A Comparative Study of the Adsorption of Methylene Blue onto Synthesized Nanoscale Zero-Valent Iron-Bamboo and Manganese-Bamboo Composites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Scanning Electron Microscopy (SEM)

2.2. Fourier Transform Infrared Spectroscopy (FTIR)
| Bamboo (cm−1) | nMn (cm−1) | nMn-Bamboo (cm−1) | nZVI (cm−1) | nZVI-Bamboo (cm−1) |
|---|---|---|---|---|
| 3417.98 | 3404.47 | 3417.98 | 3421.83 | 3441.12 |
| 2939.61 | – | 2895.25 | – | 2899.11 |
| 1728.28 | – | 1726.35 | – | 1728.28 |
| 1604.83 | 1629.9 | 1604.83 | 1637.62 | 1639.55 |
2.3. Particle Induced X-ray Emission (PIXE)
| Symbol | Concentration (mg/L) | ||||
|---|---|---|---|---|---|
| Bamboo | nMn | nMn-Bamboo | nZVI | nZVI-Bamboo | |
| Al | 4696.8 | 23,563.1 | 7369.7 | 10,785.7 | 12,088.1 |
| Si | 3047.1 | 8755.9 | 3485.9 | 12,516.6 | 2161.0 |
| Cl | 289.1 | 64,805.2 | 46,723.5 | 20,680.3 | 16,791.6 |
| K | 72.3 | nd | 62.6 | 76.0 | 79.8 |
| Ca | 110.6 | 261.1 | 345.4 | 335.0 | 598.8 |
| Ti | 9.5 | 60.8 | 35.4 | 123.9 | 89.8 |
| Cr | 13.0 | nd | nd | 68.8 | 83.5 |
| Mn | 37.5 | 336,339.4 | 109,879.9 | 1584.7 | 747.7 |
| Fe | 363.8 | 3889.4 | 4232.2 | 331,691.0 | 179,229.8 |
| Ni | nd | 320.1 | 48.0 | nd | nd |
| Zn | 7.3 | 155.4 | 93.4 | nd | 2.4 |
2.4. Effect of Initial Concentration

2.5. Effect of Adsorbent Dosage
2.6. Effect of Initial pH


2.7. Effect of Time
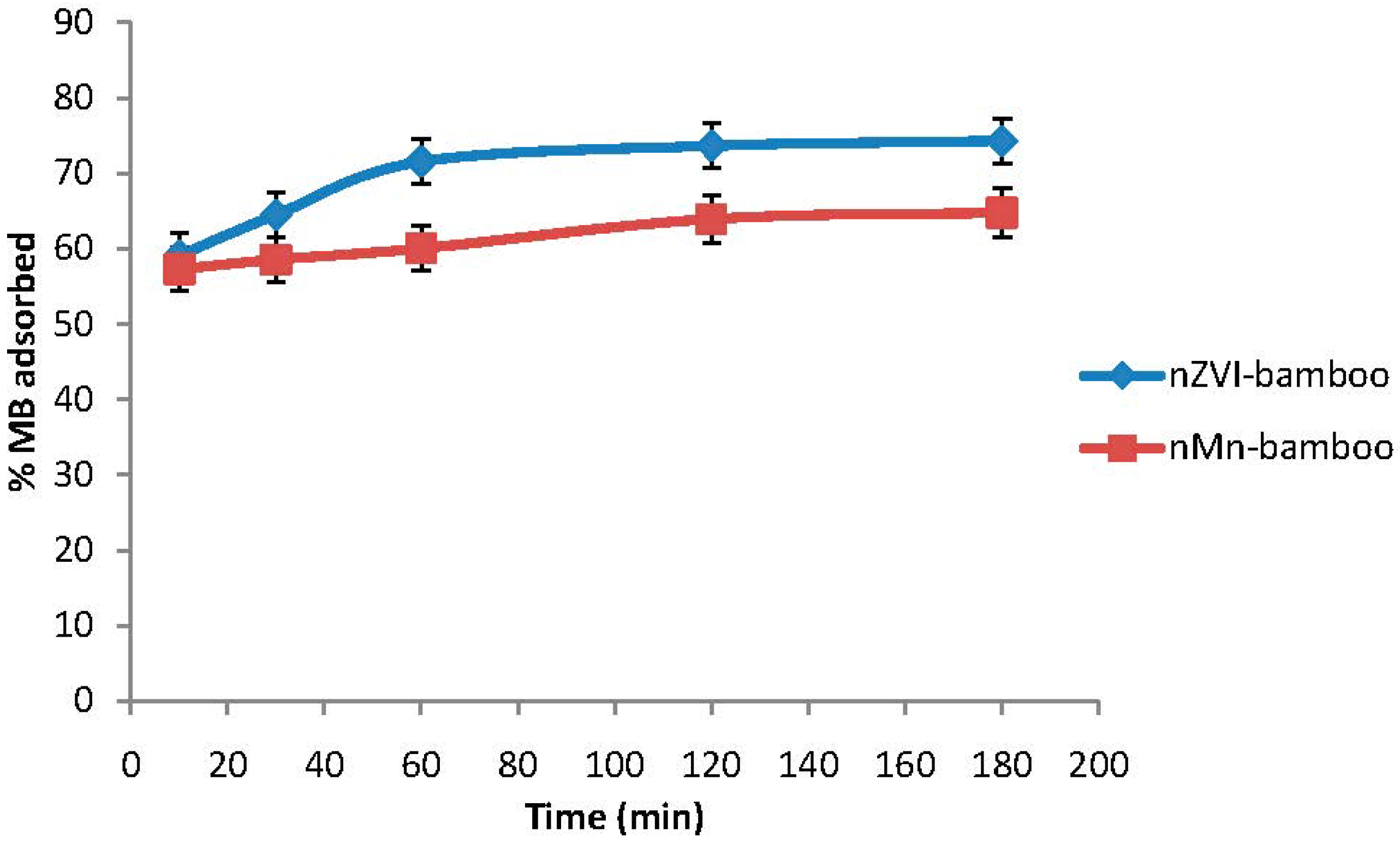
2.8. Adsorption Isotherm




| Isotherm | Parameters | Values | |
|---|---|---|---|
| (nMn-bamboo) | (nZVI-bamboo) | ||
| Freundlich | KF (mg/g(L/mg)1/n) | 9.66 | 29.4 |
| n | 1.33 | 1.78 | |
| R2 | 0.8963 | 0.9604 | |
| Langmuir | Qo (mg/g) | 263.2 | 322.6 |
| b (L/mg) | 0.0417 | 0.0583 | |
| RL | 0.13 | 0.097 | |
| R2 | 0.9281 | 0.8705 | |
| Temkin | AT (L/g) | 1.69 | 1.20 |
| bT (J/mol) | 43.9 | 40.7 | |
| R2 | 0.9041 | 0.844 | |
2.9. Kinetic Models




| Kinetic Models | Parameters | Values | |
|---|---|---|---|
| (nMn-bamboo) | (nZVI-bamboo) | ||
| Pseudo-first order | k1 (min−1) | 0.0205 | 0.0290 |
| qe,cal (mg/g) | 39.6 | 70.7 | |
| R2 | 0.9350 | 0.9850 | |
| Pseudo-second order | k2 (g/mg/min) | 0.0013 | 0.0009 |
| qe,cal (mg/g) | 232.56 | 263.16 | |
| R2 | 0.9995 | 0.9998 | |
| Elovich | β (g·min/mg) | 0.0485 | 0.1006 |
| αE (g·min2/mg) | 4.69 × 103 | 4.09 × 108 | |
| R2 | 0.8964 | 0.9694 | |
3. Experimental Section
3.1. Reagents
3.2. nZVI-Bamboo Composite Preparation
3.3. nMn-Bamboo Composite Preparation

3.4. Characterization of nZVI-Bamboo and nMn-Bamboo Composites
3.5. Adsorption Studies


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Xu, F.L.; Jorgensen, S.E.; Shimizu, Y.; Silow, E. Persistent organic pollutants in fresh water ecosystems. Sci. World J. 2013, 2013, 1–2. [Google Scholar]
- Hameed, B.H.; Ahmad, A.A.; Aziz, N. Isotherms, kinetics and thermodynamics of acid dye adsorption on activated palm ash. Chem. Eng. J. 2007, 133, 195–203. [Google Scholar] [CrossRef]
- Zawani, Z.; Chuah Luqman, A.; Thomas, S.Y.C. Equilibrium, kinetics and thermodynamic Studies: Adsorption of remazol black 5 on the palm kernel shell activated carbon (PKS-AC). Eur. J. Sci. Res. 2009, 37, 67–76. [Google Scholar]
- Nigam, P.; Banat, I.M.; Singh, D.; Marchant, R. Microbial process for decolorization of textile effluent containing azo, diazo and reactive dyes. Process Biochem. 1996, 31, 435–442. [Google Scholar] [CrossRef]
- Garg, V.K.; Gupta, R.; Yadav, B.; Kumar, R. Dye removal from aqueous solution by adsorption on treated sawdust. Bioresour. Technol. 2003, 89, 121–124. [Google Scholar] [CrossRef]
- Fatoki, O.S.; Ayanda, O.S.; Adekola, F.A.; Ximba, B.J. Sorption of triphenyltin chloride to nFe3O4, fly ash and nFe3O4/fly ash composite material in seawater. Clean Soil Air Water 2013, 42, 472–479. [Google Scholar]
- Ayanda, O.S.; Fatoki, O.S.; Adekola, F.A.; Ximba, B.J. Remediation of tributyltin contaminated seawater by adsorption using nFe3O4, activated carbon and nFe3O4/activated carbon composite material. Water Air Soil Pollut. 2013, 224. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Z.; Han, L.; Qin, C. Synthesis of nanoscale zero-valent iron supported on exfoliated graphite for removal of nitrate. Trans. Nonferr. Met. Soc. China 2006, 16, 345–349. [Google Scholar] [CrossRef]
- Sheela, T.; Arthoba Nayaka, Y.; Viswanatha, R.; Basavanna, S.; Venkatesha, T.G. Kinetics and thermodynamics studies on the adsorption of Zn(II), Cd(II) and Hg(II) from aqueous solution using zinc oxide nanoparticles. Powder Technol. 2012, 217, 163–170. [Google Scholar] [CrossRef]
- Rahmani, A.; Zavvar Mousavi, H.; Fazli, M. Effect of nanostructure alumina on adsorption of heavy metals. Desalination 2010, 253, 94–100. [Google Scholar] [CrossRef]
- Frost, R.L.; Xi, Y.; He, H. Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption. J. Colloid Interface Sci. 2010, 341, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Shahryari, Z.; Goharrizi, A.S.; Azadi, M. Experimental study of methylene blue adsorption fromaqueous solutions onto carbon nano tubes. Int. J. Water Res. Environ. Eng. 2010, 2, 016–028. [Google Scholar]
- Celebi, O.; Uzum, C.; Shahwan, T.; Erten, H.N. A radiotracer study of the adsorption behavior of aqueous Ba(2+) ions on nanoparticles of zero-valent iron. J. Hazard. Mater. 2007, 148, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Fan, M.; Brown, R.C.; van Leeuwen, L. Synthesis, properties, and environmental applications of nanoscale iron-based materials: A review. Crit. Rev. Environ. Sci. Technol. 2006, 36, 405–431. [Google Scholar] [CrossRef]
- Shirmardi, M.; Mesdaghinia, A.R.; Mahvi, H.; Nasseri, S.; Nabizadeh, R. Kinetics and equilibrium studies on adsorption of acid red 18 (azo dye) using a multiwall carbon nanotube (MWCNTs) from aqueous solution. E J. Chem. 2012, 9, 476–484. [Google Scholar]
- Cengiz, S.; Cavas, L. Removal of methylene blue by invasive marine seaweed: Caulerparacemosa var. cylindracea. Bioresour. Technol. 2008, 99, 2357–2363. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mostafapour, F.K.; Hosseini, A.R.; Khorshid, A.R.; Mahvi, A.H. Decolourisation of reactive red 120 dye using single walled carbon nanotube in aqueous solution. J. Chem. 2013, 2013, 2371–2383. [Google Scholar]
- Hu, Q.H.; Qiao, S.Z.; Haghseresht, F. Adsorption study for the removal of basic red dye using bentonite. Ind. Eng. Chem. Res. 2006, 45, 733–738. [Google Scholar] [CrossRef]
- Salman, D.D.; Ulaiwi, W.S.; Tariq, N.M. Determination the optimal conditions of methylene blue adsorption by the chicken egg shell membrane. Int. J. Poult. Sci. 2012, 11, 391–396. [Google Scholar] [CrossRef]
- Karima, B.; Mossab, B.L.; A-Hassen, M. Removal of methylene blue from aqueous solutions using an acid activated Algerian bentonite: Equilibrium and kinetic studies. In Proceedings of the International Renewable Energy Congress, Sousse, Tunisia, 5–7 November 2010.
- Jain, M.; Garg, V.K.; Krishna, K. Adsorption of hexavalent chromium from aqueous medium onto carbonaceous adsorbents prepared from waste biomass. J. Hazard. Mater. 2010, 91, 949–954. [Google Scholar]
- Han, R.P.; Zhang, J.J.; Han, P.; Wang, Y.F.; Zhao, Z.H.; Tang, M.S. Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chem. Eng. J. 2009, 145, 496–504. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, Y.; Mai, J.; Sun, W.; Zhang, C.; Wei, D.; Chen, Q.; Ni, J. Adsorption behavior of methylene blue onto titanate nanotubes. Chem. Eng. J. 2010, 156, 313–320. [Google Scholar]
- Sainz-Diaz, C.I.; Griffiths, A.J. Activated carbon from solid wastes using a pilot scale batch flaming pyrolyser. Fuel 2000, 79, 1863–1871. [Google Scholar] [CrossRef]
- Banat, F.; Al-Asheh, S.; Al-Makhadmeh, L. Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Process Biochem. 2003, 39, 193–202. [Google Scholar] [CrossRef]
- Phenrat, T.; Saleh, N.; Sirk, K.; Tilton, R.D.; Lowry, G.V. Aggregation and sedimentation of aqueous nanoscale zero valent iron dispersions. Environ. Sci. Technol. 2007, 41, 284–290. [Google Scholar] [CrossRef]
- Zheng, T.; Zhan, J.; He, J.; Day, C.; Lu, Y.; McPerson, G.L.; Piringer, G.; John, V.T. Reactivity characteristics of nanoscale zerovalent iron–silica composites for trichloroethylene remediation. Environ. Sci. Technol. 2008, 42, 4494–4499. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N. Preparation and characterization of zerovalent iron nanoparticles. Dig. J. Nanomater. Biostruct. 2011, 6, 1771–1776. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shaibu, S.E.; Adekola, F.A.; Adegoke, H.I.; Ayanda, O.S. A Comparative Study of the Adsorption of Methylene Blue onto Synthesized Nanoscale Zero-Valent Iron-Bamboo and Manganese-Bamboo Composites. Materials 2014, 7, 4493-4507. https://doi.org/10.3390/ma7064493
Shaibu SE, Adekola FA, Adegoke HI, Ayanda OS. A Comparative Study of the Adsorption of Methylene Blue onto Synthesized Nanoscale Zero-Valent Iron-Bamboo and Manganese-Bamboo Composites. Materials. 2014; 7(6):4493-4507. https://doi.org/10.3390/ma7064493
Chicago/Turabian StyleShaibu, Solomon E., Folahan A. Adekola, Halimat I. Adegoke, and Olushola S. Ayanda. 2014. "A Comparative Study of the Adsorption of Methylene Blue onto Synthesized Nanoscale Zero-Valent Iron-Bamboo and Manganese-Bamboo Composites" Materials 7, no. 6: 4493-4507. https://doi.org/10.3390/ma7064493
APA StyleShaibu, S. E., Adekola, F. A., Adegoke, H. I., & Ayanda, O. S. (2014). A Comparative Study of the Adsorption of Methylene Blue onto Synthesized Nanoscale Zero-Valent Iron-Bamboo and Manganese-Bamboo Composites. Materials, 7(6), 4493-4507. https://doi.org/10.3390/ma7064493





