Preparation and Compatibility Evaluation of Polypropylene/High Density Polyethylene Polyblends
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mechanical Properties of PP/HDPE Polyblends

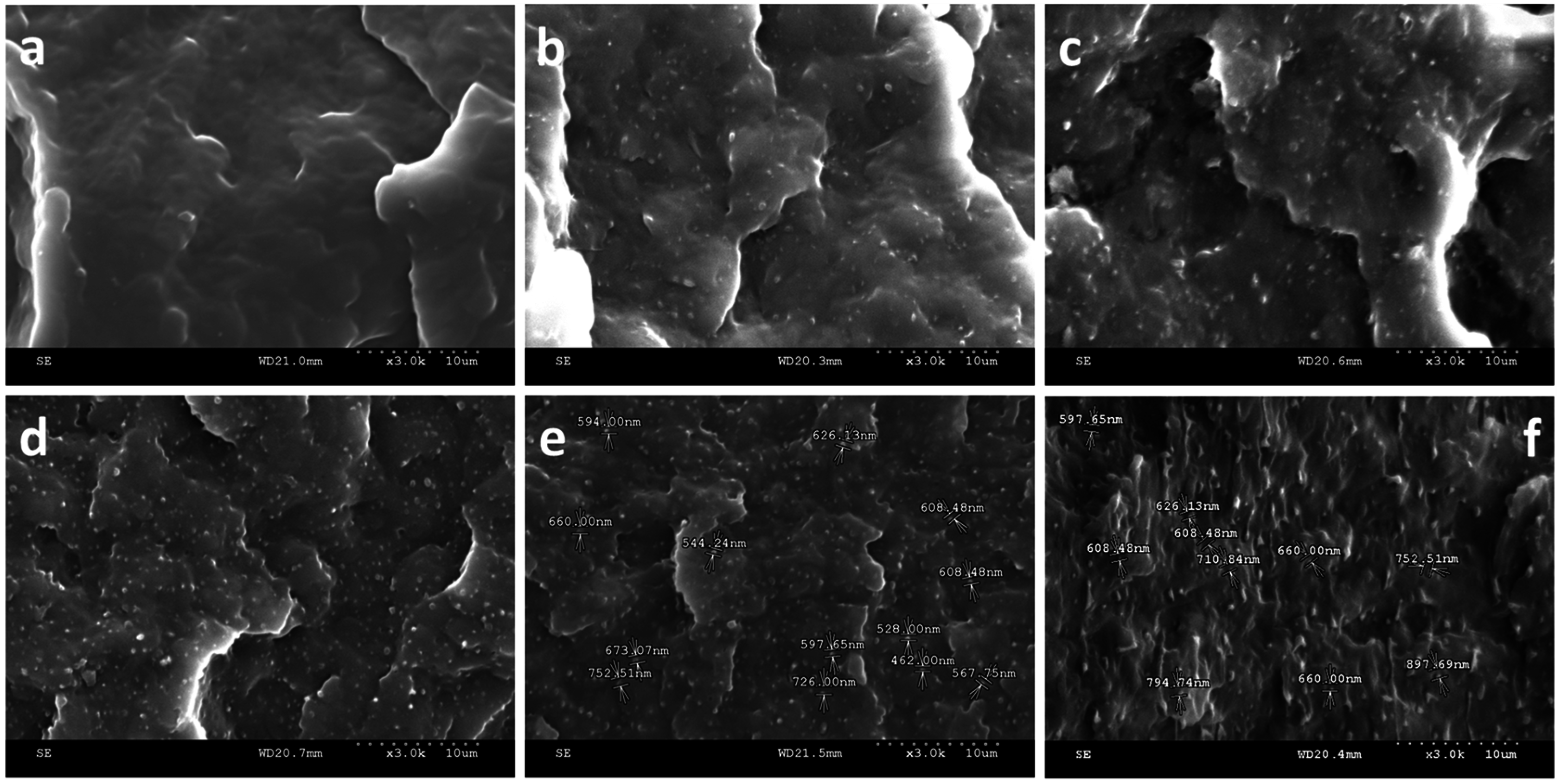
2.2. SEM of PP/HDPE Polyblends
2.3. Chemical Structure of PP/HDPE Polyblends

2.4. Non-Isothermal Crystallization and Melting Behaviors of PP/HDPE Polyblends
| Sample | ∆Hm (J/g) a | Tm (°C) a | Tc (°C) a | Xc (%) a | ∆Hm (J/g) b | Tm (°C) b | Xc (%) b |
|---|---|---|---|---|---|---|---|
| PP | 111.0 | 166.0 | 111.3 | 53.1 | - | - | - |
| HDPE | - | - | 116.5 | - | 210.3 | 132.4 | 75.1 |
| PP/HDPE-5 wt % | 126.8 | 164.8 | 117.1 | 63.9 | 8.2 | 129.7 | 58.5 |
| PP/HDPE-10 wt % | 106.1 | 166.0 | 116.7 | 56.4 | 19.6 | 131.0 | 70.0 |
| PP/HDPE-15 wt % | 121.3 | 164.8 | 117.4 | 68.3 | 31.7 | 130.5 | 75.5 |
| PP/HDPE-20 wt % | 80.2 | 164.6 | 117.8 | 63.1 | 44.9 | 130.9 | 80.2 |
| PP/HDPE-25 wt % | 70.9 | 164.1 | 116.7 | 45.2 | 50.3 | 132.3 | 71.8 |

2.5. PLM Observation of PP/HDPE Polyblends

2.6. Structure Characterization of PP/HDPE Polyblends

3. Experimental Section
3.1. Materials
| Materials | Density (g/cm3) | Melt Index (g/10 min) | Molecular Weight |
|---|---|---|---|
| PP | 0.900 | 10 (230 °C/5 Kg measured) | 80,000–90,000 |
| HDPE | 0.960 | 6 (230 °C/5 Kg measured) | 75,000–80,000 |
3.2. Preparation of Polyblends
3.3. Measurements and Characterization
3.3.1. Mechanical Properties
3.3.2. Scanning Electron Microscopy (SEM)
3.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.4. Differential Scanning Calorimetry (DSC)

3.3.5. Polarized Light Microscopy (PLM)
3.3.6. X-ray Diffraction (XRD)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bertin, S.; Robin, J.J. Study and characterization of virgin and recycled LDPE/PP blends. Eur. Polym. J. 2002, 38, 2255–2264. [Google Scholar] [CrossRef]
- Laoutid, F.; Estrada, E.; Michell, R.M.; Bonnaud, L.; Müller, A.J.; Dubois, P. The influence of nanosilica on the nucleation, crystallization and tensile properties of PP–PC and PP-PA blends. Polymer 2013, 54, 3982–3993. [Google Scholar] [CrossRef]
- Xie, B.-H.; Huang, X.; Zhang, G.-J. High thermal conductive polyvinyl alcohol composites with hexagonal boron nitride microplatelets as fillers. Compos. Sci. Technol. 2013, 85, 98–103. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, J.; Wang, X. Crystallizaion and surface morphology of poly(vinylidene fluoride)/poly(methylmethacrylate) films by solution casting on different substrates. Appl. Surf. Sci. 2008, 254, 2947–2954. [Google Scholar] [CrossRef]
- Albano, C.; González, J.; Ichazo, M.; Rosales, C.; Urbina de Navarro, C.; Parra, C. Mechanical and morphological behavior of polyolefin blends in the presence of CaCO3. Compos. Struct. 2000, 48, 49–58. [Google Scholar] [CrossRef]
- Wong, A.C.Y.; Lam, F. Study of selected thermal characteristics of polypropylene/polyethylene binary blends using DSC and TGA. Polym. Test. 2002, 21, 691–696. [Google Scholar] [CrossRef]
- Achilias, D.S.; Roupakias, C.; Megalokonomos, P.; Lappas, A.A.; Antonakou, Ε.V. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J. Hazard. Mater. 2007, 149, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Mourad, A.-H.I. Thermo-mechanical characteristics of thermally aged polyethylene/polypropylene blends. Mater. Des. 2010, 31, 918–929. [Google Scholar] [CrossRef]
- Camacho, W.; Karlsson, S. Assessment of thermal and thermo-oxidative stability of multi-extruded recycled PP, HDPE and a blend thereof. Polym. Degrad. Stab. 2002, 78, 385–391. [Google Scholar] [CrossRef]
- Wilkinson, A.N.; Laugel, L.; Clemens, M.L.; Harding, V.M.; Marin, M. Phase structure in polypropylene/PA6/SEBS blends. Polymer 1999, 40, 4971–4975. [Google Scholar] [CrossRef]
- Tseng, F.P.; Lin, J.J.; Tseng, C.R.; Chang, F.C. Poly(oxypropylene)-amide grafted polypropylene as novel compatibilizer for PP and PA6 blends. Polymer 2001, 42, 713–725. [Google Scholar] [CrossRef]
- Shi, H.; Shi, D.; Wang, X.; Yin, L.; Yin, J.; Mai, Y.-W. A facile route for preparing stable co-continuous morphology of LLDPE/PA6 blends with low PA6 content. Polymer 2010, 51, 4958–4968. [Google Scholar] [CrossRef]
- Maciel, A.; Salas, V.; Manero, O. PP/EVA blends: Mechanical properties and morphology. Effect of compatibilizers on the impact behavior. Adv. Polym. Technol. 2005, 24, 241–252. [Google Scholar] [CrossRef]
- Martins, C.G.; Larocca, N.M.; Paul, D.R.; Pessan, L.A. Nanocomposites formed from polypropylene/EVA blends. Polymer 2009, 50, 1743–1754. [Google Scholar] [CrossRef]
- Valera-Zaragoza, M.; Rivas-Vázquez, L.P.; Ramírez-Vargas, E.; Sánchez-Valdes, S.; Ramos-deValle, L.F.; Medellín-Rodríguez, F.J. Influence of morphology on the dynamic mechanical characteristics of PP-EP/EVA/organoclay nanocomposites. Compos. B Eng. 2013, 55, 506–512. [Google Scholar] [CrossRef]
- Strapasson, R.; Amico, S.C.; Pereira, M.F.R.; Sydenstricker, T.H.D. Tensile and impact behavior of polypropylene/low density polyethylene blends. Polym. Test. 2005, 24, 468–473. [Google Scholar] [CrossRef]
- Tai, C.M.; Li, R.K.Y.; Ng, C.N. Impact behaviour of polypropylene/polyethylene blends. Polym. Test. 2000, 19, 143–154. [Google Scholar] [CrossRef]
- Chiu, F.-C.; Yen, H.-Z.; Lee, C.-E. Characterization of PP/HDPE blend-based nanocomposites using different maleated polyolefins as compatibilizers. Polym. Test. 2010, 29, 397–406. [Google Scholar] [CrossRef]
- Chiu, F.-C.; Yen, H.-Z.; Chen, C.-C. Phase morphology and physical properties of PP/HDPE/organoclay (nano) composites with and without a maleated EPDM as a compatibilizer. Polym. Test. 2010, 29, 706–716. [Google Scholar] [CrossRef]
- Souza, A.M.C.; Demarquette, N.R. Influence of composition on the linear viscoelastic behavior and morphology of PP/HDPE blends. Polymer 2002, 43, 1313–1321. [Google Scholar] [CrossRef]
- Li, J.; Shanks, R.A.; Long, Y. Mechanical properties and morphology of polyethylene–polypropylene blends with controlled thermal history. J. Appl. Polym. Sci. 2000, 76, 1151–1164. [Google Scholar] [CrossRef]
- Jose, S.; Aprem, A.S.; Francis, B.; Chandy, M.C.; Werner, P.; Alstaedt, V.; Thomas, S. Phase morphology, crystallisation behaviour and mechanical properties of isotactic polypropylene/high density polyethylene blends. Eur. Polym. J. 2004, 40, 2105–2115. [Google Scholar] [CrossRef]
- Macosko, C.W.; Jeon, H.K.; Hoye, T.R. Reactions at polymer–polymer interfaces for blend compatibilization. Prog. Polym. Sci. 2005, 30, 939–947. [Google Scholar] [CrossRef]
- Saroop, M.; Mathur, G.N. Studies on the dynamically vulcanized polypropylene (PP)/butadiene styrene block copolymer (SBS) blends: Mechanical properties. J. Appl. Polym. Sci. 1997, 65, 2691–2701. [Google Scholar] [CrossRef]
- Van Puyvelde, P.; Velankar, S.; Moldenaers, P. Rheology and morphology of compatibilized polymer blends. Curr. Opin. Colloid Interface Sci. 2001, 6, 457–463. [Google Scholar] [CrossRef]
- George, J.; Varughese, K.T.; Thomas, S. Dynamically vulcanised thermoplastic elastomer blends of polyethylene and nitrile rubber. Polymer 2000, 41, 1507–1517. [Google Scholar] [CrossRef]
- Elmendorp, J.J.; Maalcke, R.J. A study on polymer blending microrheology: Part 1. Polym. Eng. Sci. 1985, 25, 1041–1047. [Google Scholar] [CrossRef]
- Plochocki, A.P.; Dagli, S.S.; Andrews, R.D. The interface in binary mixtures of polymers containing a corresponding block copolymer: Effects of industrial mixing processes and of coalescence. Polym. Eng. Sci. 1990, 30, 741–752. [Google Scholar] [CrossRef]
- Merz, E.H.; Claver, G.C.; Baer, M. Studies on heterogeneous polymeric systems. J. Polym. Sci. 1956, 22, 325–341. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Fontana, P.; Morreale, M.; Mistretta, M.C. Orientation induced brittle—Ductile transition in a polyethylene/polyamide 6 blend. Polym. Test. 2014, 36, 20–23. [Google Scholar] [CrossRef]
- Sundararaj, U.; Macosko, C.W. Drop breakup and coalescence in polymer blends: The effects of concentration and compatibilization. Macromolecules 1995, 28, 2647–2657. [Google Scholar] [CrossRef]
- Camacho, W.; Karlsson, S. NIR, DSC, and FTIR as quantitative methods for compositional analysis of blends of polymers obtained from recycled mixed plastic waste. Polym. Eng. Sci. 2001, 41, 1626–1635. [Google Scholar] [CrossRef]
- Nishino, T.; Matsumoto, T.; Nakamae, K. Surface structure of isotactic polypropylene by X-ray diffraction. Polym. Eng. Sci. 2000, 40, 336–343. [Google Scholar] [CrossRef]
- Inci, B.; Wagener, K.B. Decreasing the alkyl branch frequency in precision polyethylene: Pushing the limits toward longer run lengths. J. Am. Chem. Soc. 2011, 133, 11872–11875. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.Z.; Tjong, S.C. Mechanical and thermal performance of high-density polyethylene/alumina nanocomposites. J. Macromol. Sci. B 2012, 52, 812–825. [Google Scholar] [CrossRef]
- American Society for Testing Materials. Standard Test Method for Tensile Properties of Plastics; ASTM D638-14; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- American Society for Testing Materials. Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials; ASTM D790-10; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- American Society for Testing Materials. Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics; ASTM D256-10e1; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Arroyo, M.; Lopez-Manchado, M.A.; Avalos, F. Crystallization kinetics of polypropylene: II. Effect of the addition of short glass fibres. Polymer 1997, 38, 5587–5593. [Google Scholar] [CrossRef]
- Ajji, A.; Sammut, P.; Huneault, M.A. Elongational rheology of LLDPE/LDPE blends. J. Appl.Polym. Sci. 2003, 88, 3070–3077. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-H.; Pan, Y.-J.; Liu, C.-F.; Huang, C.-L.; Hsieh, C.-T.; Chen, C.-K.; Lin, Z.-I.; Lou, C.-W. Preparation and Compatibility Evaluation of Polypropylene/High Density Polyethylene Polyblends. Materials 2015, 8, 8850-8859. https://doi.org/10.3390/ma8125496
Lin J-H, Pan Y-J, Liu C-F, Huang C-L, Hsieh C-T, Chen C-K, Lin Z-I, Lou C-W. Preparation and Compatibility Evaluation of Polypropylene/High Density Polyethylene Polyblends. Materials. 2015; 8(12):8850-8859. https://doi.org/10.3390/ma8125496
Chicago/Turabian StyleLin, Jia-Horng, Yi-Jun Pan, Chi-Fan Liu, Chien-Lin Huang, Chien-Teng Hsieh, Chih-Kuang Chen, Zheng-Ian Lin, and Ching-Wen Lou. 2015. "Preparation and Compatibility Evaluation of Polypropylene/High Density Polyethylene Polyblends" Materials 8, no. 12: 8850-8859. https://doi.org/10.3390/ma8125496
APA StyleLin, J.-H., Pan, Y.-J., Liu, C.-F., Huang, C.-L., Hsieh, C.-T., Chen, C.-K., Lin, Z.-I., & Lou, C.-W. (2015). Preparation and Compatibility Evaluation of Polypropylene/High Density Polyethylene Polyblends. Materials, 8(12), 8850-8859. https://doi.org/10.3390/ma8125496








