G and
B of the exponential and power law terms, respectively; and power law exponent (
d). The best-fit parameters yielded the
Rg and standard deviation (σ) as indicated in the upper legend of
Figure 8a. In addition, a comparison with the scattering profiles obtained by Monte-Carlo simulation method [
36] using 50,000 particles is also shown. The two plots in
Figure 8b show the size distribution derived from the Monte-Carlo simulation method [
36]. Evidently, these size distributions are more complex than typical log-normal distribution assumed by the unified analysis [
35]. The mean particle radii from the simulations are 62.9 nm and 55.4 nm for the dry and damp conditions, respectively.
Figure 8.
(a) Normalized SAXS intensity profiles from the silicon fireball ejected by a graphite electrode in dry air and in damp air with NaHCO3 (Blue and Red curves, respectively), together with model fits; and (b) Particle size distribution derived from the best fit Monte-Carlo simulations corresponding to mean particle radii of 62.9 nm and 55.4 nm for the dry and damp conditions, respectively.
Figure 8.
(a) Normalized SAXS intensity profiles from the silicon fireball ejected by a graphite electrode in dry air and in damp air with NaHCO3 (Blue and Red curves, respectively), together with model fits; and (b) Particle size distribution derived from the best fit Monte-Carlo simulations corresponding to mean particle radii of 62.9 nm and 55.4 nm for the dry and damp conditions, respectively.
From the unified analysis, the best fit value of d for the dry air fireball (d = 3.6) is smaller than the Porod law (d = 4), suggesting that the particles have a rough fractal surface characterized by a surface fractal dimension of 2.4 (d − 6). However, in the case of the damp-air fireball, the data clearly display a Porod behavior (d = 4) indicating a smooth particle-air interface. The intermittency in this case implies that both smaller and larger particles were present. The smooth interface and narrower spread of the size distribution suggests that particles under this condition may be in the form of molten droplets. These droplets could coalesce and form the larger silica spheres observed intermittently in the SAXS analysis and seen also in the SEM images presented below.
Assuming a melt density of Si (2570 kg/m
3), the particle number densities in the relatively concentrated region are ~10
15–10
16 m
−3 for both fireball types (compared to ~10
15 m
−3 found in fireballs generated from glass substrates [
13]). The size range deduced from in-situ SAXS is consistent with the ex-situ SEM observations. For the mean radii derived from simulations,
Rm ≈ 60 nm, the maximum particle volume fraction is ~10
−6 within the fireball (as the SAXS analysis was performed using the highest levels of intensities).
3.4. SEM Observations
The SEM analysis was restricted to samples soon after microwave preparation without any modification or coating. The fireballs generated in these conditions create spherical particles of various sizes and surface roughness, including particles larger than the SAXS detection limit (~120 nm) in our experiment.
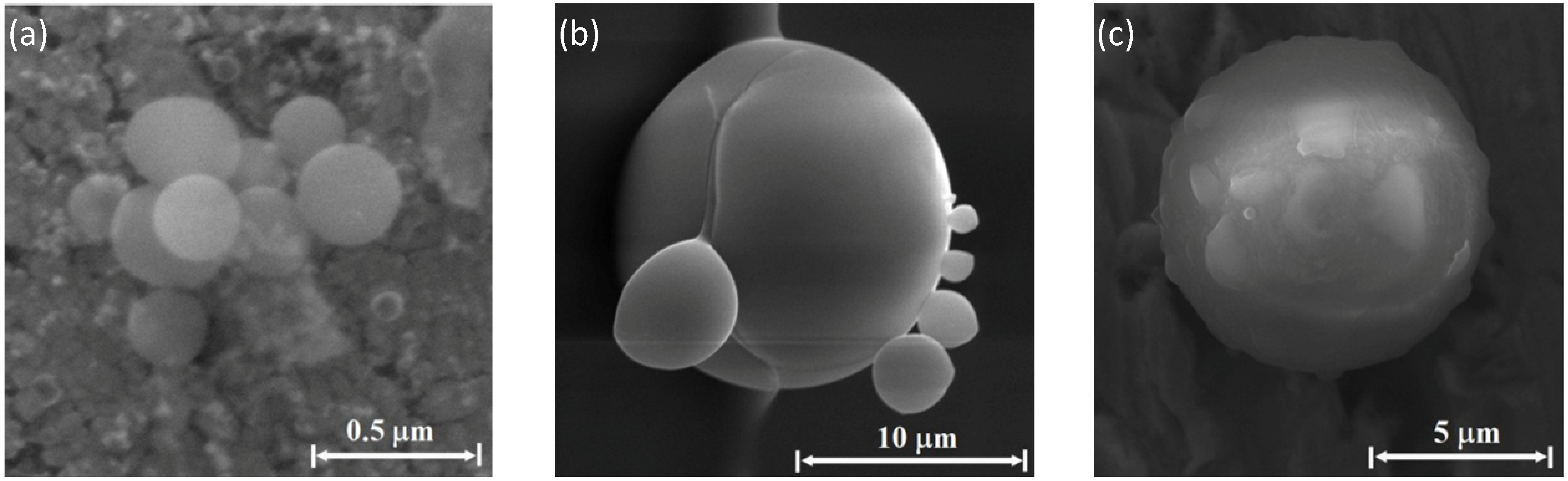
Figure 9a shows aggregates of such particles observed by SEM on the silicon emitter (the experimental setup consisted of a Si emitter, Cu collector, and C electrode, in a water-vapor sprayed atmosphere). The sub-micron spheres obtained are characterized by a rough surface, which consists of smaller particles, of the order of ~10 nm diameter. As revealed by EDS analyses, these aggregates are mostly made of silicon and oxygen. Larger spheres of several micrometers in diameter are observed in the molten region of the silicon emitters, for instance the string of micro-spheres shown in
Figure 9b. The molten surface also includes voids of similar sizes (which may indicate the origin of micro-particles that bubbled out and were ejected from the surface).
Figure 9c shows a SEM image of micro-spheres that seem to be sputtered and crushed onto the ceiling of the collector. They reside in craters that could be created by their collisions with the copper collector surface.
An SEM image of a perfectly round sphere of 8 μm in diameter, typically observed on the collector ceiling, is shown in
Figure 10a. The sub-micron particles on its outer surface may indicate that the formation of micrometer-scale spheres involves smaller particles’ agglomeration. The EDS analysis of perfect spheres mostly show Si and also O to a lesser extent, as in
Figure 10b, which may indicate surface oxidation of the bulk silicon sphere. Minor impurities of other elements, including C, Na and S, were also detected by the EDS analysis of the perfectly round sphere. The consolidation of nanoparticles to larger aggregates melted together is seen in various examples in
Figure 10c. The larger particles observed in this experiment, mostly on the upper collector, are slightly oxidized silicon spheres on the order of ~10 μm and larger.
The EDS analyses of these spheres show the presence of Si as well as O, with small amounts of other elements including Cl, C and Cu. The smaller particles, up to ~80 nm in diameter, are also observed on the silicon emitter and
in-situ by SAXS. Hence, one may deduce that the larger spheres agglomerate within the fireball while being elevated up to the collector. In view of these SEM images, one may deduce that some of these large spheres have evolved as hollow spheres with a ~1 μm thick shell, as seen by their crushed traces on the collector (
Figure 9c).
The consolidation of nanoparticles to larger aggregates melted together is seen in various examples of clusters in various stages of agglomeration in
Figure 11 and
Figure 12.
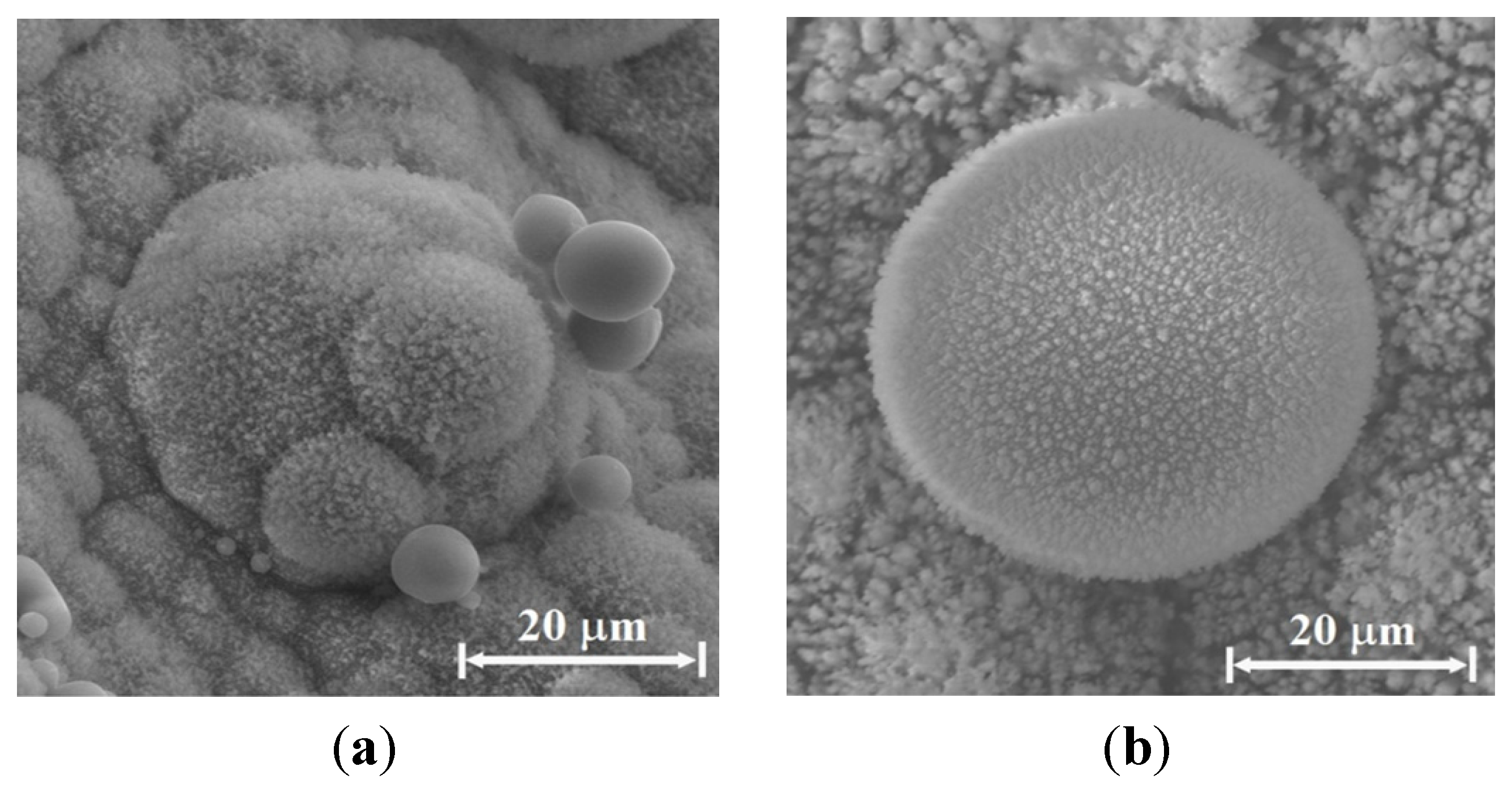
Figure 11a–c show particles observed on the Si emitter. A cluster of sub-micron spherical particles (~0.2 μm diameter each) mostly made of silicon and oxygen is shown in
Figure 11a, whereas
Figure 11b presents a larger sphere (~15 μm diameter) with several smaller spheres attached to it. Adding sodium bicarbonate (NaHCO
3) to the water sprayed vapor results in round spheres, as the ~10 μm diameter sphere presented in
Figure 11c. The latter is mostly made of silicon and oxygen, with smaller quantities of carbon.
Figure 12a,b respectively show larger sea-urchin like particles (~40 μm) observed on the copper collector surface, without and with sodium bicarbonate enrichment of the water sprayed vapor. The accumulation of smaller particles over big round particles is seen both in
Figure 11b and in
Figure 12a under the same conditions.
Figure 9.
SEM observations: (a) aggregates of spherical particles, 0.1–0.3 μm in diameter, on the silicon emitter. The inset shows a typical sphere with a rough hairy surface that consists of smaller particles, ~10 nm in diameter. EDS analysis reveals mostly silicon and oxygen in these aggregates; (b) larger spheres, on the micrometer-scale in diameter, in a molten region, and voids of similar sizes; and (c) micro-spheres that seem to be smashed onto the copper collector plate. The inset shows a typical ~10 μm sphere of silicon that seemingly was crushed into the copper plate and has created a crater by hitting the surface.
Figure 9.
SEM observations: (a) aggregates of spherical particles, 0.1–0.3 μm in diameter, on the silicon emitter. The inset shows a typical sphere with a rough hairy surface that consists of smaller particles, ~10 nm in diameter. EDS analysis reveals mostly silicon and oxygen in these aggregates; (b) larger spheres, on the micrometer-scale in diameter, in a molten region, and voids of similar sizes; and (c) micro-spheres that seem to be smashed onto the copper collector plate. The inset shows a typical ~10 μm sphere of silicon that seemingly was crushed into the copper plate and has created a crater by hitting the surface.
Figure 10.
A typical perfect sphere, 8 μm in diameter; (a) the SEM image; (b) its EDS spectrum showing mostly Si and O; and (c) examples of micro-particles observed by SEM on the copper collector as clusters in various stages of agglomeration.
Figure 10.
A typical perfect sphere, 8 μm in diameter; (a) the SEM image; (b) its EDS spectrum showing mostly Si and O; and (c) examples of micro-particles observed by SEM on the copper collector as clusters in various stages of agglomeration.
Figure 11.
The particles presented were created by a Si-emitter, C-electrode, and Cu-collector system, in water-vapor sprayed air atmosphere: (a) examples of micro-particle clusters observed by SEM on the silicon emitter; (b) a micro-particle cluster observed by SEM on the silicon emitter; and (c) around sphere obtained in the same conditions with sodium bicarbonate added to the water vapor inhalator.
Figure 11.
The particles presented were created by a Si-emitter, C-electrode, and Cu-collector system, in water-vapor sprayed air atmosphere: (a) examples of micro-particle clusters observed by SEM on the silicon emitter; (b) a micro-particle cluster observed by SEM on the silicon emitter; and (c) around sphere obtained in the same conditions with sodium bicarbonate added to the water vapor inhalator.
Figure 12.
Examples of hairy sea-urchin-like clusters observed by SEM on the copper collector, as (a) was created by a Si-emitter, C-electrode, and Cu-collector system, in water-vapor sprayed air atmosphere; and (b) was obtained in the same conditions with sodium bicarbonate added to the water vapor inhalator.
Figure 12.
Examples of hairy sea-urchin-like clusters observed by SEM on the copper collector, as (a) was created by a Si-emitter, C-electrode, and Cu-collector system, in water-vapor sprayed air atmosphere; and (b) was obtained in the same conditions with sodium bicarbonate added to the water vapor inhalator.
Similar structures with the same elemental composition were also observed on the C electrode surface. In addition to Si and O, the EDS and optical spectroscopy show traces of Na, Ca, Fe, K, Zn, P, Al and Ti, as in other silicon breakdowns in air [
37]. The silicon micro-spheres observed in this study resemble those produced by flames [
38], microwave plasmas [
15], short-pulse lasers [
39], and thermal evaporation techniques [
40]. Future SEM-EDS study would include cross-section samples for depth chemical composition profile.
3.5. Analysis
The microwave reflections measured at the input port of the cavity enable us to estimate the effective impedance of the plasmoid, and, consequently, to find its relative dielectric permittivity,
εr, the latter consists of the complex plasma permittivity
and the dust conductivity, σ
ed, thus the total effective permittivity is given by
where
ε0 is the vacuum permittivity; and
ω is the angular frequency. The dielectric permittivity of the plasma is given by its real and imaginary components,
and
, respectively, where
and
υ are the plasma and collision frequencies, respectively. The dusty plasma conductivity is given in similar conditions by [
41] as
where
is the charging factor;
and
are the electron and dust grain densities, respectively;
is the average dust particle radius;
and
are the electron charge and mass, respectively; and
k is the wave wavenumber. The electron effective collision frequency is given by
, where
is the electron thermal velocity; σ
n and
Nn are the neutrals cross-section and density, respectively;
Te is the electron temperature; and
υch is the dust charging frequency. Assuming
and
, and substituting Equation (4) into Equation (3), the latter is further reduced to
where
k is approximated by the axial wavenumber in the waveguide;
ld is defined here as the mean free path for electron-dust collisions,
and
g is a spatial profile function for the plasmoid in the waveguide.
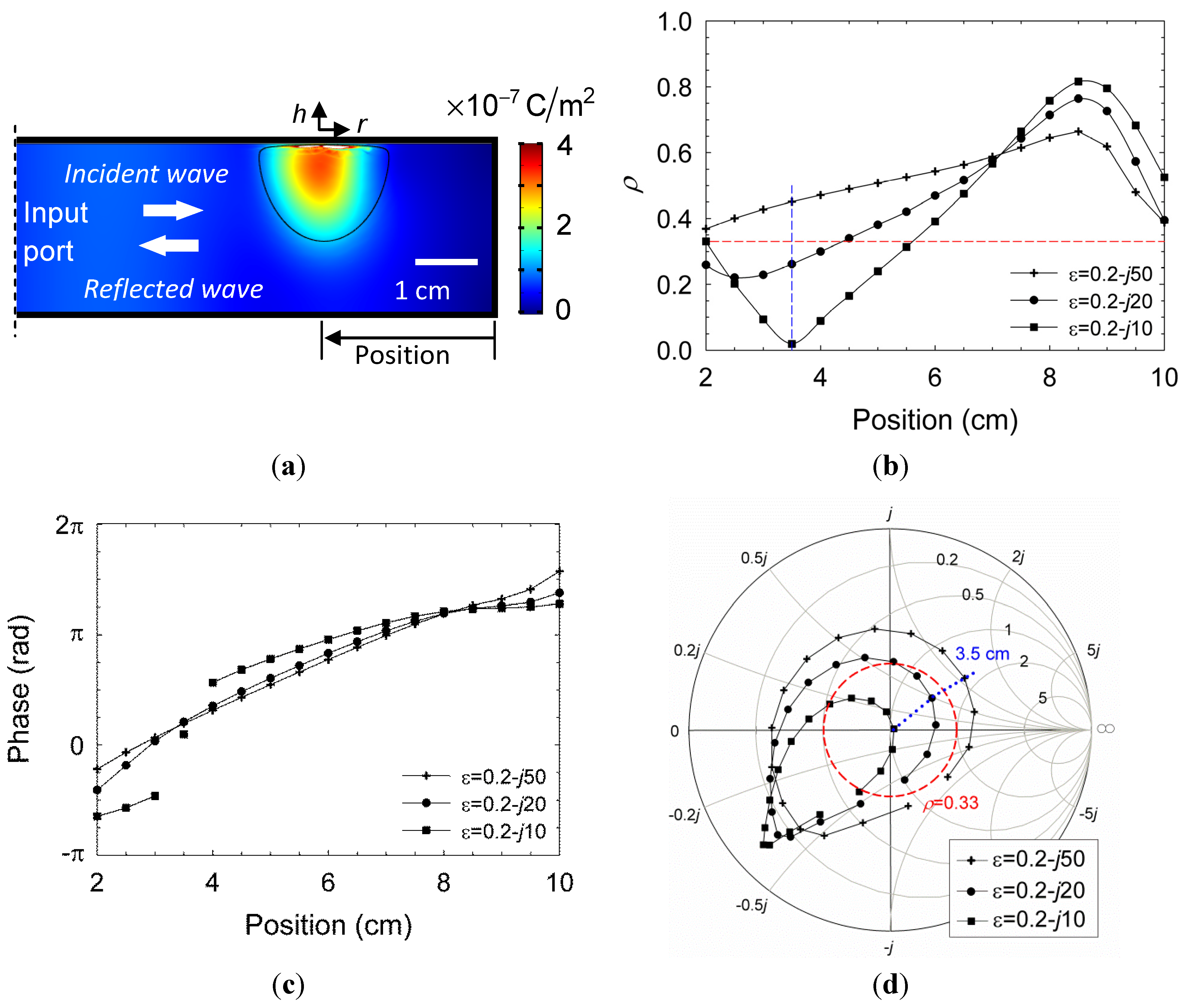
The model illustrated in
Figure 13a is employed using COMSOL MultiPhysics
TM in order to estimate the corresponding permittivity of the plasmoid, ε
r, given the measurements of the reflection coefficient,
, as in
Figure 3b. A Gaussian profile is taken for the plasmoid, similarly to
Figure 2b, with a ~2 cm core diameter (
i.e.,
where
r and
h are the plasmoid’s cylindrical coordinates, illustrated in
Figure 13a, and
wr = 1 cm and
wh = 1.6 cm are the corresponding radii of the plasmoid’s core in the radial and axial dimensions). The model also assumes a ~1 mm sheath barrier isolating the plasmoid from the waveguide ceiling. The complex ε
r space is scanned numerically by the simulation in order to find the conditions that satisfy
as observed in the experiments.
The amplitude profile of the displacement field
D =
ε0εrE along the waveguide is presented for instance in
Figure 13a for
εr = 0.2 −
j20 (in which the reflections attain
). The corresponding reflection coefficients, amplitude and phase, are shown in
Figure 13b,c, respectively, as functions of the axial position (
i.e., the distance from the end mirror as indicated in
Figure 13a) for several values of
εr A nearly perfect impedance matching (no reflection) is observed for
εr = 0.2 –
j10 at an axial position of 35 mm. Other positions or higher dielectric losses of the plasmoid decrease its microwave absorption. This effect is illustrated also by the Smith-chart presentation in
Figure 13d, which maps the complex relation between
εr and the microwave reflection coefficient
in various positions. The area within the dashed circle in Red marks the
region, with less than 10% microwave power reflection. A longitudinal periodicity of about half a wavelength is notable in
Figure 13b,c,d.
The Boltzmann-plot analysis applied under the near-LTE assumption [
33] to the spectra measured in
Section 3.2 results in electron temperatures in the range of 0.3–1.0 eV. The corresponding electron thermal velocity is
VTe ~ 2.3 × 10
5 ms
−1 hence the collision frequency is υ ~ 10
10 s
−1 (assuming σ
n ~ 4.4 × 10
−20 m
2 and
Nn ~ 10
24 m
−3 for a weakly ionized dusty plasma in air atmosphere [
41]). The dust size and number densities given by the SAXS measurements enable us to deduce the dust conductivity component in
εr (3), and hence to estimate the electron density by the remaining
εp components.
The SAXS measurements in
Section 3.3 yield dust density and size distributions with mean values in the order
nd ~ 10
15 m−
3 and
rd ~ 60 nm, respectively, for dry air hence the mean free path for electron-dust collisions (6) is
ld ~ 10
−2 m. In these conditions, for ω ≈ 1.6 × 10
10 s
−1, the real part in Equation (5),
, is significantly smaller than the imaginary part, which plays the dominant role in the microwave absorption by the plasmoid. Hence, the plasma frequency results in
s
−1 for
εr = 0.2 −
j20, and the corresponding electron density is estimated as
ne ~ 3 × 10
18 m
−3 in this case.
The resulting electron density is slightly lower than the limit derived by Griem’s criterion [
33] for LTE. However, it may satisfy the partial-LTE (pLTE) condition [
42]. In atmospheric-pressure plasmas, the LTE assumption may be valid in the core of the plasmoid, but not on its periphery [
43]. The equilibrium may exist since the higher pressure in the core increases the collision rate between the electrons and heavy particles, hence the energy transfer becomes more effective for a given electron density. The contributions of the electron and dust components to the total value of
εr in Equation (5) are comparable; hence the dust particles play a non-negligible role in the microwave absorption by the plasmoid.
The fireball attraction to the Smith-chart origin is observed experimentally as the self-impedance-matching effect presented in
Section 3.1 (
Figure 3b). This is an oscillatory effect since once the fireball is perfectly matched, more power is absorbed, which increases the electron density (e.g., along the dotted blue curve in
Figure 13d. The consequent increase in the plasmoid conductivity violates the perfect matching condition; hence the system is bouncing back and forth around its equilibrium state, as was also observed experimentally.
Figure 13.
A parametric estimation of the dusty plasmoid properties by the microwave reflection measurements: (
a) The plasmoid shape and position used in the numerical model presented by the amplitude profile of the displacement field for
εr = 0.2 −
j20; (
b,c) The amplitude and phase, respectively, of the reflection coefficient as a function of the plasmoid position, for various values of
εr; and (
d) A Smith-chart presentation for the plasmoid’s impedance. The dashed Red circle indicates the self-impedance matching region (
) as observed experimentally in
Figure 3b. The dotted Blue curve marks the 35 mm position at which a perfect match is obtained for
εr = 0.2 –
j10.
Figure 13.
A parametric estimation of the dusty plasmoid properties by the microwave reflection measurements: (
a) The plasmoid shape and position used in the numerical model presented by the amplitude profile of the displacement field for
εr = 0.2 −
j20; (
b,c) The amplitude and phase, respectively, of the reflection coefficient as a function of the plasmoid position, for various values of
εr; and (
d) A Smith-chart presentation for the plasmoid’s impedance. The dashed Red circle indicates the self-impedance matching region (
) as observed experimentally in
Figure 3b. The dotted Blue curve marks the 35 mm position at which a perfect match is obtained for
εr = 0.2 –
j10.