Conductive Polymer Synthesis with Single-Crystallinity via a Novel Plasma Polymerization Technique for Gas Sensor Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Plasma Polymer Synthesis and Measurement
2.2. Iodine Doping on Plasma Polymer Films
2.3. Scanning Electron Microscopy
2.4. Trasmission Electron Microscopy
2.5. Fourier Transform Infrared Spectroscopy
2.6. X-ray Photoelectron Spectroscopy
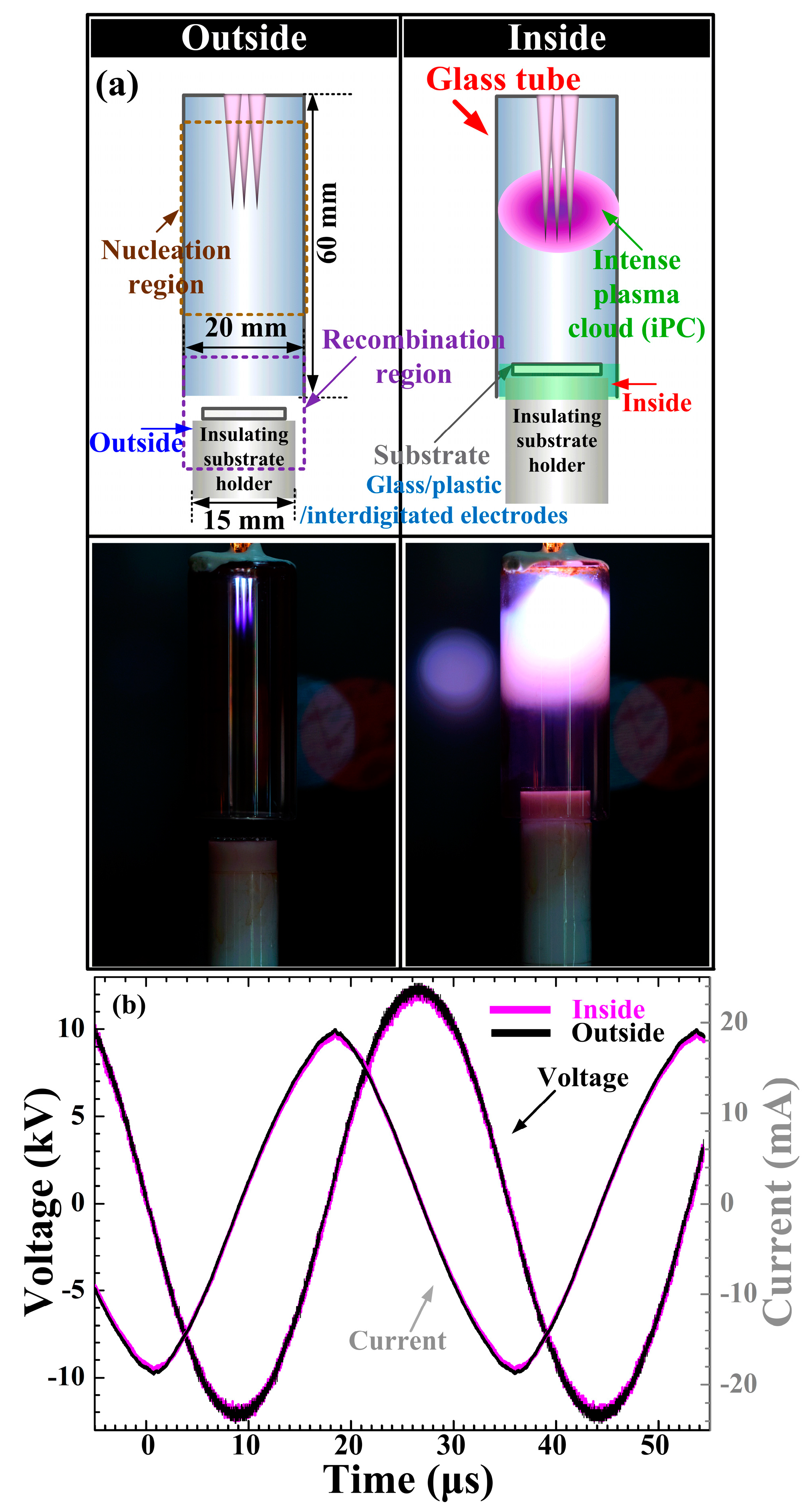
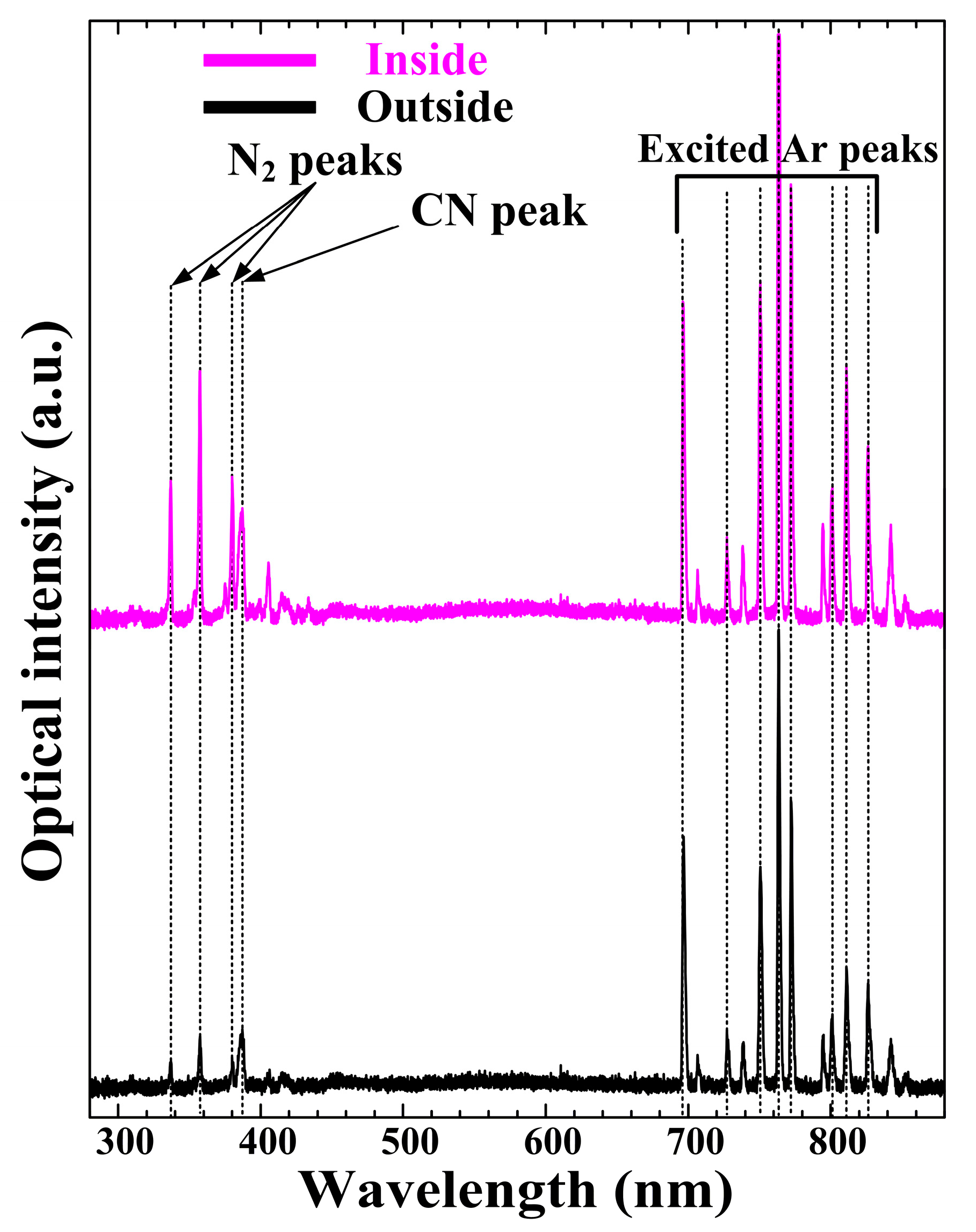
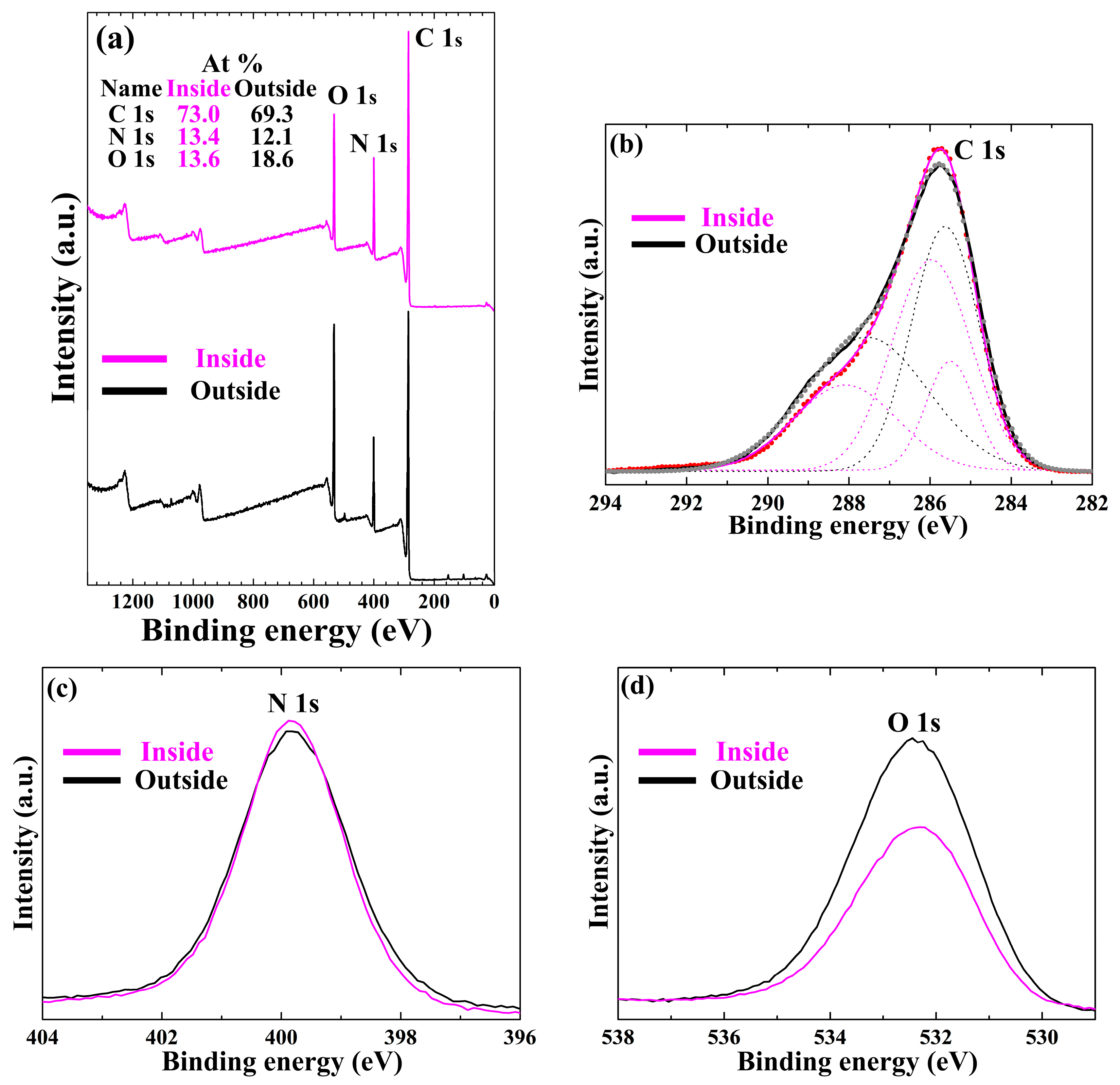
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khalil, A.; Kim, J.J.; Tuller, H.L.; Rutledge, G.C.; Hashaikeh, R. Gas sensing behavior of electrospun nickel oxide nanofibers: Effect of morphology and microstructure. Sens. Actuators B Chem. 2016, 227, 54–64. [Google Scholar] [CrossRef]
- Zanettini, S.; Dayen, J.F.; Etrillard, C.; Leclerc, N.; Kamalakar, M.V.; Doudin, B. Magnetoconductance anisotropy of a polymer thin film at the onset of metallicity. Appl. Phys. Lett. 2015, 106, 063303. [Google Scholar] [CrossRef]
- Yoon, H. Current trends in sensors based on conducting polymer nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef]
- Yoon, H.; Jang, J. Conducting-polymer nanomaterials for high-performance sensor applications: Issues and challenges. Adv. Funct. Mater. 2009, 19, 1567–1576. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas sensors based on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Gerard, M.; Chaubey, A.; Malhotra, B.D. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef]
- Virji, S.; Huang, J.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber gas sensors: Examination of response mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Adhikari, B.; Majumdar, S. Polymers in sensor applications. Prog. Polym. Sci. 2004, 29, 699–766. [Google Scholar] [CrossRef]
- Peres, L.O.; Li, R.W.C.; Yamauchi, E.Y.; Lippi, R.; Gruber, J. Conductive polymer gas sensor for quantitative detection of methanol in Brazilian sugar-cane spirit. Food Chem. 2012, 130, 1105–1107. [Google Scholar] [CrossRef]
- Rahman, M.A.; Kumar, P.; Park, D.-S.; Shim, Y.-B. Electrochemical sensors based on organic conjugated polymers. Sensors 2008, 8, 118–141. [Google Scholar] [CrossRef]
- Yoon, H.; Chang, M.; Jang, J. Formation of 1D poly(3,4-ethylenedioxythiophene) nanomaterials in reverse microemulsions and their application to chemical sensors. Adv. Funct. Mater. 2007, 17, 431–436. [Google Scholar] [CrossRef]
- Tiwari, A.; Kumar, R.; Prabaharan, M.; Pandey, R.R.; Kumari, P.; Chaturvedi, A.; Mishra, A.K. Nanofibrous polyaniline thin film prepared by plasma-induced polymerization technique for detection of NO2 gas. Polym. Adv. Technol. 2010, 21, 615–620. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Song, W.; Lu, Z. Controllable synthesis of conducting polypyrrole nanostructures. J. Phys. Chem. B 2006, 110, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yoon, H. Formation mechanism of conducting polypyrrole nanotubes in reverse micelle systems. Langmuir 2005, 21, 11484–11489. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Lian, J.; He, P.; Wang, L.M.; van Ooij, W.J.; Schulz, M.; Liu, Y.; Mast, D.B. Plasma deposition of ultrathin polymer films on carbon nanotubes. Appl. Phys. Lett. 2002, 81, 5216. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hamzah, E.; Ibrahim, Z.; Hashim, S. Synthesis of uniform polyaniline nanofibers through interfacial polymerization. Materials 2012, 5, 1487–1494. [Google Scholar] [CrossRef]
- Tatarova, E.; Dias, A.; Henriques, J.; Botelho do Rego, A.M.; Ferraria, A.M.; Abrashev, M.V.; Luhrs, C.C.; Phillips, J.; Dias, F.M.; Ferreira, C.M. Microwave plasmas applied for the synthesis of free standing graphene sheets. J. Phys. D Appl. Phys. 2014, 47, 385501. [Google Scholar] [CrossRef]
- Tsyganov, D.; Bundaleska, N.; Tatarova, E.; Dias, A.; Henriques, J.; Rego, A.; Ferraria, A.; Abrashev, M.V.; Dias, F.M.; Luhrs, C.C.; et al. On the plasma-based growth of “flowing” graphene sheets at atmospheric pressure conditions. Plasma Sources Sci. Technol. 2016, 25, 015013. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.-J.; Park, C.-S.; Shin, B.J.; Seo, J.H.; Tae, H.-S. Atmospheric pressure plasma polymerization using double grounded electrodes with He/Ar mixture. AIP Adv. 2015, 5, 097137. [Google Scholar] [CrossRef]
- Michel, M.; Bour, J.; Petersen, J.; Arnoult, C.; Ettingshausen, F.; Roth, C.; Ruch, D. Atmospheric plasma deposition: A new pathway in the design of conducting polymer-based anodes for hydrogen. Fuel Cells 2010, 10, 932–937. [Google Scholar] [CrossRef]
- Friedrich, J. Mechanisms of plasma polymerization—Reviewed from a chemical point of view. Plasma Process. Polym. 2011, 8, 783–802. [Google Scholar] [CrossRef]
- Gandhiraman, R.P.; Jayan, V.; Han, J.-W.; Chen, B.; Koehne, J.E.; Meyyappan, M. Plasma jet printing of electronic materials on flexible and nonconformal objects. ACS Appl. Mater. Interfaces 2014, 6, 20860–20867. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Ortega, M.; Ortega, M.; Morales, J.; Olayo, M.G.; Cruz, G.J.; Olayo, R. Core-shell polypyrrole nanoparticles obtained by atmospheric pressure plasma polymerization. Polym. Int. 2014, 63, 2023–2029. [Google Scholar] [CrossRef]
- Knoll, A.J.; Luan, P.; Bartis, E.A.J.; Hart, C.; Raitses, Y.; Oehrlein, G.S. Real time characterization of polymer surface modifications by an atmospheric-pressure plasma jet: Electrically coupled versus remote mode. Appl. Phys. Lett. 2014, 105, 171601. [Google Scholar] [CrossRef]
- Chen, K.S.; Liao, S.C.; Lin, S.W.; Tsao, S.H.; Ting, T.H.; Inagaki, N.; Wu, H.M.; Chen, W.Y. The film deposition via atmospheric pressure plasma from ethanol and He mixing gases. Surf. Coat. Technol. 2013, 231, 408–411. [Google Scholar] [CrossRef]
- Urabe, K.; Sands, B.L.; Ganguly, B.N.; Sakai, O. Temporally and spectrally resolved observation of a crossed-flow DBD plasma jet using pure helium and argon/acetone mixed gases. Plasma Sources Sci. Technol. 2012, 21, 034004. [Google Scholar] [CrossRef]
- Jeon, K.; Lumata, L.; Tokumoto, T.; Steven, E.; Brooks, J.; Alamo, R.G. Low electrical conductivity threshold and crystalline morphology of single-walled carbon nanotubes—High density polyethylene nanocomposites characterized by SEM, Raman spectroscopy and AFM. Polymer 2007, 48, 4751–4764. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Yang, S.-H.; Hsu, C.-S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Gurarslan, A.; Tonelli, A.E. Single-component polymer composites. Macromolecules 2011, 44, 3856–3861. [Google Scholar] [CrossRef]
- Tanaka, H.; Nishio, S.; Ito, H.; Kuroda, S.I. Microscopic signature of insulator-to-metal transition in highly doped semicrystalline conducting polymers in ionic-liquid-gated transistors. Appl. Phys. Lett. 2015, 107, 243302. [Google Scholar] [CrossRef]
- Park, C.-S.; Kim, D.; Shin, B.; Tae, H.-S. Synthesis and characterization of nanofibrous polyaniline thin film prepared by novel atmospheric pressure plasma polymerization technique. Materials 2016, 9, 39. [Google Scholar] [CrossRef]
- Groenewoud, L.M.H.; Engbers, G.H.M.; Feijen, J. Plasma polymerization of thiophene derivatives. Langmuir 2003, 19, 1368–1374. [Google Scholar] [CrossRef]
- Cruz, G.J.; Olayo, M.G.; López, O.G.; Gómez, L.M.; Morales, J.; Olayo, R. Nanospherical particles of polypyrrole synthesized and doped by plasma. Polymer 2010, 51, 4314–4318. [Google Scholar] [CrossRef]
- Dams, R.; Vangeneugden, D.; Vanderzande, D. Atmospheric pressure plasma polymerization of in situ doped polypyrrole. Open Plasma Phys.J. 2013, 6, 7–13. [Google Scholar]
- Liang, Q.; Chin, C.Y.; Lai, J.; Yan, C.-S.; Meng, Y.; Mao, H.; Hemley, R.J. Enhanced growth of high quality single crystal diamond by microwave plasma assisted chemical vapor deposition at high gas pressures. Appl. Phys. Lett. 2009, 94, 024103. [Google Scholar] [CrossRef]
- Urabe, K.; Yamada, K.; Sakai, O. Discharge-mode transition in jet-type dielectric barrier discharge using argon/acetone gas flow ignited by small helium plasma jet. Jpn. J. Appl. Phys. 2011, 50, 116002. [Google Scholar] [CrossRef]
- Cruz, G.J.; Morales, J.; Olayo, R. Films obtained by plasma polymerization of pyrrole. Thin Solid Films 1999, 342, 119–126. [Google Scholar] [CrossRef]
- Totolin, M.; Grigoraş, M. Plasma induced pyrrole polymerization. Rev. Roum. Chim. 2007, 52, 999–1005. [Google Scholar]
- Wang, J.; Neoh, K.G.; Kang, E.T. Comparative study of chemically synthesized and plasma polymerized pyrrole and thiophene thin films. Thin Solid Films 2004, 446, 205–217. [Google Scholar] [CrossRef]
- Uygun, A.; Oksuz, L.; Yavuz, A.G.; Guleç, A.; Sen, S. Characteristics of nanocomposite films deposited by atmospheric pressure uniform RF glow plasma. Curr. Appl. Phys. 2011, 11, 250–254. [Google Scholar] [CrossRef]
- Steen, M.L.; Butoi, C.I.; Fisher, E.R. Identification of gas-phase reactive species and chemical mechanisms occurring at plasma-polymer surface interfaces. Langmuir 2001, 17, 8156–8166. [Google Scholar] [CrossRef]
- Morent, R.; Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf. Interface Anal. 2008, 40, 597–600. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Chang, K.-H.; Hung, T.-S.; Chen, K.-S.; Inagaki, N. Atmospheric-pressure plasma treatment on polystyrene for the photo-induced grafting polymerization of N-isopropylacrylamide. Thin Solid Films 2010, 518, 7568–7573. [Google Scholar] [CrossRef]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.-S.; Kim, D.H.; Shin, B.J.; Kim, D.Y.; Lee, H.-K.; Tae, H.-S. Conductive Polymer Synthesis with Single-Crystallinity via a Novel Plasma Polymerization Technique for Gas Sensor Applications. Materials 2016, 9, 812. https://doi.org/10.3390/ma9100812
Park C-S, Kim DH, Shin BJ, Kim DY, Lee H-K, Tae H-S. Conductive Polymer Synthesis with Single-Crystallinity via a Novel Plasma Polymerization Technique for Gas Sensor Applications. Materials. 2016; 9(10):812. https://doi.org/10.3390/ma9100812
Chicago/Turabian StylePark, Choon-Sang, Dong Ha Kim, Bhum Jae Shin, Do Yeob Kim, Hyung-Kun Lee, and Heung-Sik Tae. 2016. "Conductive Polymer Synthesis with Single-Crystallinity via a Novel Plasma Polymerization Technique for Gas Sensor Applications" Materials 9, no. 10: 812. https://doi.org/10.3390/ma9100812





