Poly-γ-Glutamic Acid: Biodegradable Polymer for Potential Protection of Beneficial Viruses
Abstract
:1. Introduction
2. Results
2.1. Identification of γ-PGA


2.2. Effect of Temperature on Formulated and Non-Formulated Phage

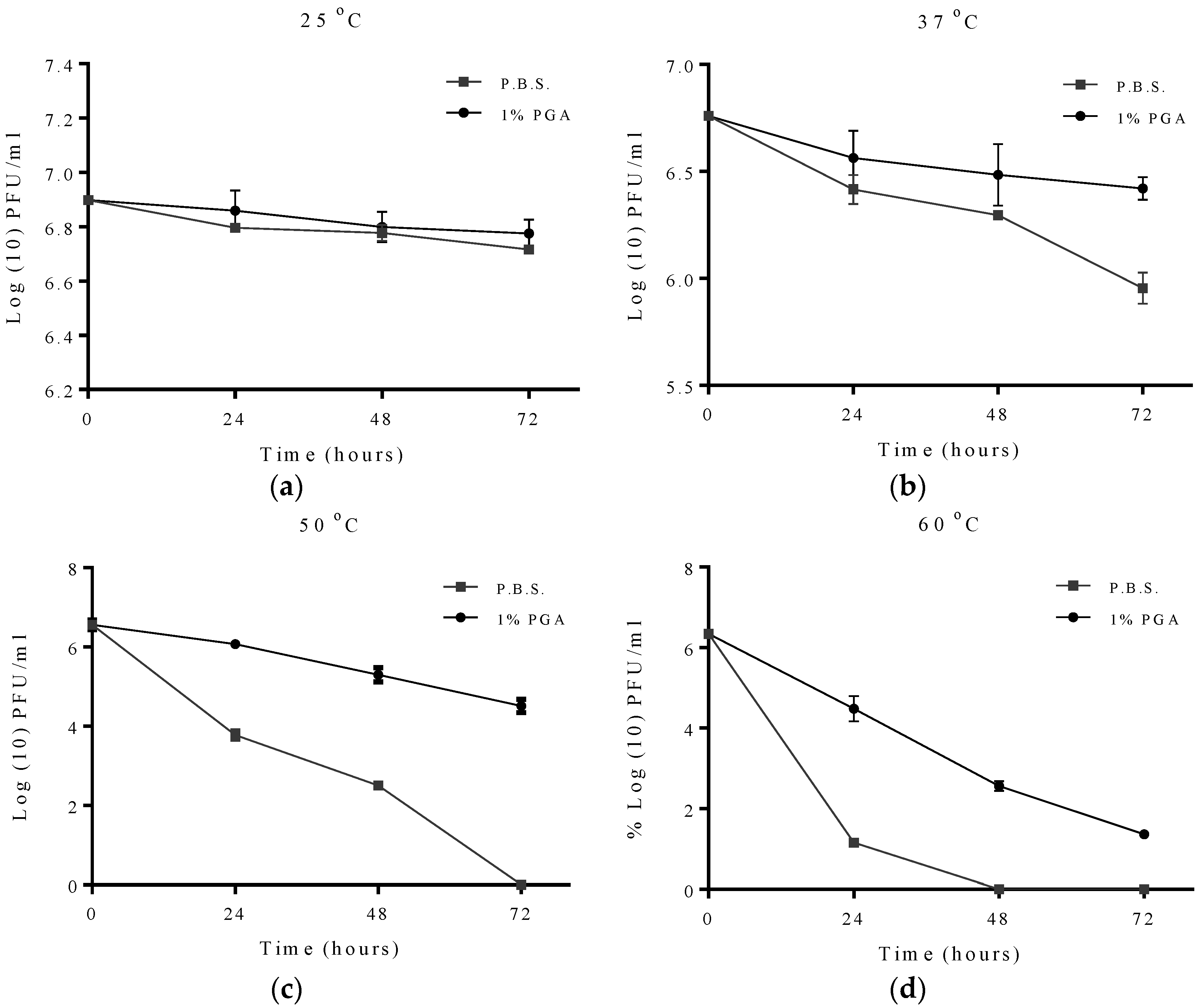

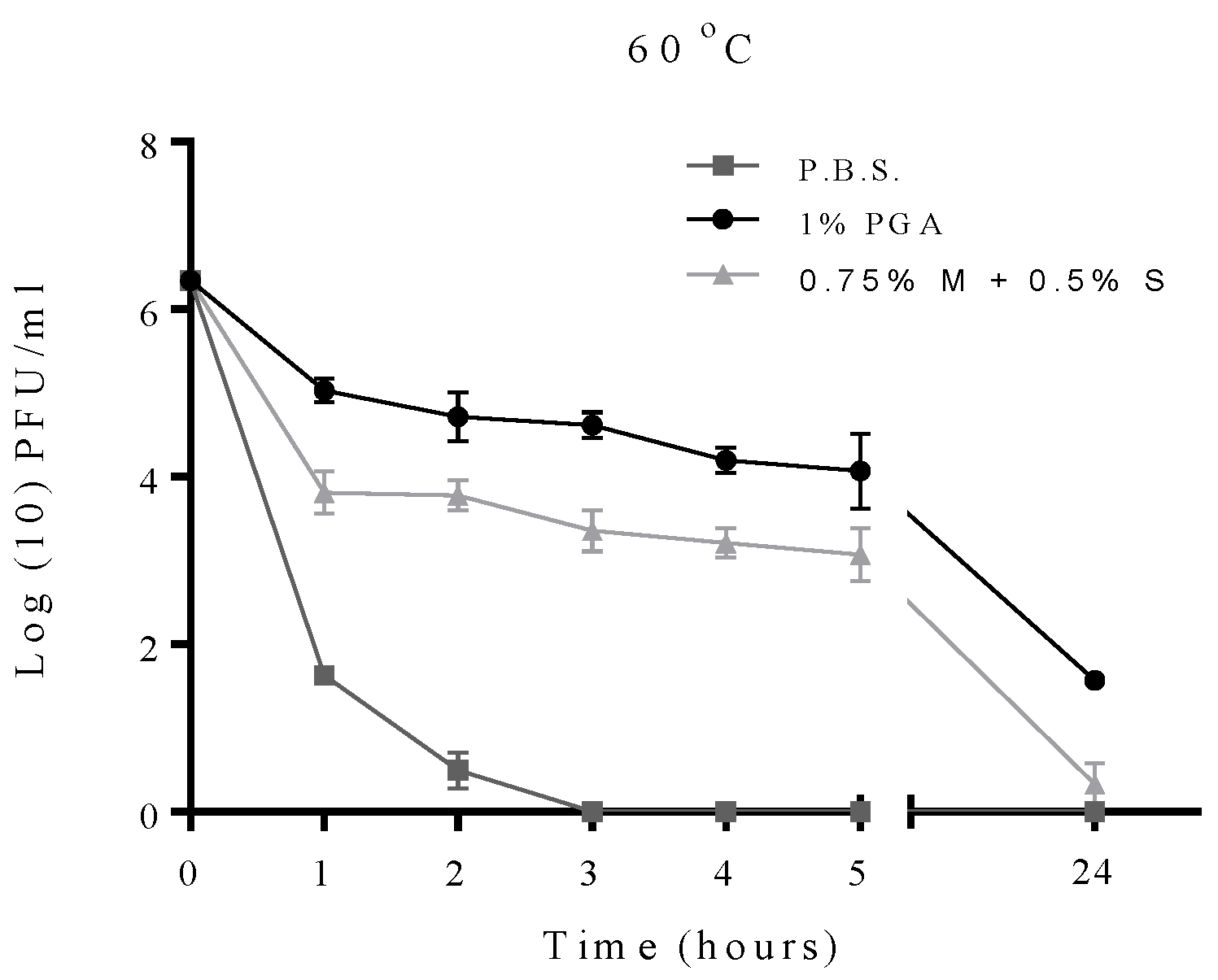
2.3. Effects of pH on Formulated and Non-Formulated Phage

2.4. Effect of UV Irradiation on Formulated and Non-Formulated Phage

2.5. Formulated and Non-formulated Phage Microscopy
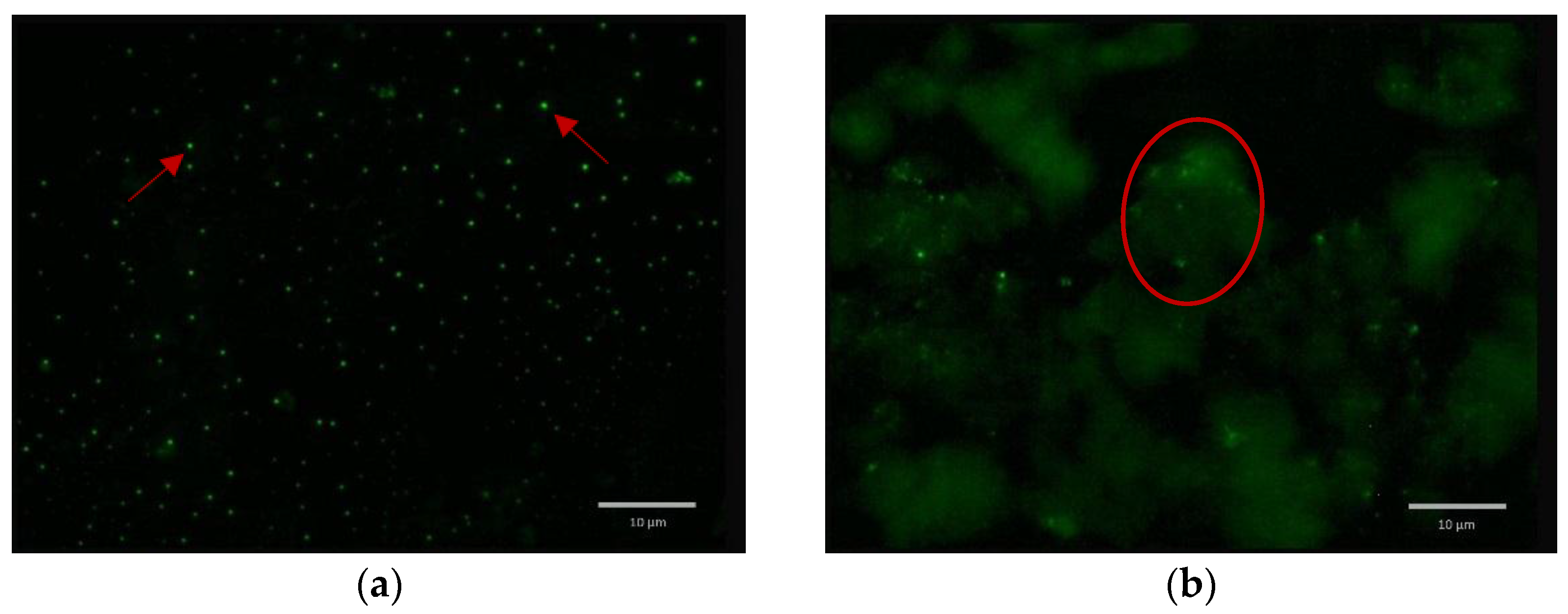
3. Discussion
4. Material and Methods
4.1. γ-PGA Production, Purification and Characterization
4.2. Bacterial Strains and Phages
4.3. Phage Amplification and Titration
4.4. Phage and γ-PGA Formulation
4.5. Effect of Elevated Temperature
4.6. Effect of UV Irradiation on Phage Viability
4.7. Effect of Different pH Values on Phage Longevity
4.8. Fluorescence Microscopy with SYBR 1 to Determine γ-PGA-Phage Interaction
4.9. Survival Experiments and Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ogunleye, A.; Bhat, A.; Irorere, V.U.; Hill, D.; Williams, C.; Radecka, I. Poly-γ-glutamic acid: Production, properties and applications. Microbiology 2015, 161, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.L.; Van, Y.T. The production of poly-(gamma-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 2001, 79, 207–225. [Google Scholar] [CrossRef]
- Ashiuchi, M. Microbial production and chemical transformation of poly-γ-glutamate. Microb. Biotechnol. 2013, 6, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Manocha, B.; Margaritis, A. Controlled release of doxorubicin from doxorubicin/-polyglutamic acid ionic complex. J. Nanomater. 2010, 2010. [Google Scholar] [CrossRef]
- Sung, M.H.; Park, C.; Kim, C.J.; Poo, H.; Soda, K.; Ashiuchi, M. Natural and edible biopolymer poly-gamma-glutamic acid: Synthesis, production, and applications. Chem. Rec. 2005, 5, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Uto, T.; Akagi, T.; Akashi, M.; Baba, M. Poly(γ-glutamic acid) nanoparticles as an efficient antigen delivery and adjuvant system: Potential for an aids vaccine. J. Med. Virology 2008, 80, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Matsusaki, M.; Akashi, M. Pharmaceutical and medical applications of poly-gamma-glutamic acid. In Amino-Acid Homopolymers Occurring in Nature; Springer: Berlin, Germany, 2010; pp. 119–153. [Google Scholar]
- Akagi, T.; Kaneko, T.; Kida, T.; Akashi, M. Preparation and characterization of biodegradable nanoparticles based on poly (γ-glutamic acid) with l-phenylalanine as a protein carrier. J. Control. Release 2005, 108, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.R.; Irorere, V.U.; Bartlett, T.; Hill, D.; Kedia, G.; Charalampopoulos, D.; Nualkaekul, S.; Radecka, I. Improving survival of probiotic bacteria using bacterial poly-γ-glutamic acid. Int. J. Food Microbiol. 2015, 196, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Irorere, V.; Bartlett, T.; Hill, D.; Kedia, G.; Morris, M.; Charalampopoulos, D.; Radecka, I. Bacillus subtilis natto: A non-toxic source of poly-γ-glutamic acid that could be used as a cryoprotectant for probiotic bacteria. AMB Expr. 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, N.M.; Parisien, A.; Lan, C.Q. Production and application of bacteriophage and bacteriophage-encoded lysins. Recent Pat. Biotechnol. 2009, 3, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Jassim, S.A.; Limoges, R.G. Natural solution to antibiotic resistance: Bacteriophages “the living drugs”. World J. Microbiol. Biotechnol. 2014, 30, 2153–2170. [Google Scholar] [CrossRef] [PubMed]
- Sillankorva, S.; Neubauer, P.; Azeredo, J. Isolation and characterization of a t7-like lytic phage for pseudomonas fluorescens. BMC Biotechnol. 2008, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tanji, Y.; Shimada, T.; Yoichi, M.; Miyanaga, K.; Hori, K.; Unno, H. Toward rational control of escherichia coli o157: H7 by a phage cocktail. Appl. Microbiol. Biotechnol. 2004, 64, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Knecht, H.J.; Kudva, I.T.; Hovde, C.J. Application of bacteriophages to control intestinal escherichia coli o157: H7 levels in ruminants. Appl. Environ. Microbiol. 2006, 72, 5359–5366. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M. Bacteriophages: Beyond antibiotics. US Pharmacist 2008, 33, 46–51. [Google Scholar]
- Viazis, S.; Akhtar, M.; Feirtag, J.; Diez-Gonzalez, F. Reduction of escherichia coli o157: H7 viability on hard surfaces by treatment with a bacteriophage mixture. Int. J. Food Microbiol. 2011, 145, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [PubMed]
- Stone, R. Bacteriophage therapy. Stalin's forgotten cure. Science 2002, 298, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Jonczyk, E.; Klak, M.; Miedzybrodzki, R.; Gorski, A. The influence of external factors on bacteriophages—Review. Folia Microbiol Praha 2011, 56, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.; Rahmanian, R.; Tam, H.; Zabetian, S. Effects of microwave irradiation and heat on t4 bacteriophage inactivation. J. Exp. Microbiol. Immunol. 2007, 11, 66–72. [Google Scholar]
- Baines, B. Comparison of the effects of microwave irradiation and heat treatment of t4 and t7 bacteriophage. J. Exp. Microbiol. Immunol. 2005, 7, 57–61. [Google Scholar]
- Iriarte, F.; Balogh, B.; Momol, M.; Smith, L.; Wilson, M.; Jones, J. Factors affecting survival of bacteriophage on tomato leaf surfaces. Appl. Environ. Microbiol. 2007, 73, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Kimura, T.; Hirofuji, Y.S.; Matsunaga, K.; Imayoshi, R.; Nagao, J.; Cho, T.; Matsumoto, H.; Ohtono, S.; Ohno, J.; et al. A poly(gamma-glutamic acid)-amphiphile complex as a novel nanovehicle for drug delivery system. J. Drug Target. 2010, 18, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Karmaker, S.; Saha, T.K.; Yoshikawa, Y.; Yasui, H.; Sakurai, H. A novel drug delivery system for type 1 diabetes: Insulin-mimetic vanadyl-poly(gamma-glutamic acid) complex. J. Inorg. Biochem. 2006, 100, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, K.; Raut, M.P.; Chandekar, R.H.; Sanmukh, S.G.; Paunikar, W.N. Novel bacteriophage therapy for controlling metallo-beta-lactamase producing pseudomonas aeruginosa infection in catfish. BMC Veterinary Res. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, J.J. Bacteriophages as model organisms for virus emergence research. Trends Microbiol. 2009, 17, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Grabow, W. Bacteriophages: Update on application as models for viruses in water. Water SA 2004, 27, 251–268. [Google Scholar] [CrossRef]
- Wick, C.H.; Elashvili, I.; McCubbin, P.E.; Birenzvige, A. Determination of ms2 Bacteriophage Stability at High Temperatures Using the Integrated Virus Detection System (IVDS); DTIC Document; Aberdeen Proving Ground: Aberdeen, MD, USA, 2005. [Google Scholar]
- Wei, X.; Tian, G.; Ji, Z.; Chen, S. A new strategy for enhancement of poly-γ-glutamic acid production by multiple physicochemical stresses in bacillus licheniformis. J. Chem. Technol. Biotechnol. 2015, 90, 709–713. [Google Scholar] [CrossRef]
- Ho, G.H.; Ho, T.I.; Hsieh, K.H.; Su, Y.C.; Lin, P.Y.; Yang, J.; Yang, K.H.; Yang, S.C. Γ-polyglutamic acid produced by bacillus subtilis (natto): Structural characteristics, chemical properties and biological functionalities. J. Chin. Chem. Soc. 2006, 53, 1363–1384. [Google Scholar] [CrossRef]
- Pham, M.; Mintz, E.A.; Nguyen, T.H. Deposition kinetics of bacteriophage ms2 to natural organic matter: Role of divalent cations. J. Colloid Interface Sci. 2009, 338, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stanley, N.R.; Lazazzera, B.A. Defining the genetic differences between wild and domestic strains of bacillus subtilis that affect poly-γ-dl-glutamic acid production and biofilm formation. Mol. Microbiol. 2005, 57, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Folimonov, A.S.; Folimonova, S.Y.; Bar-Joseph, M.; Dawson, W.O. A stable RNA virus-based vector for citrus trees. Virology 2007, 368, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Kedia, G.; Hill, D.; Hill, R.; Radecka, I. Production of poly-γ-glutamic acid by bacillus subtilis and bacillus licheniformis with different growth media. J. Nanosci. Nanotechnol. 2010, 10, 5926–5934. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.T.; Fuhrman, J.A. Use of sybr green i for rapid epifluorescence counts of marine viruses and bacteria. Aquatic Microb. Ecology 1998, 14, 113–118. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, I.R.; Irorere, V.U.; Radecka, I.; Burns, A.T.H.; Kowalczuk, M.; Mason, J.L.; Khechara, M.P. Poly-γ-Glutamic Acid: Biodegradable Polymer for Potential Protection of Beneficial Viruses. Materials 2016, 9, 28. https://doi.org/10.3390/ma9010028
Khalil IR, Irorere VU, Radecka I, Burns ATH, Kowalczuk M, Mason JL, Khechara MP. Poly-γ-Glutamic Acid: Biodegradable Polymer for Potential Protection of Beneficial Viruses. Materials. 2016; 9(1):28. https://doi.org/10.3390/ma9010028
Chicago/Turabian StyleKhalil, Ibrahim R., Victor U. Irorere, Iza Radecka, Alan T. H. Burns, Marek Kowalczuk, Jessica L. Mason, and Martin P. Khechara. 2016. "Poly-γ-Glutamic Acid: Biodegradable Polymer for Potential Protection of Beneficial Viruses" Materials 9, no. 1: 28. https://doi.org/10.3390/ma9010028
APA StyleKhalil, I. R., Irorere, V. U., Radecka, I., Burns, A. T. H., Kowalczuk, M., Mason, J. L., & Khechara, M. P. (2016). Poly-γ-Glutamic Acid: Biodegradable Polymer for Potential Protection of Beneficial Viruses. Materials, 9(1), 28. https://doi.org/10.3390/ma9010028






