2. Results and Discussion
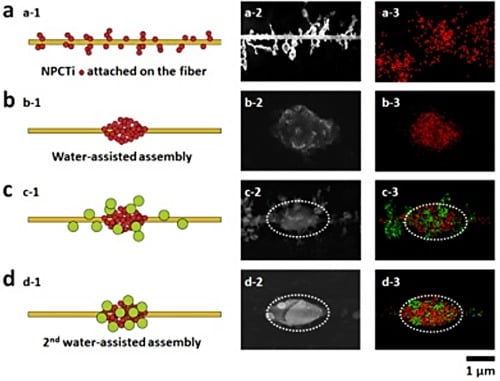
A metal shelf was put under the needle as the collector, on which the parallel PMMA electrospun fibers (0.562 ± 0.035 μm in diameter) can be collected between the two strings (
Figure 1a). When the fibers were put into a chamber with two apertures (one for gas in and the other for gas out) and exposed in the smog of the hydrolysis of TiCl
4, NPCTi, with the average diameter of 0.233 ± 0.054 μm, would be adsorbed onto and randomly surround the fiber, forming a particle-on-string structure (
Figure 1b and
Figure 2a). By the time the water stream flowed into the chamber from one side and passed through these fibers (the first wet-assembly process), the NPCTi would assemble into highly-ordered micro-humps (
Figure 2b, 1.326 ± 0.223 μm in length, 0.885 ± 0.174 μm in width) due to the collection and coalescence of the tiny water droplets. Then, the hump-on-string fibers were put into a similar chamber with apertures with smog caused by hydrolysis process of TMA (
Figure 1d). As expected, a series of nanoparticles (
Figure 2c, 0.677 ± 0.145 μm in diameter) could be caught by the humps and fibers, which could be identified as NPCAl attaching on the NPCTi-humps via EDS mapping (
Figure 2c-3, white oval,
Figure S1).
The Ti-hump with a conical shape can cause a difference in the pressure and such a conical shape with a curvature gradient will give rise to a difference in Laplace pressure (Δ
P) acting on a water drop [
2,
14]:
where
γ,
r,
R0,
β, z are the surface tension of the drop, the radius of the fiber, the radius of the spindle, the half apex-angle of the spindle and the direction along the fiber, respectively. In addition, the Ti-hump is more hydrophilic due to its rougher surface and chemical composition and has a higher apparent surface energy than the fiber, Thus, the force generated by a surface energy gradient that arises from a difference in surface roughness and chemical composition can be given by [
2,
15,
16]:
where
γ is the surface tension of water;
θs and
θf are the contact angles of water drop on the spindle and the fiber. According to cos
θw = εcos
θ (ε is the roughness of the surface),
θs <
θf, so
F has a direction pointing to the spindle. Under the efforts of the collaboration of Δ
P (geometric gradient) and
F (chemical gradient), those larger NPCAl can gather around the Ti-humps (the second wet-assembly process), forming the multi-scale heterogeneous hump-on-string fibers (MHHFs,
Figure 1e and
Figure 2d, 2.136 ± 0.267 μm in length, 1.045 ± 0.155 μm in width).
Figure 1.
Illustrations of fabrication process of multi-scale heterogeneous hump-on-string fibers (MHHFs). (a) Electrospinning process; (b) nanoparticles containing Ti (NPCTi) absorption; (c) The first wet-assembly process; (d) nanoparticles containing Al (NPCAl) absorption; (e) The second wet-assembly process.
Figure 1.
Illustrations of fabrication process of multi-scale heterogeneous hump-on-string fibers (MHHFs). (a) Electrospinning process; (b) nanoparticles containing Ti (NPCTi) absorption; (c) The first wet-assembly process; (d) nanoparticles containing Al (NPCAl) absorption; (e) The second wet-assembly process.
Figure 2.
Illustrations and SEM images of the multi-scale heterogeneous hump-on-string fiber. (a) NPCTi are adsorbed onto the smooth PMMA fiber; (b) the Ti-humps are fabricated after the 1st wet-assisted assembly; (c) NPCAl are adsorbed onto the fiber with Ti-humps; (d) the multi-scale heterogeneous hump-on-string fibers are formed after the 2nd wet-assisted assembly.
Figure 2.
Illustrations and SEM images of the multi-scale heterogeneous hump-on-string fiber. (a) NPCTi are adsorbed onto the smooth PMMA fiber; (b) the Ti-humps are fabricated after the 1st wet-assisted assembly; (c) NPCAl are adsorbed onto the fiber with Ti-humps; (d) the multi-scale heterogeneous hump-on-string fibers are formed after the 2nd wet-assisted assembly.
Considering that MHHFs are fabricated by NPCAl attaching on the NPCTi-humps (Four main peaks appear in the spectrum in
Figure 3, which can be seen as the combination of the spectrum of NPCAl (Al peak and O peak) and NPCTi (Ti peak; Cl peak and O peak)); the ratio of Ti and Al can be controlled by adjusting the volume of hydrolyzed TMA and TiCl
4. When the volume of TMA varies from 0.2 to 1.2 mL while TiCl
4 is set to 1 mL, the Al peak (E
Kα = 1.49 KeV) rises obviously (Ti peak (E
Kα = 4.51 KeV) is set to the same height during the characterization); with the ratio of Ti and Al decreasing from 3.92:1 (
Figure 3, red line at the bottom) to 0.65:1 (
Figure 3, orange line at the top). Besides, a linear correlation is found between (
r2 ≈ 0.98) the ratio of TiCl
4 and TMA and the ratio of Ti and Al, which may be attributed to the similar process of the adsorption and wet-assisted assembly of NPCTi and NPCAl.
Figure 3.
EDS spectrums (a) and ratio-control of Ti and Al (b) in different fibers (the error bars indicate the standard deviations of the results).
Figure 3.
EDS spectrums (a) and ratio-control of Ti and Al (b) in different fibers (the error bars indicate the standard deviations of the results).
Based on a similar mechanism of wet-induced assembly, the distances and volumes of the humps on MHHFs can also be adjusted by controlling the humidity of the chamber (noted as RH in
Figure 4) and the wetting time (noted as W.T. in
Figure 4). As for the distances (
Figure 4a), the intervals between humps on MHHFs are from ~5 μm when RH ~ 60% to over 15 μm when RH reaches ~ 80% at a short wetting time (
i.e., 5 s) and can be from near 10 to 24 μm with the RH increasing also from ~60% to ~ 80% at a longer wetting time (10 s). Moreover, their trends are nearly the same as those for humps composed of single NPCTi, which may due to the similar collecting-coalescence process of these two kinds of fibers. However, the volumes of the humps are larger than the humps composed of single NPCTi. As RH ~ 60% in wetting time of 5 s, the volumes of humps of NPCTi are only 3.127 ± 0.285 μm
3 while the volume of humps on MHHFs can be 8.144 ± 0.725 μm
3. Despite the diversity between the values of volumes, the two kinds of fibers also shared a similar trend line when the RH increases (
Figure 4b, the dashes).
Compared with fibers with beads composed of single NPCTi, the water collection efficiency of MHHFs is affected by not only the surface area of the beads, but also the ratio of Ti and Al. The results of the water collection efficiency of MHHFs are shown in
Figure 5. According to the scaling law of water droplet growth, the efficiency (
η) can be roughly defined as
η = d
V/d
t =
nS
VM [
17], which means the small change of the drop volume (d
V) during a short time period (d
t) can be estimated by the mole number (
n) and the molar volume (
VM) of the liquid, as well as the effective collection surface (
S) of the beads. In this article, we take
η = Vw/
t = 4/3 π (
rw)
3/
t (where
Vw,
rw and
t represent the total volume and radius of the drop and the water collecting time) and S = π (
l2+
αw2/tan
α)/2, where
α = arcos (
l/w),
l and
w are length and width of bead, respectively. The water collection efficiency of MHHFs increases from ~0.5 × 10
4 to ~2.5 × 10
4 μm
3 when the average collection surface area changes from ~100 to ~500 μm
2 (
Figure 5), which indicates that larger beads have a higher efficiency in water collection than that of smaller ones due to stronger water capturing ability [
17,
18,
19,
20,
21]. Besides, when the ratio of Ti decreases from 3.92 to 0.65, the water collection efficiency goes up (
Figure 5), which may be a result of the chemical and geometric gradient due to the heterogeneous and multi-scale on MHHFs [
5].
Figure 4.
Comparison of distances (a) and volumes of humps (b) composed of (NPCAl + NPCTi) and NPCTi at different humidity (the error bars indicate the standard deviations of the results).
Figure 4.
Comparison of distances (a) and volumes of humps (b) composed of (NPCAl + NPCTi) and NPCTi at different humidity (the error bars indicate the standard deviations of the results).
Figure 5.
Water collection efficiency of the MHHFs at different ratios of Ti and Al (the error bars indicate the standard deviations of the results).
Figure 5.
Water collection efficiency of the MHHFs at different ratios of Ti and Al (the error bars indicate the standard deviations of the results).
In order to explain the wet-induced assembly process and water collection property more theoretically, a numerical simulative calculation based on computational fluid dynamics (CFD) and the Navier-Stokes equation (N-S equation) [
22] is introduced to simulate the flow field, including the distribution of the pressure and the velocity. As depicted in
Figure S2, periodic conditions are taken at the lateral boundaries. The velocity of air is assigned to be 1 m/s at the inlet and a zero diffusion flux for all flow variables is specified at the outflow. Symmetry conditions are employed at the upper and lower boundaries. For the fiber surfaces, no-slip wall boundary conditions are applied, and the wall temperature is assumed to be 298K, which equals to the temperature of the free stream.
The comparisons of the CFD simulation nephograms between the bead-on-string fiber with humped and smooth surface reveal the reason why the MHHFs have a stronger water collection ability (
Figure 6). A larger area with higher pressure occurs on the surface of the bead-on-string fibers with nanoparticles attached (
Figure 6a, red zone,
Pmax = 1.6 Pa) than the bead-on-string fibers, which have a smooth surface (
Figure 6b,
Pmax = 0.6 Pa). Such a difference in the distribution in pressure can also be reflected by a more chaotic and denser distribution of streamline. As a result of the blocking and interference effect of the particles, the streamlines have much more curved traces (
Figure 6c, grayscale lines with arrows) than those passing through the smooth surface (
Figure 6d, grayscale lines with arrows), making both
Pmax and
Pmin on the surface of humped bead-on-surface fibers higher than those on the fibers with smooth beads at where the particles attached (hump:
Figure 6,
Pmax = 1.7 Pa,
Pmin = 0.1 Pa; smooth:
Figure 6d,
Pmax = 0.6 Pa,
Pmin = −1.0 Pa) and the middle of the beads (hump:
Figure 6e,
Pmax = 1.7 Pa,
Pmin = 0.1 Pa; smooth:
Figure 6f,
Pmax = 0.6 Pa,
Pmin = −1.0 Pa). From the view of diffusion, the Brownian motion becomes intense with decrease of fragment size according to Stokes-Einstein equation [
23], which proves that the tiny water drops are much more easily to be caught by the particles than the fiber [
18,
19,
20,
21].
Figure 6.
Computational fluid dynamics (CFD) simulation nephograms of bead-on-string fiber with humped and smooth surface. Perspective view of the bead-on-string fibers with humped (a) and smooth surface (b); Comparison of the side view of the fibers in the humped position (c) and the same position on the smooth fiber (d); Comparison of the distribution of streamlines at the beads with maximum diameter on the humped (e) and smooth (f) fiber.
Figure 6.
Computational fluid dynamics (CFD) simulation nephograms of bead-on-string fiber with humped and smooth surface. Perspective view of the bead-on-string fibers with humped (a) and smooth surface (b); Comparison of the side view of the fibers in the humped position (c) and the same position on the smooth fiber (d); Comparison of the distribution of streamlines at the beads with maximum diameter on the humped (e) and smooth (f) fiber.
Here, from another perspective of thermodynamics, according the Kelvin’s Law:
where
pr,
p∞ represent the pressure of the drops (radius =
r) and the pressure of the flat surface (radius = ∞);
M,
ρ,
R,
T are the mole mass, density of the stream, ideal gas constant, and temperature, respectively, which can be seen as constant in this situation. Such a higher pressure field around the MHHFs may be benefit for the water condensation and leads to a stronger ability in water collection.