1. Introduction
Important customer issues in pH measurement are a decrease in pH sensitivity and an increase in pH response time, which mainly arise from the fouling due to contamination of the responsive glass membrane and liquid junction, and from the change in concentration of the internal liquid [
1]. To avoid these issues, customers have to maintain their pH glass electrodes. This is troublesome, especially in industrial uses, because it is not easy to remove the accumulated stain from pH glass electrodes. For this reason, we have developed novel pH glass electrodes, such as TiO
2-P
2O
5 (TP) glasses [
2,
3,
4,
5], with a self-cleaning property based on photocatalytic activity and photo-induced hydrophilicity [
6]. TP glasses with low electrical resistivity gave a high pH sensitivity and short pH response time.
On the other hand, we have also reported that Bi
2O
3-B
2O
3 (BiB) glasses show hydrophobicity (contact angle of 90°) so far [
7]. Materials with hydrophobicity have been used for anti-fouling and anti-fogging [
8]. Glasses with hydrophobicity such as BiB glasses may be candidates of pH glass electrodes with an anti-fouling property based on their hydrophobicity, because pH measurement is basically carried out for aqueous solutions. The anti-fouling effect based on hydrophobicity may become remarkable, when pH of products and waste is monitored in fluid system as in industrial uses. Electric resistivity lower than 10
10 Ω·cm, which is a representative value for commercially available pH glass electrode, is desirable in practical use. However, BiB glasses are undesirable for pH glass electrodes because of the relatively high electric resistivity (>10
11 Ω·cm) [
9]. It is well known that addition of transition metal oxides into glass composition causes the electronic conduction to the glasses as in TiO
2-P
2O
5 glasses [
6]. V
2O
5 and Fe
2O
3 are the most familiar additives as an origin of electronic conduction. Accordingly, Fe
2O
3-Bi
2O
3-B
2O
3 (FeBiB) glasses have been developed as novel pH responsive glasses with an anti-fouling property based on their hydrophobicity [
10]. The electrical resistivity and pH response time of FeBiB glasses decreased with increasing Fe
2O
3 content, while their pH repeatability for standard solutions increased with increasing Bi
2O
3 content. FeBiB glasses showed a pH sensitivity close to that of commercial pH responsive glass, and shorter pH response time than that of a commercial glass. The contact angle for water of FeBiB glasses was relatively high (about 90°), similar to BiB glasses, and tended to increase slightly with increasing Bi
2O
3 content, regardless of Fe
2O
3 content. In this sense, we consider that Fe
2O
3 and Bi
2O
3 play an important role in electrical conduction in the bulk glass, and in the pH response and hydrophobicity, respectively. Such novel lithium-free nonsilicate pH responsive glasses are also expected to show a short pH response time, because they are a new type of pH glass electrodes based on “electronic conduction” that is different from the “ionic conduction” present in commercial lithium silicate glasses.
In the present study, the influence of Bi2O3 (or B2O3) content on the pH responsivity, electrical resistivity, and hydrophobicity (contact angle to water) for 20Fe2O3·yBi2O3·(80 − y)B2O3 (20FeyBiB, y = 70–80 mol %) glasses was investigated in order to reveal the role of each glass component.
2. Experimental
20Fe
2O
3·
yBi
2O
3·(80 −
y)B
2O
3 (20Fe
yBiB,
y = 79, 79.5, 79.7, 79.8, 79.9 and 80 mol %) glasses were produced by a conventional melt-quenching method in 30 g batches under the following preparation conditions: melting at 1100 °C for 1 h, annealing at 350 °C for 1 h. Fe
2O
3 (99.9%, Kojundo Chemical Lab. Co., Ltd., Sakado, Japan), Bi
2O
3 (99.9%, Kojundo Chemical Lab. Co., Ltd., Sakado, Japan) and B
2O
3 (90%, guaranteed reagent grade, Nacalai Tesque, Inc., Kyoto, Japan) were used as raw materials. For example, 20Fe
2O
3·70Bi
2O
3·10B
2O
3 glass is abbreviated to 20Fe70BiB as a sample name in
Table 1.
Potentiometric measurements for the 20Fe
yBiB glasses was carried out at 25 °C, at time intervals of 3 s and 0.5 s using a pH meter F-73 (HORIBA, Ltd., Kyoto, Japan) and a portable multi logger ZR-RX20 (OMRON Corp., Kyoto, Japan) equipped with a handmade cell with a glass membrane of 1mm thickness, respectively. The details of the characterization of the pH responsivity (pH sensitivity and pH response time) were described in [
8].
The direct current (DC) electrical resistivity of 20Fe
yBiB glasses with ~1 mm thickness and an Ag electrode of 6 mm φ on both sides was measured at 25 °C using a super megohm meter SM-8215 (HIOKI E.E. Corp., Ueda, Japan). The contact angle for ~2 μL of water on 20Fe
yBiB glasses was measured at 25 °C using a mobile contact angle meter PG-3 (Matsubo Corp., Tokyo, Japan) as a measure of hydrophobicity. The density was measured at 25 °C by Archimedes' method in order to conveniently estimate the distance between Fe ions linked by oxygen ions [
11,
12].
3. Results and Discussion
Figure 1 indicates the change in potential with measurement time for 20Fe
yBiB glasses in pH7, pH4 and pH9 buffer solutions. When the Bi
2O
3 content increases to 79.7 mol %, the B
2O
3 content decreases to 0.3 mol %, the change in potential related to pH sensitivity drastically decreased. The dependence of the pH4-9 sensitivity (left axis) between pH4 and pH9, and the pH4-7/pH7-9 sensitivity ratio (right axis) on the Bi
2O
3 and B
2O
3 contents for the 20Fe
yBiB glasses is shown in
Figure 2. It can be seen from the left axis of
Figure 2 that the pH4-9 sensitivity decreases to almost zero when the Bi
2O
3 content increases to 80 mol %. At this time, the pH4-7/pH7-9 ratio decreases with increasing Bi
2O
3 content. This corresponds to the decrease in H
+ ion-selectivity for acid solutions (right axis of
Figure 2).
Figure 1.
Change in potential with measurement time for 20FeyBiB glasses in pH7, pH4 and pH9 buffer solutions.
Figure 1.
Change in potential with measurement time for 20FeyBiB glasses in pH7, pH4 and pH9 buffer solutions.
Figure 2.
Dependence of pH4-9 sensitivity (left axis) between pH4 and pH9, and pH4-7/pH7-9 sensitivity ratio (right axis) on Bi2O3 and B2O3 contents for 20FeyBiB glasses.
Figure 2.
Dependence of pH4-9 sensitivity (left axis) between pH4 and pH9, and pH4-7/pH7-9 sensitivity ratio (right axis) on Bi2O3 and B2O3 contents for 20FeyBiB glasses.
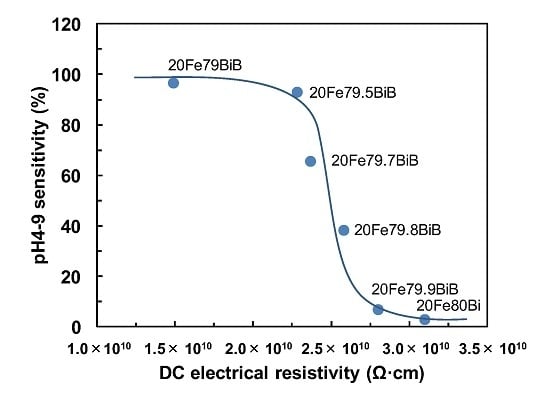
Figure 3 presents the relationship between the pH4-9 sensitivity and DC electrical resistivity for the 20Fe
yBiB glasses. The pH4-9 sensitivity decreased with increases in the DC electrical resistivity. In our previous work [
10], the pH4-9 sensitivity of
xFe
yBiB glasses was not so strongly affected by the glass compositions. However, a shortage of B
2O
3 seems to affect the pH4-9 sensitivity strongly in the present case. This suggests that a small amount of the B
2O
3 component (B-OH sites) may play an important role in the pH4-9 sensitivity. The reason for the decrease in pH4-9 sensitivity is complicated, because it decreases both with decreasing B
2O
3 content and with increasing DC electrical resistivity. Moreover, it should be noted that the decrease in pH4-7/pH7-9 ratio as a measure of H
+ ion-selectivity for acid solutions is observed as the pH4-9 sensitivity decreases. Therefore, the pH4-9 sensitivity may be related to the specific glass compositions, such as the amount of B
2O
3, rather than the DC electrical resistivity.
Figure 3.
Relationship between pH4-9 sensitivity and DC electrical resistivity for 20FeyBiB glasses.
Figure 3.
Relationship between pH4-9 sensitivity and DC electrical resistivity for 20FeyBiB glasses.
Table 1.
DC electrical resistivity, pH4-9 sensitivity, pH response time and contact angle for 20FeyBiB glasses and related glasses.
Table 1.
DC electrical resistivity, pH4-9 sensitivity, pH response time and contact angle for 20FeyBiB glasses and related glasses.
| Sample Name | DC Electrical Resistivity (Ω·cm) | pH4-9 Sensitivity (%) | pH Response Time (s) | Contact Angle (°) |
|---|
| 70BiB | 1.97 × 1010 | - *1 | - *1 | 93.3 |
| 10Fe50BiB | 1.78 × 109 | (92.4) *2 | 144 | 85.9 |
| 10Fe80BiB | 1.68 × 109 | (92.3) *2 | >180 | 93.1 |
| 15Fe70BiB | 6.96 × 108 | (97.3) *2 | 39 | 93.6 |
| 20Fe20BiB | 9.67 × 107 | 88.7 | 15 | 87.5 |
| 20Fe50BiB | 1.93 × 108 | 92.6 | 10 | 87.9 |
| 20Fe60BiB | 1.33 × 108 | 92.6 | 9 | 93.5 |
| 20Fe70BiB | 2.88 × 108 | 90.2 | 10 | 94.0 |
| 20Fe79BiB | 1.49 × 1010 | 96.6 | 22 | 82.8 |
| 20Fe79.5BiB | 2.28 × 1010 | 92.9 | 18 | 78.8 |
| 20Fe79.7BiB | 2.37 × 1010 | 65.5 | 20 | 80.1 |
| 20Fe79.8BiB | 2.58 × 1010 | 38.3 | 15 | 79.8 |
| 20Fe79.9BiB | 2.80 × 1010 | 6.7 | (3) *3 | 83.5 |
| 20Fe80Bi | 3.10 × 1010 | 2.7 | (6) *3 | 79.0 |
| 70TP [6] | 3.72 × 109 | 80.7 | 12 | 58.8 (6.6) *4 |
| Reference (HORIBA) | 1.06 × 1010 | 99.2 | 27 | 30.2 |
Table 1 lists the DC electrical resistivity, pH4-9 sensitivity between pH4 and pH9, pH response time and contact angles of
xFe
yBiB and related glasses [
10] along with TP glass and a reference glass (HORIBA, Ltd., Kyoto, Japan). The DC electrical resistivity of 20Fe
yBiB glasses changed from 9.67 × 10
7 Ω·cm for 20Fe20BiB glass to 3.10 × 10
10 Ω·cm for 20Fe80Bi glass (the second column in
Table 1).
Figure 4 shows the dependence of the DC electrical resistivity on the Fe-Fe distance for the 20Fe
yBiB glasses. It is seen from
Figure 4 that the DC electrical resistivity increases with increasing Fe-Fe distance (increasing Bi
2O
3 content). This is because electron hopping of from Fe
2+ to Fe
3+ via O
2 ion becomes more difficult. This result is consistent with the data in [
9,
10].
Figure 4.
Dependence of DC electrical resistivity on Fe-Fe distance for 20FeyBiB glasses.
Figure 4.
Dependence of DC electrical resistivity on Fe-Fe distance for 20FeyBiB glasses.
We have previously reported that TP glasses with low DC electrical resistivity show a high pH4-9 sensitivity and short pH response time [
6]. In the present work, the third column of
Table 1 shows that
xFe
yBiB glasses with a low DC electrical resistivity did not always show a high pH4-9 sensitivity. This suggests that the pH responsive sites (B-OH and or Bi-OH) may differ from the conductive sites (Fe-OH) in
xFe
yBiB glasses, whereas the pH responsive and conductive sites are both Ti-OH in TP glasses. Taking the results of
Figure 2 and
Figure 3 into consideration, the most important pH responsive sites are B-OH in
xFe
yBiB glasses.
On the other hand, the pH response time of 20Fe
yBiB glasses (
Table 1, column 4) tended to increase from 10 to 20 s on increasing the Bi
2O
3 content from 50 to 80 mol % (decreasing B
2O
3 content). We conclude that the pH response time of
xFe
yBiB glasses is mainly determined by both (a) the dissociation rate of pH responsive sites, such as B-OH, at glass surfaces, and (b) the conduction rate of carriers (e) though the bulk glass. The latter is the predominant rate-determining process for the pH response of
xFe
yBiB glasses, as well as in TP glasses [
6]. However, the 20Fe20BiB glass with the lowest DC electrical resistivity did not show the shortest pH response time among
xFe
yBiB glasses. This may suggest that the rate-determining process changes from a conduction process (bulk) to a pH response process (surface) with decreasing DC electrical resistivity in
xFe
yBiB glasses.
Thus, a moderate amount of Fe
2O
3 and a small amount of B
2O
3 results in electronic conduction through the bulk glass and a pH response on glass surfaces, respectively. Because the remaining components can be selected freely, this result is a very useful in order to develop novel pH glass electrodes with functionalities such as self-cleaning [
6], an anti-fouling ability [
10], and so on.
So far,
xFe
yBiB glasses with a Bi
2O
3 content larger than 60 mol % have shown contact angles higher than 90° [
10]. Moreover, the fifth column in
Table 1 reveals that a B
2O
3 concentration larger than 10 mol % is necessary for hydrophobicity. Thus, the present results suggest that using B
2O
3 as a glass former may play an important role in hydrophobicity as well as in pH4-9 sensitivity. Based on our results [
7], Fe
2O
3-ZnO-B
2O
3 and Fe
2O
3-ZnO-Bi
2O
3-B
2O
3 glasses are candidate for pH glass electrodes with an anti-fouling property based on their hydrophobicity.