Constructing Biopolymer-Inorganic Nanocomposite through a Biomimetic Mineralization Process for Enzyme Immobilization
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Catalyzing Function of NH2-Alginate
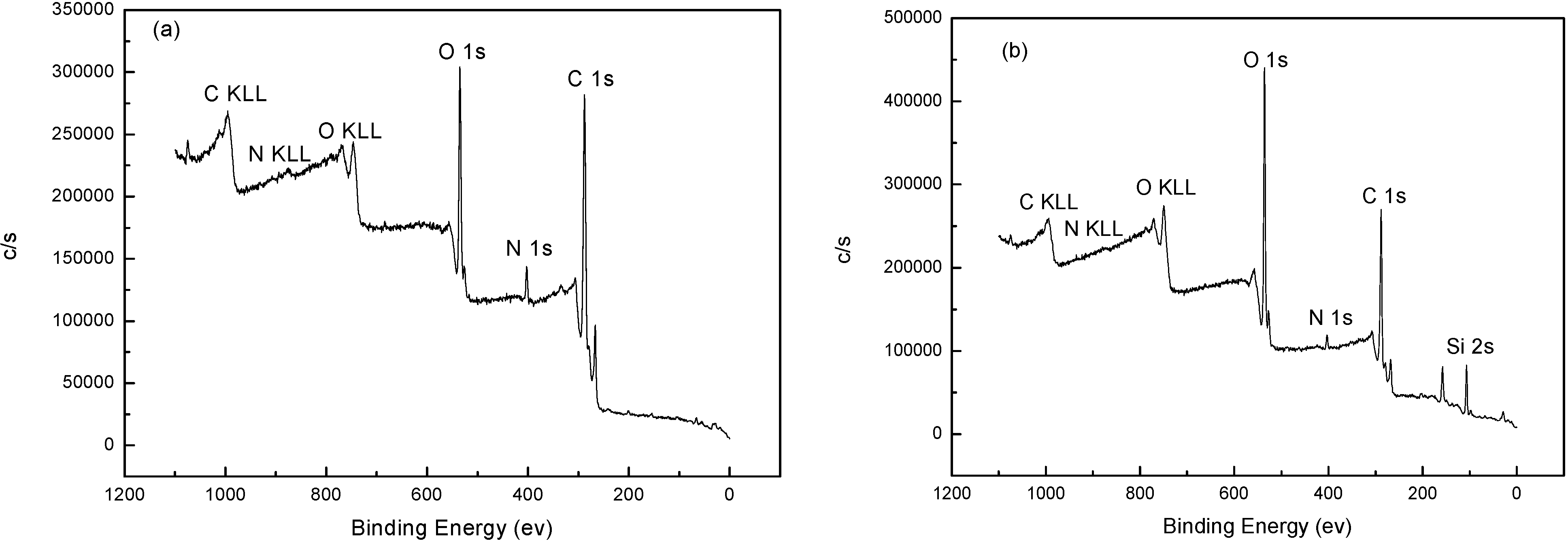

2.2. The Template Function of NH2-Alginate
| No. | [IO4−]/[alginate unit] | Amination Agent | Diameter (nm) |
|---|---|---|---|
| 1# | 1 | 1,2-ethylenediamine | 216.1 |
| 2# | 1 | Diethylenetriamine | 173.6 |
| 3# | 1 | Spermine | 145.0 |
| 4# | 0.25 | 1,2-ethylenediamine | 404.9 |
| 5# | 0.25 | Diethylenetriamine | 344.5 |
| 6# | 0.25 | Spermine | 331.9 |
| 7# | 0.1 | 1,2-ethylenediamine | 1535 |
| 8# | 0.1 | Diethylenetriamine | 1160 |
| 9# | 0.1 | Spermine | 598.7 |
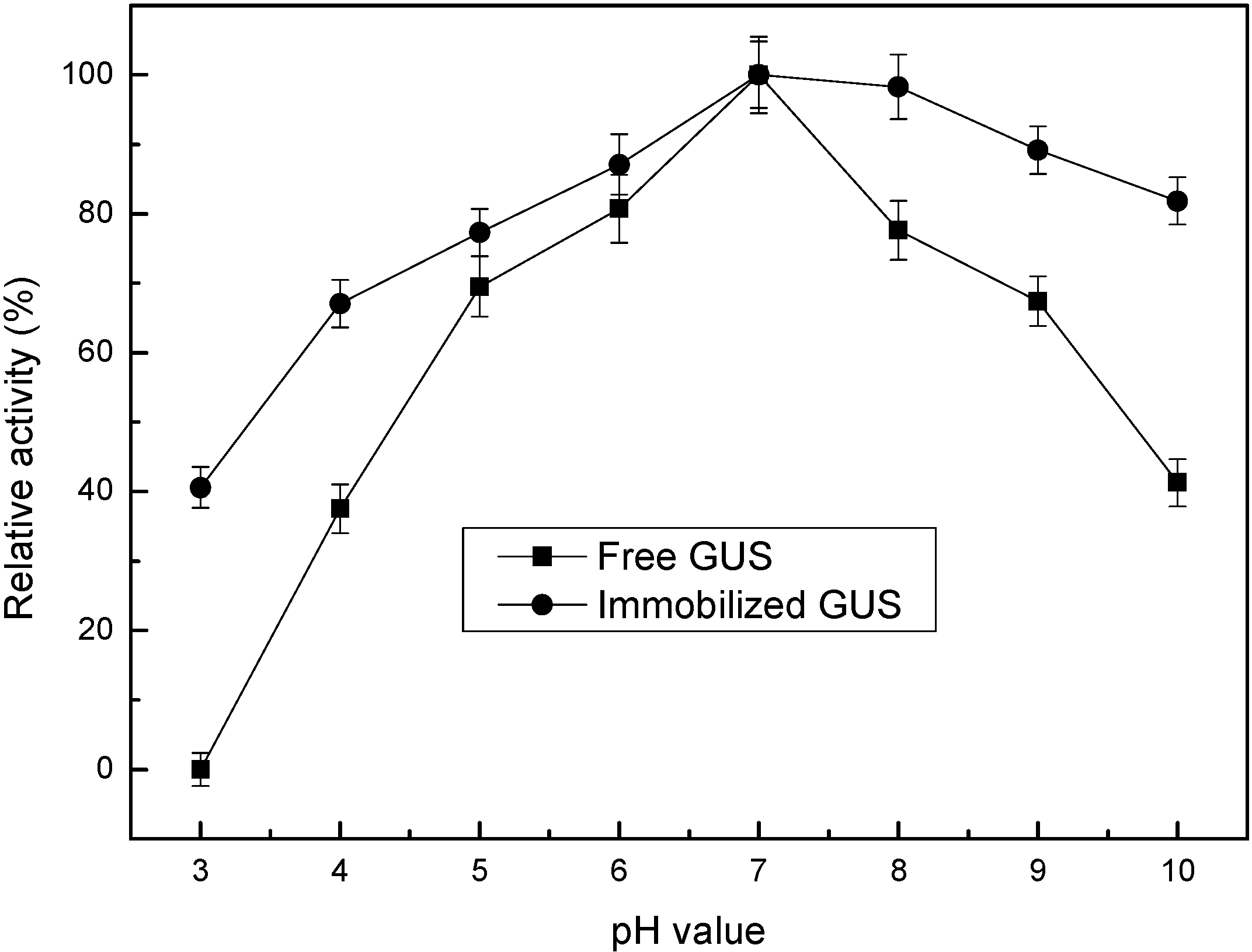
2.3. Enzyme Activity Assay


| GUS | Km (mM) | Vmax (μmol/min·mg GUS) |
|---|---|---|
| free | 67.52 | 135.29 |
| immobilized | 69.33 | 91.57 |

3. Experimental Section
3.1. Materials
3.2. Synthesis of NH2-Alginate

3.2.1. Oxidation
3.2.2. Amination
3.2.3. Reduction
3.3. Preparation of NH2-Alginate/Silica Nanocomposite and Encapsulation of GUS
3.4. Characterizations
3.5. Enzyme Activity Assay
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, J.E.; Lee, N.; Kim, T.; Kim, J.; Hyeon, T. Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc. Chem. Res. 2011, 44, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Beland, F.; Ilharco, L.M.; Pagliaro, M. The sol-gel route to advanced silica-based materials and recent applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef] [PubMed]
- Ab Rahman, I.; Padavettan, V. Synthesis of silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—A review. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Bounor-Legare, V.; Cassagnau, P. In situ synthesis of organic-inorganic hybrids or nanocomposites from sol-gel chemistry in molten polymers. Prog. Polym. Sci. 2014, 39, 1473–1497. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Aranda, P.; Ariga, K. Advances in biomimetic and nanostructured biohybrid materials. Adv. Mater. 2010, 22, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Cai, Q.W.; Du, M.X.; Cao, M.W.; Xu, H. Biomimetic mineralization of silica. Prog. Chem. 2015, 27, 229–241. [Google Scholar]
- Cha, J.N.; Shimizu, K.; Zhou, Y.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D.; Morse, D.E. Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Kroger, N.; Deutzmann, R.; Sumper, M. Polycationic peptides from diatom biosilica that direct silica nanosphere formation. Science 1999, 286, 1129–1132. [Google Scholar] [PubMed]
- Liu, X.L.; Zhu, P.X.; Gao, Y.F.; Jin, R.H. Polyamine-promoted growth of one-dimensional nanostructure-based silica and its feature in catalyst design. Materials 2012, 5, 1787–1799. [Google Scholar] [CrossRef]
- Shiomi, T.; Tsunoda, T.; Kawai, A.; Mizukami, F.; Sakaguchi, K. Biomimetic synthesis of lysozyme-silica hybrid hollow particles using sonochemical treatment: Influence of pH and lysozyme concentration on morphology. Chem. Mater. 2007, 19, 4486–4493. [Google Scholar] [CrossRef]
- Leng, B.X.; Chen, X.; Shao, Z.Z.; Ming, W.H. Biomimetic synthesis of silica with chitosan-mediated morphology. Small 2008, 4, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, Y.; Kugimiya, S.; Nakamura, H.; Kato, K. Enzyme encapsulation in silica gel prepared by polylysine and its catalytic activity. Appl. Surf. Sci. 2014, 314, 64–70. [Google Scholar] [CrossRef]
- Dolatabadi, J.E.N.; de la Guardia, M. Applications of diatoms and silica nanotechnology in biosensing, drug and gene delivery, and formation of complex metal nanostructures. Trac. Trend. Anal. Chem. 2011, 30, 1538–1548. [Google Scholar] [CrossRef]
- Patwardhan, S.V. Biomimetic and bioinspired silica: Recent developments and applications. Chem. Commun. 2011, 47, 7567–7582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Li, J.; Li, L.; Jiang, Y.; Jiang, Y.; Jiang, Z. Protamine-templated biomimetic hybrid capsules: Efficient and stable carrier for enzyme encapsulation. Chem. Mater. 2007, 20, 1041–1048. [Google Scholar]
- Jiang, Y.; Yang, D.; Zhang, L.; Sun, Q.; Zhang, Y.; Li, J.; Jiang, Z. Biomimetic synthesis of titania nanoparticles induced by protamine. Dalton Trans. 2008, 31, 4165–4171. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.M.; Zhou, Y.; Yang, W.J.; Morse, D.E. Bifunctional small molecules are biomimetic catalysts for silica synthesis at neutral pH. J. Am. Chem. Soc. 2005, 127, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Sumper, M. A phase separation model for the nanopatterning of diatom biosilica. Science 2002, 295, 2430–2433. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.M.; Coradin, T.; Aime, C. Self-assembly in biosilicification and biotemplated silica materials. Nanomaterials 2014, 4, 792–812. [Google Scholar] [CrossRef]
- Poulsen, N.; Sumper, M.; Kroger, N. Biosilica formation in diatoms: Characterization of native silaffin-2 and its role in silica morphogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 12075–12080. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Lutz, K.; Sumper, M. Biomimetic synthesis of silica nanospheres depends on the aggregation and phase separation of polyamines in aqueous solution. Phys. Chem. Chem. Phys. 2004, 6, 854–857. [Google Scholar] [CrossRef]
- Lutz, K.; Groger, C.; Sumper, M.; Brunner, E. Biomimetic silica formation: Analysis of the phosphate-induced self-assembly of polyamines. Phys. Chem. Chem. Phys. 2005, 7, 2812–2815. [Google Scholar] [CrossRef] [PubMed]
- Kroger, N.; Deutzmann, R.; Sumper, M. Silica-precipitating peptides from diatoms-the chemical structure of silaffin-1a from cylindrotheca fusiformis. J. Biol. Chem. 2001, 276, 26066–26070. [Google Scholar] [CrossRef] [PubMed]
- Kroger, N.; Lorenz, S.; Brunner, E.; Sumper, M. Self-assembly of highly phosphorylated silaffins and their function in biosilica morphogenesis. Science 2002, 298, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Groger, C.; Lutz, K.; Brunner, E. NMR studies of biomineralisation. Prog. Nucl. Magn. Reson. Spectrosc. 2009, 54, 54–68. [Google Scholar] [CrossRef]
- Yuan, J.J.; Jin, R.H. Temporally and spatially controlled silicification for self-generating polymer@silica hybrid nanotube on substrates with tunable film nanostructure. J. Mater. Chem. 2012, 22, 5080–5088. [Google Scholar] [CrossRef]
- Yuan, J.J.; Zhu, P.X.; Fukazawa, N.; Jin, R.H. Synthesis of nanofiber-based silica networks mediated by organized poly(ethylene imine): Structure, properties, and mechanism. Adv. Funct. Mater. 2006, 16, 2205–2212. [Google Scholar] [CrossRef]
- Shiu, C.C.; Wang, S.A.; Chang, C.H.; Jan, J.S. Poly(L-glutamic acid)-decorated hybrid colloidal particles from complex particle-templated silica mineralization. J. Phys. Chem. B 2013, 117, 10007–10016. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Hsieh, Y.H.; Jan, J.S. Polyelectrolyte complex-silica hybrid colloidal particles decorated with different polyelectrolytes. J. Colloid Interface Sci. 2015, 438, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Kaigler, D.; Kim, K.; Mooney, D.J. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules 2004, 5, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Boontheekul, T.; Kong, H.J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, S.V.; Clarson, S.J.; Perry, C.C. On the role(s) of additives in bioinspired silicification. Chem. Commun. 2005, 9, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, M.M.; Glawe, D.D.; Drummy, L.F.; Lawrence, C.G.; Stone, M.O.; Perry, C.C.; Pochan, D.J.; Deming, T.J.; Naik, R.R. Polypeptide-templated synthesis of hexagonal silica platelets. J. Am. Chem. Soc. 2005, 127, 12577–12582. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wei, B.; Zhang, X.T.; Li, C. Bifunctional graphene/gamma-Fe2O3 hybrid aerogels with double nanocrystalline networks for enzyme immobilization. Small 2013, 9, 2331–2340. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Z.; Wu, H.; Long, L.; Jiang, Y.; Zhang, L. Improving the recycling and storage stability of enzyme by encapsulation in mesoporous CaCO3-alginate composite gel. Compos. Sci. Technol. 2009, 69, 539–544. [Google Scholar] [CrossRef]
- Ravindra, R.; Shuang, Z.; Gies, H.; Winter, R. Protein encapsulation in mesoporous silicate: The effects of confinement on protein stability, hydration, and volumetric properties. J. Am. Chem. Soc. 2004, 126, 12224–12225. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Liang, Y.; Jiang, Z.; Jiang, Y.; Zhang, L. Facile fabrication of organic-inorganic hybrid beads by aminated alginate enabled gelation and biomimetic mineralization. J. Biomater. Sci. Polym. Ed. 2013, 24, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Song, X.K.; Wu, H.; Shi, J.F.; Wang, X.L.; Zhang, W.Y.; Ai, Q.H.; Jiang, Z.Y. Facile fabrication of organic-inorganic composite beads by gelatin induced biomimetic mineralization for yeast alcohol dehydrogenase encapsulation. J. Mol. Catal. B. Enzym. 2014, 100, 49–58. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z.; Wu, H.; Zhang, L.; Long, L.; Jiang, Y. Constructing inorganic shell onto LBL microcapsule through biomimetic mineralization: A novel and facile method for fabrication of microbioreactors. Soft Matter 2010, 6, 542–550. [Google Scholar] [CrossRef]
- Lai, J.K.; Chuang, T.H.; Jan, J.S.; Wang, S.S.S. Efficient and stable enzyme immobilization in a block copolypeptide vesicle-templated biomimetic silica support. Colloid Surf. B 2010, 80, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Luckarift, H.R.; Spain, J.C.; Naik, R.R.; Stone, M.O. Enzyme immobilization in a biomimetic silica support. Nat. Biotechnol. 2004, 22, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.R.; Tomczak, M.M.; Luckarift, H.R.; Spain, J.C.; Stone, M.O. Entrapment of enzymes and nanoparticles using biomimetically synthesized silica. Chem. Commun. 2004, 15, 1684–1685. [Google Scholar] [CrossRef] [PubMed]
- Yudovin-Farber, I.; Azzam, T.; Metzer, E.; Taraboulos, A.; Domb, A.J. Cationic polysaccharides as antiprion agents. J. Med. Chem. 2005, 48, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, H.; Li, L.; Li, J.; Jiang, Z.; Jiang, Y.; Chen, Y. Enzymatic conversion of baicalin into baicalein by β-glucuronidase encapsulated in biomimetic core-shell structured hybrid capsules. J. Mol. Catal. B Enzym. 2009, 57, 130–135. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ma, J.; Jiang, T.; Wang, Y.; Wen, X.; Li, G. Constructing Biopolymer-Inorganic Nanocomposite through a Biomimetic Mineralization Process for Enzyme Immobilization. Materials 2015, 8, 6004-6017. https://doi.org/10.3390/ma8095286
Li J, Ma J, Jiang T, Wang Y, Wen X, Li G. Constructing Biopolymer-Inorganic Nanocomposite through a Biomimetic Mineralization Process for Enzyme Immobilization. Materials. 2015; 8(9):6004-6017. https://doi.org/10.3390/ma8095286
Chicago/Turabian StyleLi, Jian, Jun Ma, Tao Jiang, Yanhuan Wang, Xuemei Wen, and Guozhu Li. 2015. "Constructing Biopolymer-Inorganic Nanocomposite through a Biomimetic Mineralization Process for Enzyme Immobilization" Materials 8, no. 9: 6004-6017. https://doi.org/10.3390/ma8095286





