Removal of Chromium(VI) from Aqueous Solutions Using Fe3O4 Magnetic Polymer Microspheres Functionalized with Amino Groups
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphologies of the Particles

2.2. Magnetism Analysis

2.3. X-ray Diffraction

2.4. Fourier Transform Infrared (FT-IR) Analysis

2.5. Adsorption
2.5.1. Effect of pH on Cr(VI) Adsorption and Sorption Mechanism

2.5.2. Effect of Contact on MPMs and EDA-MPMs

2.5.3. Adsorption Kinetics of the EDA-MPMs

| C0 (mg/g) | qe, exp a (mg/g) | Pseudo-First Order Model | Pseudo-Second Order Model | ||||
|---|---|---|---|---|---|---|---|
| qe,cal (mg/g) | k1 | R2 | qe,cal b (mg/g) | k2 (g·mg−1·min−1) × 10−4 | R2 | ||
| 100 | 50.5 | 14.206 | 0.0319 | 0.803 | 53.333 | 25.539 | 0.998 |
| 200 | 73.9 | 56.179 | 0.0321 | 0.968 | 80.451 | 8.718 | 0.999 |
| 300 | 145.2 | 141.206 | 0.0303 | 0.985 | 169.205 | 2.295 | 0.997 |
2.5.4. Adsorption Isotherm
| Temperature (K) | Langmuir Equation | Freundich Equation | ||||
|---|---|---|---|---|---|---|
| qmax (mg/g) | b (L/mg) | R2 | KF | n | R2 | |
| 298 K | 236.9 | 0.0752 | 0.999 | 110.132 | 7.512 | 0.901 |
| 308 K | 242.1 | 0.127 | 0.999 | 84.612 | 5.011 | 0.944 |
| 318 K | 253.2 | 0.183 | 0.999 | 105.314 | 6.288 | 0.922 |

| Adsorbent | pH | Adsorption Capacity (mg/g) | References |
|---|---|---|---|
| MSCGE | 2.0 | 171.5 | [32] |
| Magnetic poly(MA-DVB) microspheres | 3 | 231.8 | [44] |
| Aminofunctionalized titanate nanotubes | 5.4 | 153.85 | [45] |
| Polyethylenimine-modified magnetic nanoparticle | 2–3 | 83.33 | [46] |
| EDA-MPMs | 2 | 253 | This Study |
2.5.5. Adsorption Thermodynamics

| Temperature (K) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol/K) | R2 |
|---|---|---|---|---|
| 298 | −20.492 | 35.085 | 186.69 | 0.996 |
| 308 | −22.522 | |||
| 318 | −24.219 |
2.6. Desorption and Reusability Study

3. Experimental Section
3.1. Materials
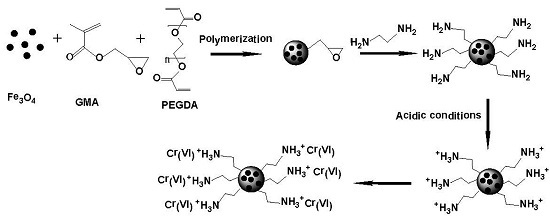
3.2. Synthesis of the Adsorbent

3.2.1. Preparation of Fe3O4 Nanoparticles Modified by SDS and PEG
3.2.2. Synthesis of Magnetic Polymer Microspheres
3.2.3. Ring-Opening Reaction of the Ester Groups
3.3. Characterization
3.4. Batch Adsorption Experiments
3.5. Desorption Experiments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, W.; Gong, X.; Li, X.; Zhang, D.; Gong, H. Removal of Cr(VI) from low-temperature micro-polluted surface water by tannic acid immobilized powdered activated carbon. Bioresour. Technol. 2012, 113, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Al-Othman, Z.A.; Ali, R.; Naushad, M. Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: Adsorption kinetics, equilibrium and thermodynamic studies. Chem. Eng. J. 2012, 184, 238–247. [Google Scholar] [CrossRef]
- Karimi, M.; Shojaei, A.; Nematollahzadeh, A.; Abdekhodaie, M.J. Column study of Cr(VI) adsorption onto modified silica–polyacrylamide microspheres composite. Chem. Eng. J. 2012, 210, 280–288. [Google Scholar] [CrossRef]
- Samani, M.R.; Borghei, S.M.; Olad, A.; Chaichi, M.J. Removal of chromium from aqueous solution using polyaniline-poly ethylene glycol composite. J. Hazard. Mater. 2010, 184, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Nima, J.; Divya, P.L. Adsorption of chromium(VI) from aqueous solutions by glycidylmethacrylate-grafted-densified cellulose with quaternary ammonium groups. Appl. Surf. Sci. 2013, 279, 441–449. [Google Scholar] [CrossRef]
- Deveci, H.; Kar, Y. Adsorption of hexavalent chromium from aqueous solutions by bio-chars obtained during biomass pyrolysis. J. Ind. Eng. Chem. 2013, 19, 190–196. [Google Scholar] [CrossRef]
- Ramos-Ramírez, E.; Ortega, N.L.G.; Soto, C.A.C.; Gutiérrez, M.T.O. Adsorption isotherm studies of chromium(VI) from aqueous solutions using sol-gel hydrotalcite-like compounds. J. Hazard. Mater. 2009, 172, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmi, A.; Rajesh, S.; Senthilkumar, S.; Mohan, D. Epoxy functionalized poly(ether-sulfone) incorporated cellulose acetate ultrafiltration membrane for the removal of chromium ions. Sep. Purif. Technol. 2012, 90, 120–132. [Google Scholar] [CrossRef]
- Gherasim, C.V.; Bourceanu, G.; Olariu, R.I.; Arsene, C. A novel polymer inclusion membrane applied in chromium(VI) separation from aqueous solutions. J. Hazard. Mater. 2011, 197, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Golbaz, S.; Jafari, A.J.; Rafiee, M.; Kalantary, R.R. Separate and simultaneous removal of phenol, chromium, and cyanide from aqueous solution by coagulation/precipitation: Mechanisms and theory. Chem. Eng. J. 2014, 253, 251–257. [Google Scholar] [CrossRef]
- Golder, A.K.; Samanta, A.N.; Ray, S. Removal of trivalent chromium by electrocoagulation. Sep. Purif. Technol. 2007, 53, 33–41. [Google Scholar] [CrossRef]
- Alvarado, L.; Torres, I.R.; Chen, A. Integration of ion exchange and electrodeionization as a new approach for the continuous treatment of hexavalent chromium wastewater. Sep. Purif. Technol. 2013, 105, 55–62. [Google Scholar] [CrossRef]
- Kamakura, N.; Inui, T.; Kitano, M.; Nakamura, T. Determination of chromium(III), chromium(VI), and chromium(III) acetylacetonate in water by ion-exchange disk extraction/metal furnace atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2014, 93, 28–33. [Google Scholar] [CrossRef]
- Venkateswaran, P.; Palanivelu, K. Solvent extraction of hexavalent chromium with tetrabutyl ammonium bromide from aqueous solution. Sep. Purif. Technol. 2004, 40, 279–284. [Google Scholar] [CrossRef]
- Yavuz, A.G.; Dincturk-Atalay, E.; Uygun, A.; Gode, F.; Aslan, E. A comparison study of adsorption of Cr(VI) from aqueous solutions onto alkyl-substituted polyaniline/chitosan composites. Desalination 2011, 279, 325–331. [Google Scholar] [CrossRef]
- Zhong, Q.Q.; Yue, Q.Y.; Li, Q.; Gao, B.Y.; Xu, X. Removal of Cu(II) and Cr(VI) from wastewater by an amphoteric sorbent based on cellulose-rich biomass. Carbohyd. Polym. 2014, 111, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Gueye, M.; Richardson, Y.; Kafack, F.T.; Blin, J. High efficiency activated carbons from African biomass residues for the removal of chromium(VI) from wastewater. J. Environ. Chem. Eng. 2014, 2, 273–281. [Google Scholar] [CrossRef]
- Murugesan, A.; Vidhyadevi, T.; Kirupha, S.D.; Ravikumar, L.; Sivanesan, S. Removal of chromium(VI) from aqueous solution using chemically modified corncorb-activated carbon: Equilibrium and kinetic studies. Environ. Prog. Sustain. Energy 2013, 32, 673–680. [Google Scholar] [CrossRef]
- Kumar, K.Y.; Muralidhara, H.B.; Nayaka, Y.A.; Balasubramanyam, J.; Hanumanthappa, H. Low-cost synthesis of metal oxide nanoparticles and their application in adsorption of commercial dye and heavy metal ion in aqueous solution. Powder Technol. 2013, 246, 125–136. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Yang, Z.; Mangwandi, C.; Glocheux, Y.; Walker, G.; Ahmad, M.N.M. Experimental design and batch experiments for optimization of Cr(VI) removal from aqueous solutions by hydrous cerium oxide nanoparticles. Chem. Eng. Res. Des. 2014, 92, 1354–1362. [Google Scholar] [CrossRef]
- Sun, X.; Yang, L.; Xing, H.; Zhao, J.; Li, X.; Huang, Y.; Liu, H. High capacity adsorption of Cr(VI) from aqueous solution using polyethylenimine-functionalized poly(glycidyl methacrylate) microspheres. Colloids Surf. A Physicochem. Eng. Aspects 2014, 457, 160–168. [Google Scholar] [CrossRef]
- Kaya, K.; Pehlivan, E.; Schmidt, C.; Bahadir, M. Use of modified wheat bran for the removal of chromium(VI) from aqueous solutions. Food Chem. 2014, 158, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Jiang, J.; Xu, R. Adsorption of Cr(III) from acidic solutions by crop straw derived biochars. J. Environ. Sci. 2013, 25, 1957–1965. [Google Scholar] [CrossRef]
- Bansal, M.; Singh, D.; Garg, V.K. A comparative study for the removal of hexavalent chromium from aqueous solution by agriculture wastes’ carbons. J. Hazard. Mater. 2009, 171, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Brum, M.C.; Capitaneo, J.L.; Oliveira, J.F. Removal of hexavalent chromium from water by adsorption onto surfactant modified montmorillonite. Miner. Eng. 2010, 23, 270–272. [Google Scholar] [CrossRef]
- Guan, Q.; Wu, D.; Lin, Y.; Chen, X.; Wang, X.; Li, C.; He, S.; Kong, H. Application of zeolitic material synthesized from thermally treated sediment to the removal of trivalent chromium from wastewater. J. Hazard. Mater. 2009, 167, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Ballav, N.; Maity, A.; Mishra, S.B. High efficient removal of chromium(VI) using glycine doped polypyrrole adsorbent from aqueous solution. Chem. Eng. J. 2012, 198–199, 536–546. [Google Scholar] [CrossRef]
- Cui, L.; Meng, Q.; Zheng, J.; Wei, X.; Ye, Z. Adsorption of Cr(VI) on 1,2-ethylenediamine-aminated macroporous polystyrene particles. Vacuum 2013, 89, 1–6. [Google Scholar] [CrossRef]
- Wang, Q.; Guan, Y.; Liu, X.; Yang, M.; Ren, X. Micron-sized magnetic polymer microspheres for adsorption and separation of Cr(VI) from aqueous solution. Chin. J. Chem. Eng. 2012, 20, 105–110. [Google Scholar] [CrossRef]
- Uğuzdoğan, E.; Denkbaş, E.B.; Kabasakal, O.S. The use of polyethyleneglycolmethacrylate-co-vinylimidazole (PEGMA-co-VI) microspheres for the removal of nickel(II) and chromium(VI) ions. J. Hazard. Mater. 2010, 177, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.W.; Kwak, N.S.; Hwang, T.S. Preparation of poly(GMA-co-PEGDA) microbeads modified with iminodiacetic acid and their indium adsorption properties. Chem. Eng. J. 2013, 226, 79–86. [Google Scholar] [CrossRef]
- Sun, X.; Yang, L.; Li, Q.; Zhao, J.; Li, X.; Wang, X.; Liu, H. Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr(VI): Synthesis and adsorption studies. Chem. Eng. J. 2014, 241, 175–183. [Google Scholar] [CrossRef]
- Yan, H.; Yang, L.; Yang, Z.; Yang, H.; Li, A.; Cheng, R. Preparation of chitosan/poly(acrylic acid) magnetic composite microspheres and applications in the removal of copper(II) ions from aqueous solutions. J. Hazard. Mater. 2012, 229–230, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, L.; Xiao, A.; Gao, J.; Ding, W.; Yu, H.; Huo, J.; Ericson, M. Templated preparation of porous magnetic microspheres and their application in removal of cationic dyes from wastewater. J. Hazard. Mater. 2010, 181, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, W.; Wu, Y.; Zhu, Q.; Lu, Z.; Du, G. Preparation and characterization of chitosan/montmorillonite magnetic microspheres and its application for the removal of Cr(VI). Chem. Eng. J. 2013, 221, 8–15. [Google Scholar] [CrossRef]
- Van Nguyen, N.; Lee, J.C.; Jeong, J.; Pandey, B.D. Enhancing the adsorption of chromium(VI) from the acidic chloride media using solvent impregnated resin (SIR). Chem. Eng. J. 2013, 219, 174–182. [Google Scholar] [CrossRef]
- Sun, X.; Yang, L.; Xing, H.; Zhao, J.; Li, X.; Huang, Y.; Liu, H. Synthesis of polyethylenimine-functionalized poly(glycidyl methacrylate) magnetic microspheres and their excellent Cr(VI) ion removal properties. Chem. Eng. J. 2013, 234, 338–345. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Lv, Z.; Lu, F.; Qiu, H. Removal of Ag+ from water environment using a novel magnetic thiourea-chitosan imprinted Ag+. J. Hazard. Mater. 2011, 194, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.L.; Zhao, X.D.; Li, Y.H.; Du, Q.J.; Sun, J.K.; Wang, Y.H.; Wang, X.; Xia, Y.Z.; Wang, Z.H.; Xia, L.H. Adsorption properties of doxorubicin hydrochloride onto graphene oxide: Equilibrium, kinetic and thermodynamic studies. Materials 2013, 6, 2026–2042. [Google Scholar] [CrossRef]
- Hena, S. Removal of chromium hexavalent ion from aqueous solutions using biopolymer chitosan coated with poly 3-methyl thiophene polymer. J. Hazard. Mater. 2010, 181, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J.; Wang, J.S.; Liu, Y.G.; Li, X.; Zeng, G.M.; Bao, Z.L.; Zeng, X.X.; Chen, A.W.; Long, F. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2011, 185, 306–314. [Google Scholar] [CrossRef] [PubMed]
- García, E.R.; Medina, R.L.; Lozano, M.M.; Pérez, I.H.; Valero, M.J.; Franco, A.M.M. Adsorption of azo-dye orange II from aqueous solutions using a metal-organic framework material: Iron-benzenetricarboxylate. Materials 2014, 7, 8037–8057. [Google Scholar] [CrossRef]
- Liao, D.; Zheng, W.; Li, X.; Yang, Q.; Yue, X.; Guo, L.; Zeng, G. Removal of lead(II) from aqueous solutions using carbonate hydroxyapatite extracted from eggshell waste. J. Hazard. Mater. 2010, 177, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guan, Y.; Liu, X.; Ren, X.; Yang, M. High-capacity adsorption of hexavalent chromium from aqueous solution using magnetic microspheres by surface dendrimer graft modification. J. Colloid Interface Sci. 2012, 375, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, W.; Wang, T.; Ni, J. Highly efficient adsorption of Cr(VI) from aqueous solutions by amino-functionalized titanate nanotubes. Chem. Eng. J. 2013, 225, 153–163. [Google Scholar] [CrossRef]
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Liu, Z.; Xiong, Y. Preparation and application of stability enhanced magnetic nanoparticles for rapid removal of Cr(VI). Chem. Eng. J. 2011, 175, 222–227. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Qiu, G.; Cao, H.; Jin, R. Removal of Chromium(VI) from Aqueous Solutions Using Fe3O4 Magnetic Polymer Microspheres Functionalized with Amino Groups. Materials 2015, 8, 8378-8391. https://doi.org/10.3390/ma8125461
Wang K, Qiu G, Cao H, Jin R. Removal of Chromium(VI) from Aqueous Solutions Using Fe3O4 Magnetic Polymer Microspheres Functionalized with Amino Groups. Materials. 2015; 8(12):8378-8391. https://doi.org/10.3390/ma8125461
Chicago/Turabian StyleWang, Kai, Guangming Qiu, Hongyu Cao, and Ruifa Jin. 2015. "Removal of Chromium(VI) from Aqueous Solutions Using Fe3O4 Magnetic Polymer Microspheres Functionalized with Amino Groups" Materials 8, no. 12: 8378-8391. https://doi.org/10.3390/ma8125461
APA StyleWang, K., Qiu, G., Cao, H., & Jin, R. (2015). Removal of Chromium(VI) from Aqueous Solutions Using Fe3O4 Magnetic Polymer Microspheres Functionalized with Amino Groups. Materials, 8(12), 8378-8391. https://doi.org/10.3390/ma8125461