Nanocarbon-Coated Porous Anodic Alumina for Bionic Devices
Abstract
:1. Introduction

2. Results and Discussion
2.1. Conductivity of the Electrodes
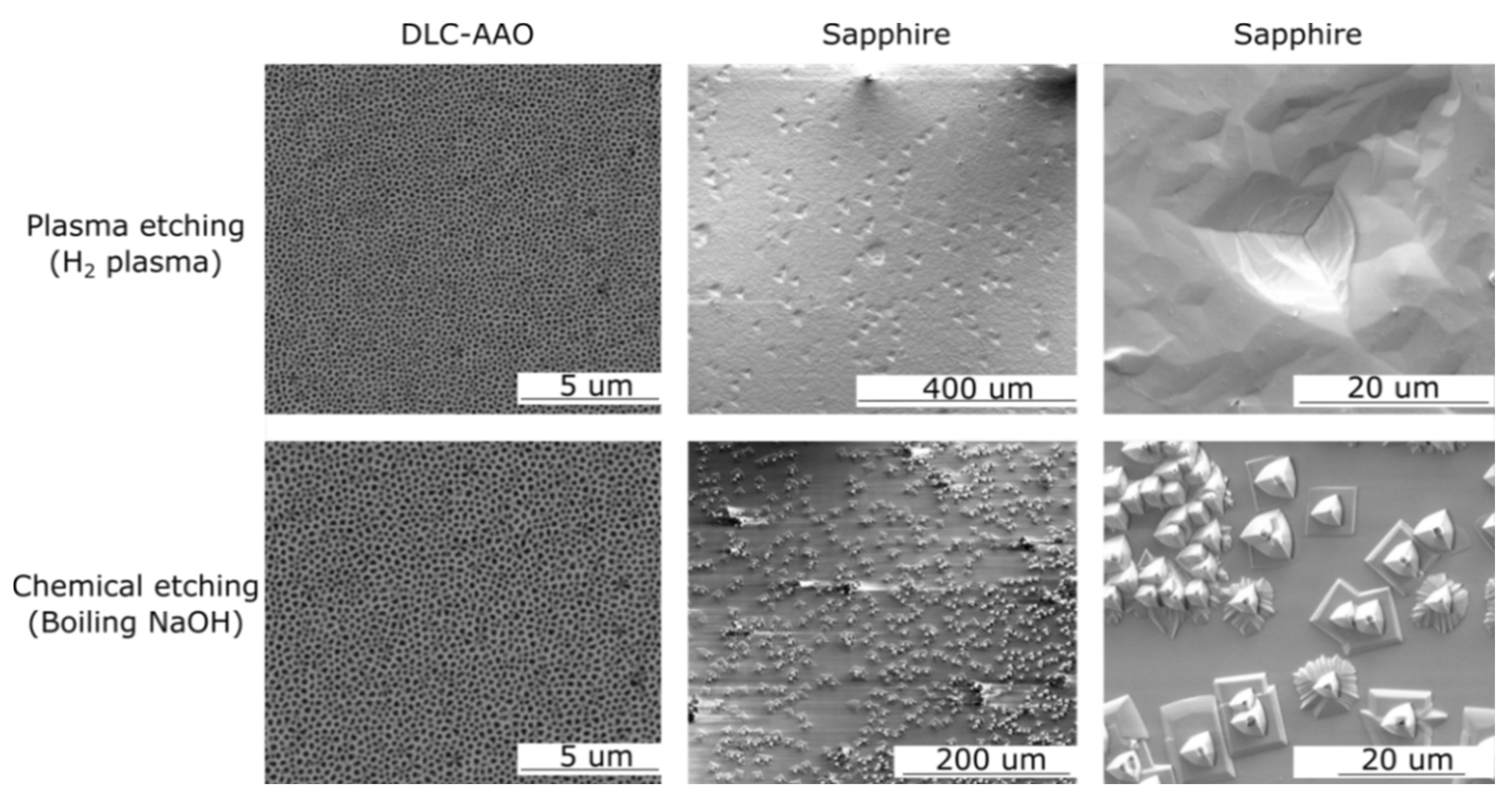
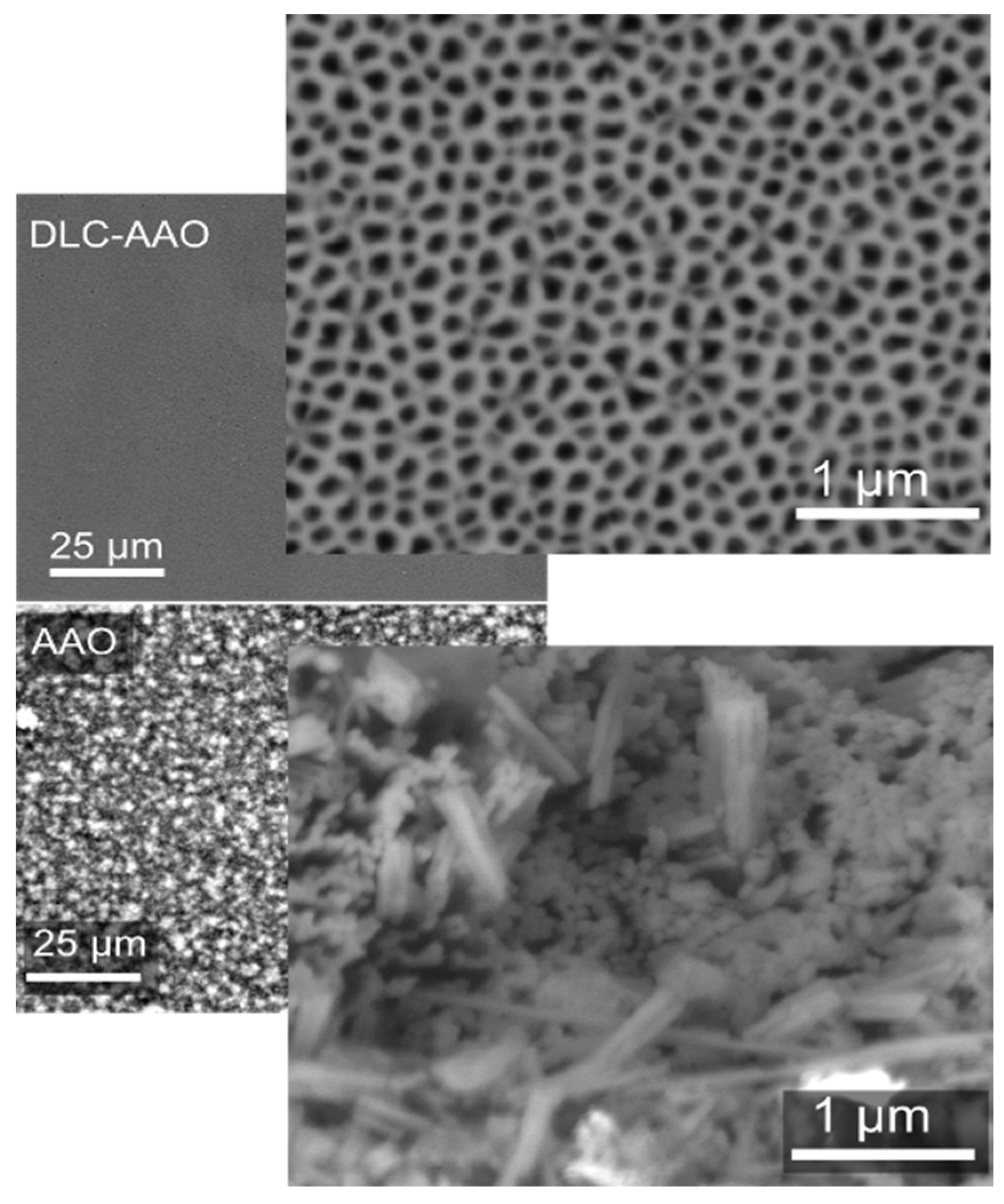
2.2. Chemical Stability
| Chemical | Time | pH | T (°C) | AAO | DLC-AAO | Sapphire | Diamond |
|---|---|---|---|---|---|---|---|
| Saturated Potassium/Sodium Hydroxide (KOH/NaOH) | 24 h | 14 | 25 | etched ** | resistant ** | resistant | resistant |
| Saturated Potassium/Sodium Hydroxide (KOH/NaOH) | 2 h | 14 | 80 | etched | resistant | damaged | resistant |
| Phosphoric Acid (10% vol.) | 12 h | 4 | 60 | etched | resistant | resistant | resistant |
| Perchloric acid (HClO4 25% vol.) | 1 h | 1 | 25 | etched | resistant | resistant | resistant |
| Hydrofluoric Acid (HF 40% vol.) | 72 h | 3.5 | 25 | etched | resistant | damaged ** | resistant |
| Sulfuric Acid and Sodium Nitrate (1 mL H2SO4 + 0.25 mg NaNO3) | 1 h | 1 | 200 | etched | resistant | damaged | resistant |
| Accelerated aging * (Saline buffer) | 6 months in vivo | 5.5 | 80 | damaged | resistant | resistant | resistant |

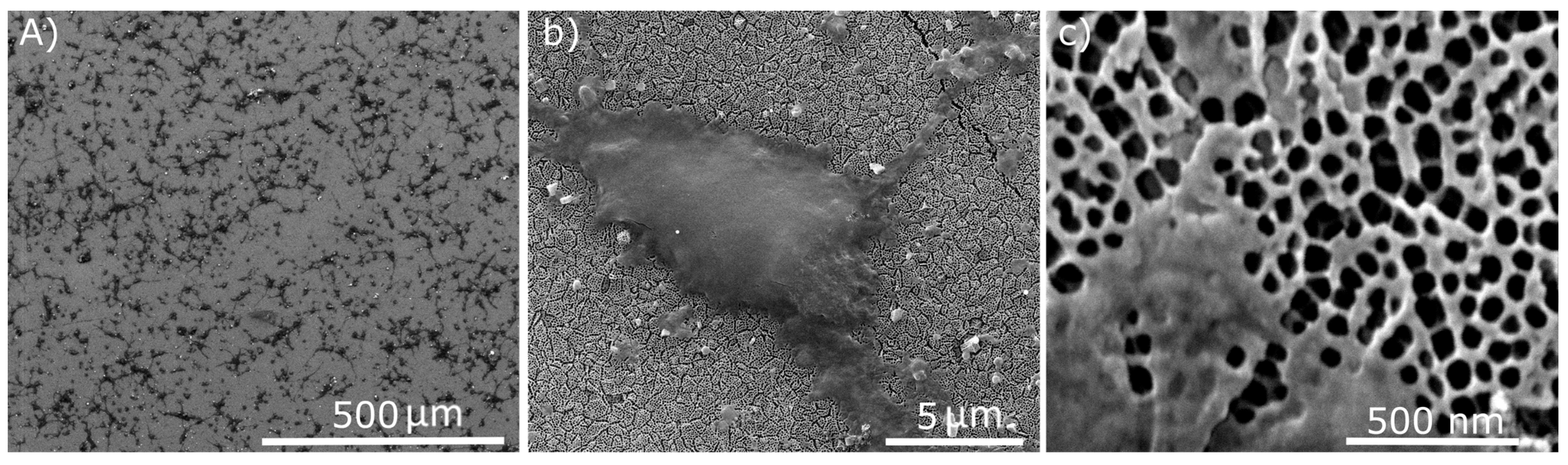
2.3. Cytotoxicity


3. Experimental Section
3.1. DLC-AAO Fabrication
3.2. Electrical Conductivity Measurement
3.3. Chemical Tests
3.4. XPS
3.5. Non-Cytotoxicity Test
3.6. Neuron Growth
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clem, W.C.; Chowdhury, S.; Catledge, S.A.; Weimer, J.J.; Shaikh, F.M.; Hennessy, K.M.; Konovalov, V.V.; Hill, M.R.; Waterfeld, A.; Bellis, S.L. Mesenchymal stem cell interaction with ultra-smooth nanostructured diamond for wear-resistant orthopaedic implants. Biomaterials 2008, 29, 3461–3468. [Google Scholar] [CrossRef] [PubMed]
- Grill, A. Diamond-like carbon coatings as biocompatible materials—An overview. Diamond Relat. Mater. 2003, 12, 166–170. [Google Scholar] [CrossRef]
- Love, C.; Cook, R.B.; Harvey, T.; Dearnley, P.; Wood, R. Diamond like carbon coatings for potential application in biological implants—A review. Tribol. Int. 2013, 63, 141–150. [Google Scholar] [CrossRef]
- Roy, R.K.; Lee, K.R. Biomedical applications of diamond-like carbon coatings: A review. J. Biomed. Mater. Res. Part B 2007, 83, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.A.; Law, F.C.; Rushton, N.; Franks, J. Biocompatibility of diamond-like carbon coating. Biomaterials 1991, 12, 37–40. [Google Scholar] [CrossRef]
- Aramesh, M.; Shimoni, O.; Ostrikov, K.; Prawer, S.; Cervenka, J. Surface charge effects in protein adsorption on nanodiamonds. Nanoscale 2015, 7, 5726–5736. [Google Scholar] [CrossRef] [PubMed]
- Miksovsky, J.; Voss, A.; Kozarova, R.; Kocourek, T.; Pisarik, P.; Ceccone, G.; Kulisch, W.; Jelinek, M.; Apostolova, M.; Reithmaier, J. Cell adhesion and growth on ultrananocrystalline diamond and diamond-like carbon films after different surface modifications. Appl. Surf. Sci. 2014, 297, 95–102. [Google Scholar] [CrossRef]
- Shi, B.; Jin, Q.; Chen, L.; Woods, A.S.; Schultz, A.J.; Auciello, O. Cell growth on different types of ultrananocrystalline diamond thin films. J. Funct. Biomater. 2012, 3, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Fox, K.; Ganesan, K.; Turnley, A.M.; Shimoni, O.; Tran, P.A.; Lohrmann, A.; McFarlane, T.; Ahnood, A.; Garrett, D.J.; et al. Fabrication of planarised conductively patterned diamond for bio-applications. Mater. Sci. Eng. C 2014, 43, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lee, D.C.; Hsiao, C.Y.; Chung, Y.F.; Chen, H.C.; Thomas, J.P.; Pong, W.F.; Tai, N.H.; Lin, I.N.; Chiu, I.M. The effect of ultra-nanocrystalline diamond films on the proliferation and differentiation of neural stem cells. Biomaterials 2009, 30, 3428–3435. [Google Scholar] [CrossRef] [PubMed]
- Härtl, A.; Schmich, E.; Garrido, J.A.; Hernando, J.; Catharino, S.C.; Walter, S.; Feulner, P.; Kromka, A.; Steinmüller, D.; Stutzmann, M. Protein-modified nanocrystalline diamond thin films for biosensor applications. Nat. Mater. 2004, 3, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Tachiki, M.; Kaibara, Y.; Sumikawa, Y.; Shigeno, M.; Banno, T.; Song, K.S.; Umezawa, H.; Kawarada, H. Diamond nanofabrication and characterization for biosensing application. Phys. Status Solidi A 2003, 199, 39–43. [Google Scholar] [CrossRef]
- Luo, D.; Wu, L.; Zhi, J. Fabrication of boron-doped diamond nanorod forest electrodes and their application in nonenzymatic amperometric glucose biosensing. ACS Nano 2009, 3, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Aramesh, M.; Shimoni, O.; Fox, K.; Karle, T.; Lohrmann, A.; Ostrikov, K.; Prawer, S.; Cervenka, J. Ultra-high-density 3D DNA arrays within nanoporous biocompatible membranes for single-molecule-level detection and purification of circulating nucleic acids. Nanoscale 2015, 7, 5998–6006. [Google Scholar] [CrossRef] [PubMed]
- Aramesh, M.; Cervenka, J.; Roberts, A.; Djalalian-Assl, A.; Rajasekharan, R.; Fang, J.; Ostrikov, K.; Prawer, S. Coupling of a single-photon emitter in nanodiamond to surface plasmons of a nanochannel-enclosed silver nanowire. Opt. Express 2014, 22, 15530–15541. [Google Scholar] [CrossRef] [PubMed]
- Aramesh, M.; Djalalian-Assl, A.; Cervenka, J.; Roberts, A.; Ostrikov, K.; Prawer, S. Coupling of a quantum emitter to the surface plasmons of a nanowire. In Proceedings of the 2014 IEEE Conference on Optoelectronic and Microelectronic Materials & Devices (COMMAD 2014), Perth, Australia, 14–17 December 2014; pp. 238–241.
- Allen, M.; Myer, B.; Rushton, N. In vitro and in vivo investigations into the biocompatibility of diamond-like carbon (dlc) coatings for orthopedic applications. J. Biomed. Mater. Res. 2001, 58, 319–328. [Google Scholar] [CrossRef]
- Ahnood, A.; Escudie, M.; Cicione, R.; Abeyrathne, C.; Ganesan, K.; Fox, K.; Garrett, D.; Stacey, A.; Apollo, N.; Lichter, S. Ultrananocrystalline diamond-cmos device integration route for high acuity retinal prostheses. Biomed. Microdevices 2015, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Garrett, D.J.; Ahnood, A.; Shivdasani, M.N.; Tong, W.; Turnley, A.M.; Fox, K.; Meffin, H.; Prawer, S. An all-diamond, hermetic electrical feedthrough array for a retinal prosthesis. Biomaterials 2014, 35, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Garrett, D.J.; Ganesan, K.; Stacey, A.; Fox, K.; Meffin, H.; Prawer, S. Ultra-nanocrystalline diamond electrodes: Optimization towards neural stimulation applications. J. Neural. Eng. 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Garrett, D.J.; Saunders, A.L.; McGowan, C.; Specks, J.; Ganesan, K.; Meffin, H.; Williams, R.A.; Nayagam, D.A. In vivo biocompatibility of boron doped and nitrogen included conductive-diamond for use in medical implants. J. Biomed. Mater. Res. B 2015. [Google Scholar] [CrossRef]
- Friehs, G.M.; Zerris, V.A.; Ojakangas, C.L.; Fellows, M.R.; Donoghue, J.P. Brain–machine and brain–computer interfaces. Stroke 2004, 35, 2702–2705. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, M.A.; Nicolelis, M.A. Brain–machine interfaces: Past, present and future. Trends Neurosci. 2006, 29, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Adamschik, M.; Hinz, M.; Maier, C.; Schmid, P.; Seliger, H.; Hofer, E.; Kohn, E. Diamond micro system for bio-chemistry. Diamond Related Mater. 2001, 10, 722–730. [Google Scholar] [CrossRef]
- Moxon, K.; Kalkhoran, N.M.; Markert, M.; Sambito, M.; McKenzie, J.; Webster, J.T. Nanostructured surface modification of ceramic-based microelectrodes to enhance biocompatibility for a direct brain-machine interface. IEEE Trans. Biomed. Eng. 2004, 51, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Kotov, N.A.; Winter, J.O.; Clements, I.P.; Jan, E.; Timko, B.P.; Campidelli, S.; Pathak, S.; Mazzatenta, A.; Lieber, C.M.; Prato, M. Nanomaterials for neural interfaces. Adv. Mater. 2009, 21, 3970–4004. [Google Scholar] [CrossRef]
- Kim, M.-H.; Park, M.; Kang, K.; Choi, I.S. Neurons on nanometric topographies: Insights into neuronal behaviors in vitro. Biomater. Sci. 2014, 2, 148–155. [Google Scholar] [CrossRef]
- Seidlits, S.K.; Lee, J.Y.; Schmidt, C.E. Nanostructured scaffolds for neural applications. Nanomedicine 2008, 3, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, D. Nanoporous aluminium oxide membranes as cell interfaces. J. Nanomater. 2013, 2013, 1–18. [Google Scholar] [CrossRef]
- Ni, S.; Li, C.; Ni, S.; Chen, T.; Webster, T.J. Understanding improved osteoblast behavior on select nanoporous anodic alumina. Int. J. Nanomed. 2014, 9, 3325–3334. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Liu, H.Y.; Mamiya, P.C.; Ponery, A.S.; Babu, A.N.; Weik, T.; Schindler, M.; Meiners, S. Three-dimensional nanofibrillar surfaces covalently modified with tenascin-c-derived peptides enhance neuronal growth in vitro. J. Biomed. Mater. Res. A 2006, 76, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Sabri, F.; Cole, J.A.; Scarbrough, M.C.; Leventis, N. Investigation of polyurea-crosslinked silica aerogels as a neuronal scaffold: A pilot study. PLoS ONE 2012, 7, e33242. [Google Scholar] [CrossRef] [PubMed]
- Aramesh, M.; Fox, K.; Lau, D.W.; Fang, J.; Ostrikov, K.K.; Prawer, S.; Cervenka, J. Multifunctional three-dimensional nanodiamond-nanoporous alumina nanoarchitectures. Carbon 2014, 75, 452–464. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic alumina: A versatile platform for optical biosensors. Materials 2014, 7, 4297–4320. [Google Scholar] [CrossRef]
- Aramesh, M.; Cervenka, J. Surface modification of porous anodic alumina for medical and biological applications. In Nanomedicine; One Central Press (OCP): Manchester, UK, 2014; pp. 439–467. [Google Scholar]
- La Flamme, K.E.; Popat, K.C.; Leoni, L.; Markiewicz, E.; LaTempa, T.J.; Roman, B.B.; Grimes, C.A.; Desai, T.A. Biocompatibility of nanoporous alumina membranes for immunoisolation. Biomaterials 2007, 28, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Popat, K.C.; Mor, G.; Grimes, C.A.; Desai, T.A. Surface modification of nanoporous alumina surfaces with poly(ethylene glycol). Langmuir 2004, 20, 8035–8041. [Google Scholar] [CrossRef] [PubMed]
- Flamme, K.E.L.; Mor, G.; Gong, D.; Tempa, T.L.; Fusaro, V.A.; Grimes, C.A.; Desai, T.A. Nanoporous alumina capsules for cellular macroencapsulation: Transport and biocompatibility. Diabetes Technol. Ther. 2005, 7, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Santos, A.; Kaur, G.; Evdokiou, A.; Losic, D. Structurally engineered anodic alumina nanotubes as nano-carriers for delivery of anticancer therapeutics. Biomaterials 2014, 35, 5517–5526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kaur, G.; Zysk, A.; Liapis, V.; Hay, S.; Santos, A.; Losic, D.; Evdokiou, A. Systematic in vitro nanotoxicity study on anodic alumina nanotubes with engineered aspect ratio: Understanding nanotoxicity by a nanomaterial model. Biomaterials 2015, 46, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Hemmerich, K.J. General aging theory and simplified protocol for accelerated aging of medical devices. Med. Plastic Biomater. 1998, 5, 16–23. [Google Scholar]
- Tang, L.; Tsai, C.; Gerberich, W.W.; Kruckeberg, L.; Kania, D.R. Biocompatibility of chemical-vapour-deposited diamond. Biomaterials 1995, 16, 483–488. [Google Scholar] [CrossRef]
- Cho, W.K.; Kang, K.; Kang, G.; Jang, M.J.; Nam, Y.; Choi, I.S. Pitch-dependent acceleration of neurite outgrowth on nanostructured anodized aluminum oxide substrates. Angew. Chem. Int. Ed. Engl. 2010, 49, 10114–10118. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Choi, S.E.; Jang, H.S.; Cho, W.K.; Nam, Y.; Choi, I.S.; Lee, J.S. In vitro developmental acceleration of hippocampal neurons on nanostructures of self-assembled silica beads in filopodium-size ranges. Angew. Chem. Int. Ed. Engl. 2012, 51, 2855–2858. [Google Scholar] [CrossRef] [PubMed]
- Cyster, L.A.; Parker, K.G.; Parker, T.L.; Grant, D.M. The effect of surface chemistry and nanotopography of titanium nitride (tin) films on primary hippocampal neurones. Biomaterials 2004, 25, 97–107. [Google Scholar] [CrossRef]
- Gentile, F.; Tirinato, L.; Battista, E.; Causa, F.; Liberale, C.; di Fabrizio, E.M.; Decuzzi, P. Cells preferentially grow on rough substrates. Biomaterials 2010, 31, 7205–7212. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Regan, E.M.; Uney, J.B.; Dick, A.D.; McGeehan, J.P.; Bristol Biochip, G.; Mayer, E.J.; Claeyssens, F. Patterned growth of neuronal cells on modified diamond-like carbon substrates. Biomaterials 2008, 29, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Sapelkin, A.V.; Bayliss, S.C.; Unal, B.; Charalambou, A. Interaction of B50 rat hippocampal cells with stain-etched porous silicon. Biomaterials 2006, 27, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Ariano, P.; Budnyk, O.; Dalmazzo, S.; Lovisolo, D.; Manfredotti, C.; Rivolo, P.; Vittone, E. On diamond surface properties and interactions with neurons. Eur. Phys. J. E Soft Matter 2009, 30, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Bruder, J.M.; Lee, A.P.; Hoffman-Kim, D. Biomimetic materials replicating schwann cell topography enhance neuronal adhesion and neurite alignment in vitro. J. Biomater. Sci. Polym. Ed. 2007, 18, 967–982. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Newaz, G. A comprehensive review of surface modification for neural cell adhesion and patterning. J. Biomed. Mater. Res. A 2010, 93, 1209–1224. [Google Scholar] [CrossRef] [PubMed]
- Edgington, R.J.; Thalhammer, A.; Welch, J.O.; Bongrain, A.; Bergonzo, P.; Scorsone, E.; Jackman, R.B.; Schoepfer, R. Patterned neuronal networks using nanodiamonds and the effect of varying nanodiamond properties on neuronal adhesion and outgrowth. J. Neural. Eng. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Jiang, X. Micro/nano-scale materials and structures for constructing neuronal networks and addressing neurons. J. Mater. Chem. C 2013, 1, 7652–7662. [Google Scholar] [CrossRef]
- Lord, M.S.; Foss, M.; Besenbacher, F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010, 5, 66–78. [Google Scholar] [CrossRef]
- Gabay, T.; Jakobs, E.; Ben-Jacob, E.; Hanein, Y. Engineered self-organization of neural networks using carbon nanotube clusters. Physica A 2005, 350, 611–621. [Google Scholar] [CrossRef]
- Guan, Y.; Li, M.; Dong, K.; Ren, J.; Qu, X. Nir-responsive upconversion nanoparticles stimulate neurite outgrowth in PC12 cells. Small 2014, 10, 3655–3661. [Google Scholar] [CrossRef] [PubMed]
- Variola, F.; Vetrone, F.; Richert, L.; Jedrzejowski, P.; Yi, J.-H.; Zalzal, S.; Clair, S.; Sarkissian, A.; Perepichka, D.F.; Wuest, J.D.; et al. Improving biocompatibility of implantable metals by nanoscale modification of surfaces: An overview of strategies, fabrication methods, and challenges. Small 2009, 5, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Mogilner, A.; Rubinstein, B. The physics of filopodial protrusion. Biophys. J. 2005, 89, 782–795. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aramesh, M.; Tong, W.; Fox, K.; Turnley, A.; Seo, D.H.; Prawer, S.; Ostrikov, K. Nanocarbon-Coated Porous Anodic Alumina for Bionic Devices. Materials 2015, 8, 4992-5006. https://doi.org/10.3390/ma8084992
Aramesh M, Tong W, Fox K, Turnley A, Seo DH, Prawer S, Ostrikov K. Nanocarbon-Coated Porous Anodic Alumina for Bionic Devices. Materials. 2015; 8(8):4992-5006. https://doi.org/10.3390/ma8084992
Chicago/Turabian StyleAramesh, Morteza, Wei Tong, Kate Fox, Ann Turnley, Dong Han Seo, Steven Prawer, and Kostya (Ken) Ostrikov. 2015. "Nanocarbon-Coated Porous Anodic Alumina for Bionic Devices" Materials 8, no. 8: 4992-5006. https://doi.org/10.3390/ma8084992
APA StyleAramesh, M., Tong, W., Fox, K., Turnley, A., Seo, D. H., Prawer, S., & Ostrikov, K. (2015). Nanocarbon-Coated Porous Anodic Alumina for Bionic Devices. Materials, 8(8), 4992-5006. https://doi.org/10.3390/ma8084992