Gas-Phase Deposition of Ultrathin Aluminium Oxide Films on Nanoparticles at Ambient Conditions
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Setup
2.2. Design of the Experiments
2.3. Characterization of the Coating
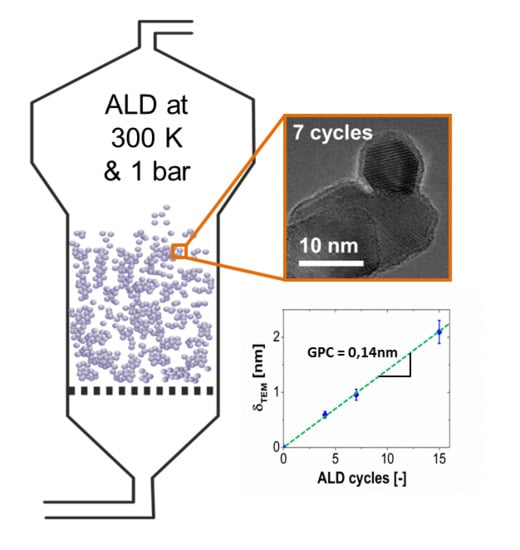
3. Results and Discussion
3.1. Base Case Coating Experiments


3.2. Coating Experiments with Excess of Precursors at Ambient Conditions


3.3. Coating with Excess of Precursors at 1 bar and 170 °C

3.4. Alumina Film Density
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- King, D.M.; Liang, X.; Weimer, A.W. Functionalization of fine particles using atomic and molecular layer deposition. Powder Technol. 2012, 221, 13–25. [Google Scholar] [CrossRef]
- Van Ommen, J.R.; Valverde, J.M.; Pfeffer, R. Fluidization of nanopowders: A review. J. Nanopart. Res. 2012, 14, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Goulas, A.; van Ommen, J.R. Scalable production of nanostructured particles using atomic layer deposition. KONA Powder Part. J. 2014, 31, 234–246. [Google Scholar] [CrossRef]
- Longrie, D.; Deduytsche, D.; Detavernier, C. Reactor concepts for atomic layer deposition on agitated particles: A review. J. Vac. Sci. Technol. A 2014, 32, 010802. [Google Scholar] [CrossRef]
- Moghtaderi, B.; Shames, I.; Doroodchi, E. Combustion prevention of iron powders by a novel coating method. Chem. Eng. Technol. 2006, 29, 97–103. [Google Scholar] [CrossRef]
- Hakim, L.F.; Vaughn, C.L.; Dunsheath, H.J.; Carney, C.S.; Liang, X.; Li, P.; Weimer, A.W. Synthesis of oxidation-resistant metal nanoparticles via atomic layer deposition. Nanotechnology 2007, 18, 345603. [Google Scholar] [CrossRef]
- King, D.M.; Liang, X.; Burton, B.B.; Kamal Akhtar, M.; Weimer, A.W. Passivation of pigment-grade TiO2 particles by nanothick atomic layer deposited SiO2 films. Nanotechnology 2008, 19, 255604. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; King, D.M.; Groner, M.D.; Blackson, J.H.; Harris, J.D.; George, S.M.; Weimer, A.W. Barrier properties of polymer/alumina nanocomposite membranes fabricated by atomic layer deposition. J. Membr. Sci. 2008, 322, 105–112. [Google Scholar] [CrossRef]
- Liang, X.; Lynn, A.D.; King, D.M.; Bryant, S.J.; Weimer, A.W. Biocompatible interface films deposited within porous polymers by atomic layer deposition (ALD). ACS Appl. Mater. Interfaces 2009, 1, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Lichty, P.; Liang, X.; Muhich, C.; Evanko, B.; Bingham, C.; Weimer, A.W. Atomic layer deposited thin film metal oxides for fuel production in a solar cavity reactor. Int. J. Hydrog. Energy 2012, 37, 16888–16894. [Google Scholar] [CrossRef]
- Li, J.; Liang, X.; King, D.M.; Jiang, Y.B.; Weimer, A.W. Highly dispersed Pt nanoparticle catalyst prepared by atomic layer deposition. Appl. Catal. B Environ. 2010, 97, 220–226. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Gobin, A.M.; Lowery, A.R.; Tam, F.; Drezek, R.A.; Halas, N.J.; West, J.L. Metal nanoshells. Ann. Biomed. Eng. 2006, 34, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Bawarski, W.E.; Chidlowsky, E.; Bharali, D.J.; Mousa, S.A. Emerging nanopharmaceuticals. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 273–282. [Google Scholar] [CrossRef]
- Elam, J.W.; Dasgupta, N.P.; Prinz, F.B. ALD for clean energy conversion, utilization, and storage. MRS Bull. 2011, 36, 899–906. [Google Scholar] [CrossRef]
- Sveshnikova, G.V.; Kol’Tsov, S.I.; Aleskovskii, V.B. Formation of a silica layer of predetermined thickness on silicon by the Molecular-Layering method. Zh Prikl Khim 1970, 43, 1150–1152. [Google Scholar]
- Suntola, T. Atomic layer epitaxy. Thin Solid Films 1992, 216, 84–89. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, Y.; Li, J.; Weimer, A. Reaction mechanism studies for platinum nanoparticle growth by atomic layer deposition. J. Nanopart. Res. 2011, 13, 3781–3788. [Google Scholar] [CrossRef]
- Goulas, A.; van Ommen, J.R. Atomic layer deposition of platinum clusters on titania nanoparticles at atmospheric pressure. J. Mater. Chem. A 2013, 1, 4647–4650. [Google Scholar] [CrossRef]
- Hakim, L.F.; King, D.M.; Zhou, Y.; Gump, C.J.; George, S.M.; Weimer, A.W. Nanoparticle coating for advanced optical, mechanical and rheological properties. Adv. Funct. Mater. 2007, 17, 3175–3181. [Google Scholar] [CrossRef]
- Puurunen, R.L. Growth per cycle in atomic layer deposition: A theoretical model. Chem. Vap. Depos. 2003, 9, 249–257. [Google Scholar] [CrossRef]
- Profijt, H.B.; Potts, S.E.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-assisted atomic layer deposition: Basics, opportunities, and challenges. J. Vac. Sci. Technol. A 2011, 29, 050801. [Google Scholar] [CrossRef]
- Potts, S.E.; Kessels, W.M.M. Energy-enhanced atomic layer deposition for more process and precursor versatility. Coord. Chem. Rev. 2013, 257, 3254–3270. [Google Scholar] [CrossRef]
- Puurunen, R.L. Correlation between the growth-per-cycle and the surface hydroxyl group concentration in the atomic layer deposition of aluminum oxide from trimethylaluminum and water. Appl. Surf. Sci. 2005, 245, 6–10. [Google Scholar] [CrossRef]
- Philip, A.; Thomas, S.; Kumar, K.R. Calculation of growth per cycle (GPC) of atomic layer deposited aluminium oxide nanolayers and dependence of GPC on surface OH concentration. Pram. J. Phys. 2014, 82, 563–569. [Google Scholar] [CrossRef]
- Wank, J.R.; George, S.M.; Weimer, A.W. Coating fine nickel particles with Al2O3 utilizing an atomic layer deposition-fluidized bed reactor (ALD-FBR). J. Am. Ceram. Soc. 2004, 87, 762–765. [Google Scholar] [CrossRef]
- Wank, J.R.; George, S.M.; Weimer, A.W. Nanocoating individual cohesive boron nitride particles in a fluidized bed by ALD. Powder Technol. 2004, 142, 59–69. [Google Scholar] [CrossRef]
- Hakim, L.F.; George, S.M.; Weimer, A.W. Conformal nanocoating of zirconia nanoparticles by atomic layer deposition in a fluidized bed reactor. Nanotechnology 2005, 16, S375–S381. [Google Scholar] [CrossRef] [PubMed]
- Hakim, L.F.; Blackson, J.; George, S.M.; Weimer, A.W. Nanocoating individual silica nanoparticles by atomic layer deposition in a fluidized bed reactor. Chem. Vap. Depos. 2005, 11, 420–425. [Google Scholar] [CrossRef]
- Hakim, L.F.; McCormick, J.A.; Zhan, G.D.; Weimer, A.W.; Li, P.; George, S.M. Surface modification of titania nanoparticles using ultrathin ceramic films. J. Am. Ceram. Soc. 2006, 89, 3070–3075. [Google Scholar] [CrossRef]
- Liang, X.; Zhan, G.D.; King, D.M.; McCormick, J.A.; Zhang, J.; George, S.M.; Weimer, A.W. Alumina atomic layer deposition nanocoatings on primary diamond particles using a fluidized bed reactor. Diam. Relat. Mater. 2008, 17, 185–189. [Google Scholar] [CrossRef]
- Beetstra, R.; Lafont, U.; Nijenhuis, J.; Kelder, E.M.; van Ommen, J.R. Atmospheric pressure process for coating particles using atomic layer deposition. Chem. Vap. Depos. 2009, 15, 227–233. [Google Scholar] [CrossRef]
- Liang, X.; George, S.M.; Weimer, A.W.; Li, N.H.; Blackson, J.H.; Harris, J.D.; Li, P. Synthesis of a novel porous polymer/ceramic composite material by low-temperature atomic layer deposition. Chem. Mater. 2007, 19, 5388–5394. [Google Scholar] [CrossRef]
- Liang, X.; Hakim, L.F.; Zhan, G.D.; McCormick, J.A.; George, S.M.; Weimer, A.W.; Spencer Ii, J.A.; Buechler, K.J.; Blackson, J.; Wood, C.J.; et al. Novel processing to produce polymer/ceramic nanocomposites by atomic layer deposition. J. Am. Ceram. Soc. 2007, 90, 57–63. [Google Scholar] [CrossRef]
- Groner, M.D.; Fabreguette, F.H.; Elam, J.W.; George, S.M. Low-temperature Al2O3 atomic layer deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Potts, S.E.; Profijt, H.B.; Roelofs, R.; Kessels, W.M.M. Room-temperature ALD of metal oxide thin films by energy-enhanced ALD. Chem. Vap. Depos. 2013, 19, 125–133. [Google Scholar] [CrossRef]
- Tang, X.; Francis, L.A.; Simonis, P.; Haslinger, M.; Delamare, R.; Deschaume, O.; Flandre, D.; Defrance, P.; Jonas, A.M.; Vigneron, J.P.; et al. Room temperature atomic layer deposition of Al2O3 and replication of butterfly wings for photovoltaic application. J. Vac. Sci. Technol. A 2012, 30, 01A146. [Google Scholar] [CrossRef]
- Jur, J.S.; Parsons, G.N. Atomic layer deposition of Al2O3 and ZnO at atmospheric pressure in a flow tube reactor. ACS Appl. Mater. Interfaces 2011, 3, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.B.M.; Oldham, C.J.; Parsons, G.N. Atmospheric pressure atomic layer deposition of Al2O3 using trimethyl aluminum and ozone. Langmuir 2014, 30, 3741–3748. [Google Scholar] [CrossRef] [PubMed]
- Skoog, S.A.; Elam, J.W.; Narayan, R.J. Atomic layer deposition: Medical and biological applications. Int. Mater. Rev. 2013, 58, 113–129. [Google Scholar] [CrossRef]
- Salameh, S.; Schneider, J.; Laube, J.; Alessandrini, A.; Facci, P.; Seo, J.W.; Ciacchi, L.C.; Mädler, L. Adhesion mechanisms of the contact interface of TiO2 nanoparticles in films and aggregates. Langmuir 2012, 28, 11457–11464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, M.B.M.; Oldham, C.J.; Jur, J.S.; Parsons, G.N. Effect of temperature and gas velocity on growth per cycle during Al2O3 and ZnO atomic layer deposition at atmospheric pressure. J. Vac. Sci. Technol. A. 2012, 30, 01A155. [Google Scholar] [CrossRef]
- De Martín, L.; Bouwman, W.G.; van Ommen, J.R. Multidimensional nature of fluidized nanoparticle agglomerates. Langmuir 2014, 30, 12696–12702. [Google Scholar]
- Hakim, L.F.; Portman, J.L.; Casper, M.D.; Weimer, A.W. Fluidization behavior and conformal coating of nanoparticles in fluidized beds by ALD. In Proceedings of the 2004 AIChE Annual Meeting, Austin, TX, USA, 7–12 November 2004; pp. 2339–2359.
- Mueller, R.; Kammler, H.K.; Wegner, K.; Pratsinis, S.E. OH surface density of SiO2 and TiO2 by thermogravimetric analysis. Langmuir 2003, 19, 160–165. [Google Scholar] [CrossRef]
- Gow, T.R.; Lin, R.; Cadwell, L.A.; Lee, F.; Backman, A.L.; Masel, R.I. Decomposition of trimethylaluminum on silicon(100). Chem. Mater. 1989, 1, 406–411. [Google Scholar] [CrossRef]
- Travis, C.; Adomaitis, R. Dynamic modeling for the design and cyclic operation of an atomic layer deposition (ALD) reactor. Processes 2013, 1, 128–152. [Google Scholar] [CrossRef]
- Henrickson, C.H.; Eyman, D.P. Lewis acidity of alanes. Interactions of trimethylalane with sulfides. Inorg. Chem. 1967, 6, 1461–1465. [Google Scholar] [CrossRef]
- Laubengayer, A.W.; Gilliam, W.F. The alkyls of the third group elements. I. Vapor phase studies of the alkyls of aluminum, gallium and indium1. J. Am. Chem. Soc. 1941, 63, 477–479. [Google Scholar] [CrossRef]
- Mayer, B.; Collins, C.C.; Walton, M. Transient analysis of carrier gas saturation in liquid source vapor generators. J. Vac. Sci. Technol. A 2001, 19, 329–344. [Google Scholar] [CrossRef]
- Kunii, D.; Levenspiel, O. Fluidization Engineering, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1991. [Google Scholar]
- Grillo, F.; Kreutzer, M.T.; van Ommen, J.R. Modeling the precursor utilization in atomic layer deposition on nanostructured materials in fluidized bed reactors. Chem. Eng. J. 2015, 268, 384–398. [Google Scholar] [CrossRef]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdesueiro, D.; Meesters, G.M.H.; Kreutzer, M.T.; Van Ommen, J.R. Gas-Phase Deposition of Ultrathin Aluminium Oxide Films on Nanoparticles at Ambient Conditions. Materials 2015, 8, 1249-1263. https://doi.org/10.3390/ma8031249
Valdesueiro D, Meesters GMH, Kreutzer MT, Van Ommen JR. Gas-Phase Deposition of Ultrathin Aluminium Oxide Films on Nanoparticles at Ambient Conditions. Materials. 2015; 8(3):1249-1263. https://doi.org/10.3390/ma8031249
Chicago/Turabian StyleValdesueiro, David, Gabrie M. H. Meesters, Michiel T. Kreutzer, and J. Ruud Van Ommen. 2015. "Gas-Phase Deposition of Ultrathin Aluminium Oxide Films on Nanoparticles at Ambient Conditions" Materials 8, no. 3: 1249-1263. https://doi.org/10.3390/ma8031249