Tribological Performance of Green Lubricant Enhanced by Sulfidation IF-MoS2
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- (1)
- High-density composite material MoS2/MoO3 particles were successfully produced via the simple sulfidation method.
- (2)
- The thin film of lubricating material was produced with excellent homogeneity from the biological polymer material HPMC with NP additives.
- (3)
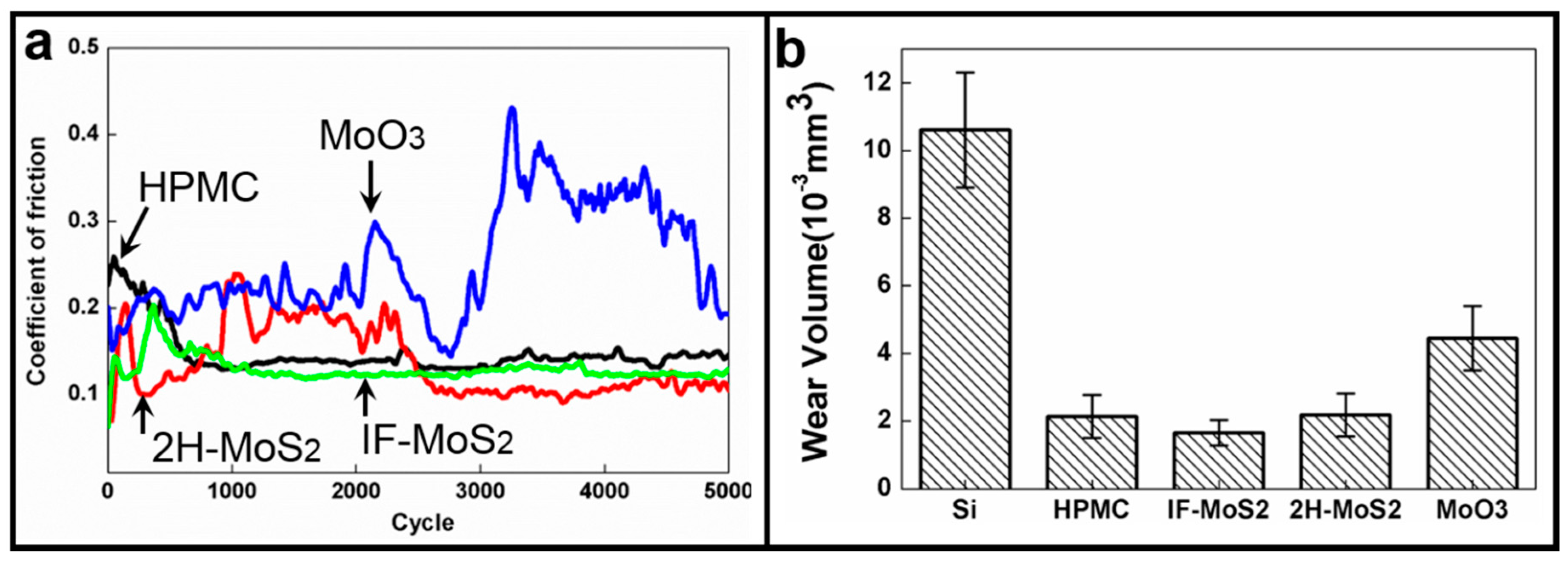
- MoS2 particles were added to HPMC, and friction and wear were reduced by more than 50%. A more stable COF was also achieved when MoO3 was added.
- (4)
- The study showed that IF-MoS2 NPs have multiple lubricating mechanisms, and superior and more stable lubricating properties compared with 2H-MoS2 MPs and MoO3 NPs.
- (5)
- It is hypothesized that the IF-MoS2/HPMC thin film showed the best tribological performance in our experiment because its wear debris can easily enter the contact area to form a tribofilm/transfer film with good coverage; IF-MoS2 NPs have multiple friction mechanisms, which are unique to this additive’s size, shape, and structure.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NCKU | National Cheng Kung University |
| HPMC | hydroxypropyl methylcellulose |
| NP | nanoparticle |
| IF | inorganic fullerene |
| 2H | hexagonal |
| COF | coefficient of friction |
| SEM | scanning electron microscope |
| EDS | energy dispersive spectroscopy |
References
- Jovane, F.; Westkämper, E.; Williams, D. The Manufuture Road: Towards Competitive and Sustainable High-Adding-Value Manufacturing; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Bartz, W.J. Lubricants and the environment. Tribol. Int. 1998, 31, 35–47. [Google Scholar] [CrossRef]
- Bartz, W.J. Ecotribology: Environmentally acceptable tribological practices. Tribol. Int. 2006, 39, 728–733. [Google Scholar] [CrossRef]
- Palacio, M.; Bhushan, B. A review of ionic liquids for green molecular lubrication in nanotechnology. Tribol. Lett. 2010, 40, 247–268. [Google Scholar] [CrossRef]
- Lovell, M.; Higgs, C.; Deshmukh, P.; Mobley, A. Increasing formability in sheet metal stamping operations using environmentally friendly lubricants. J. Mater. Process. Technol. 2006, 177, 87–90. [Google Scholar] [CrossRef]
- Lovell, M.R.; Kabir, M.; Menezes, P.L.; Higgs, C.F. Influence of boric acid additive size on green lubricant performance. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2010, 368, 4851–4868. [Google Scholar] [CrossRef] [PubMed]
- Fahs, A.; Brogly, M.; Bistac, S.; Schmitt, M. Hydroxypropyl methylcellulose (HPMC) formulated films: Relevance to adhesion and friction surface properties. Carbohydr. Polym. 2010, 80, 105–114. [Google Scholar] [CrossRef]
- Al Mahmud, K.; Kalam, M.; Masjuki, H.; Abdollah, M. Tribological study of a tetrahedral diamond-like-carbon coating under vegetable oil-based lubricated condition. Tribol. Trans. 2015, 58, 907–913. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Reddyhoff, T.; Gallegos, C.; Spikes, H.A. Tribological studies of potential vegetable oil-based lubricants containing environmentally friendly viscosity modifiers. Tribol. Int. 2014, 69, 110–117. [Google Scholar] [CrossRef]
- Rani, S.; Joy, M.; Nair, K.P. Evaluation of physiochemical and tribological properties of rice bran oil-biodegradable and potential base stoke for industrial lubricants. Ind. Crops Prod. 2015, 65, 328–333. [Google Scholar] [CrossRef]
- Padgurskas, J.; Rukuiža, R.; Meškinis, A.; Kreivaitis, R.; Spruogis, B. Influence of manufacturing methods on the tribological properties of rapeseed oil lubricants. Transport 2016, 31, 56–62. [Google Scholar] [CrossRef]
- Koshy, C.P.; Rajendrakumar, P.K.; Thottackkad, M.V. Evaluation of the tribological and thermo-physical properties of coconut oil added with MoS2 nanoparticles at elevated temperatures. Wear 2015, 330–331, 288–308. [Google Scholar] [CrossRef]
- Su, Y.L.; Kao, W. Optimum multilayer TiN–TiCN coatings for wear resistance and actual application. Wear 1998, 223, 119–130. [Google Scholar] [CrossRef]
- Banerji, A.; Bhowmick, S.; Alpas, A. High temperature tribological behavior of w containing diamond-like carbon (DLC) coating against titanium alloys. Surf. Coat. Technol. 2014, 241, 93–104. [Google Scholar] [CrossRef]
- Beckford, S.; Cai, J.; Chen, J.; Zou, M. Use of Au nanoparticle-filled PTFE films to produce low-friction and low-wear surface coatings. Tribol. Lett. 2014, 56, 223–230. [Google Scholar] [CrossRef]
- Wu, Y.; Tsui, W.; Liu, T. Experimental analysis of tribological properties of lubricating oils with nanoparticle additives. Wear 2007, 262, 819–825. [Google Scholar] [CrossRef]
- Gara, L.; Zou, Q. Friction and wear characteristics of water-based ZnO and Al2O3 nanofluids. Tribol. Trans. 2012, 55, 345–350. [Google Scholar] [CrossRef]
- Gara, L.; Zou, Q. Friction and wear characteristics of oil-based ZnO nanofluids. Tribol. Trans. 2013, 56, 236–244. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Wang, Y.; Wang, W.; Yan, L.; Luo, J. An investigation on the tribological properties of multilayer graphene and MoS2 nanosheets as additives used in hydraulic applications. Tribol. Int. 2016, 97, 14–20. [Google Scholar] [CrossRef]
- Novak, C.; Kingman, D.; Stern, K.; Zou, Q.; Gara, L. Tribological properties of paraffinic oil with nanodiamond particles. Tribol. Trans. 2014, 57, 831–837. [Google Scholar] [CrossRef]
- Hsin, Y.L.; Chu, H.-Y.; Jeng, Y.-R.; Huang, Y.-H.; Wang, M.H.; Chang, C.K. In situ de-agglomeration and surface functionalization of detonation nanodiamond, with the polymer used as an additive in lubricant oil. J. Mater. Chem. 2011, 21, 13213–13222. [Google Scholar] [CrossRef]
- Omrani, E.; Barari, B.; Dorri Moghadam, A.; Rohatgi, P.K.; Pillai, K.M. Mechanical and tribological properties of self-lubricating bio-based carbon-fabric epoxy composites made using liquid composite molding. Tribol. Int. 2015, 92, 222–232. [Google Scholar] [CrossRef]
- Sorrentino, A.; Altavilla, C.; Merola, M.; Senatore, A.; Ciambelli, P.; Iannace, S. Nanosheets of MoS2-oleylamine as hybrid filler for self-lubricating polymer composites: Thermal, tribological, and mechanical properties. Polym. Compos. 2015, 36, 1124–1134. [Google Scholar] [CrossRef]
- Zalaznik, M.; Novak, S.; Huskić, M.; Kalin, M. Tribological behaviour of a peek polymer containing solid MoS2 lubricants. Lubr. Sci. 2016, 28, 27–42. [Google Scholar] [CrossRef]
- Narayanunni, V.; Kheireddin, B.A.; Akbulut, M. Influence of surface topography on frictional properties of Cu surfaces under different lubrication conditions: Comparison of dry, base oil, and ZnS nanowire-based lubrication system. Tribol. Int. 2011, 44, 1720–1725. [Google Scholar] [CrossRef]
- Kheireddin, B.A.; Narayanunni, V.; Akbulut, M. Influence of shearing surface topography on frictional properties of ZnS nanowire-based lubrication system across ductile surfaces. J. Tribol. 2012, 134, 022001. [Google Scholar] [CrossRef]
- Reeves, C.J.; Menezes, P.L.; Lovell, M.R.; Jen, T.-C. The influence of surface roughness and particulate size on the tribological performance of bio-based multi-functional hybrid lubricants. Tribol. Int. 2015, 88, 40–55. [Google Scholar] [CrossRef]
- Ewen, J.P.; Gattinoni, C.; Thakkar, F.M.; Morgan, N.; Spikes, H.A.; Dini, D. Nonequilibrium molecular dynamics investigation of the reduction in friction and wear by carbon nanoparticles between iron surfaces. Tribol. Lett. 2016, 63, 38. [Google Scholar] [CrossRef]
- Conte, M.; Igartua, A. Study of PTFE composites tribological behavior. Wear 2012, 296, 568–574. [Google Scholar] [CrossRef]
- Schroeder, R.; Torres, F.; Binder, C.; Klein, A.; de Mello, J. Failure mode in sliding wear of peek based composites. Wear 2013, 301, 717–726. [Google Scholar] [CrossRef]
- Chang, L.; Friedrich, K. Enhancement effect of nanoparticles on the sliding wear of short fiber-reinforced polymer composites: A critical discussion of wear mechanisms. Tribol. Int. 2010, 43, 2355–2364. [Google Scholar] [CrossRef]
- Tanaka, K.; Kawakami, S. Effect of various fillers on the friction and wear of polytetrafluoroethylene-based composites. Wear 1982, 79, 221–234. [Google Scholar] [CrossRef]
- Burris, D.L.; Sawyer, W.G. Improved wear resistance in alumina-PTFE nanocomposites with irregular shaped nanoparticles. Wear 2006, 260, 915–918. [Google Scholar] [CrossRef]
- Ye, J.; Khare, H.; Burris, D. Transfer film evolution and its role in promoting ultra-low wear of a PTFE nanocomposite. Wear 2013, 297, 1095–1102. [Google Scholar] [CrossRef]
- Ye, J.; Burris, D.L.; Xie, T. A review of transfer films and their role in ultra-low-wear sliding of polymers. Lubricants 2016, 4, 4. [Google Scholar] [CrossRef]
- Bahadur, S. The development of transfer layers and their role in polymer tribology. Wear 2000, 245, 92–99. [Google Scholar] [CrossRef]
- Bahadur, S.; Tabor, D. The wear of filled polytetrafluoroethylene. Wear 1984, 98, 1–13. [Google Scholar] [CrossRef]
- Bahadur, S.; Gong, D. The action of fillers in the modification of the tribological behavior of polymers. Wear 1992, 158, 41–59. [Google Scholar] [CrossRef]
- Zak, A.; Feldman, Y.; Alperovich, V.; Rosentsveig, R.; Tenne, R. Growth mechanism of MoS2 fullerene-like nanoparticles by gas-phase synthesis. J. Am. Chem. Soc. 2000, 122, 11108–11116. [Google Scholar] [CrossRef]
- Zuo, X.; Chang, K.; Zhao, J.; Xie, Z.; Tang, H.; Li, B.; Chang, Z. Bubble-template-assisted synthesis of hollow fullerene-like MoS2 nanocages as a lithium ion battery anode material. J. Mater. Chem. A 2016, 4, 51–58. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W. l-cysteine-assisted synthesis of layered MoS2/graphene composites with excellent electrochemical performances for lithium ion batteries. ACS Nano 2011, 5, 4720–4728. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Yan, L.; Yu, J.; Tian, G.; Zhou, L.; Zheng, X.; Zhang, X.; Yong, Y.; Li, J.; Gu, Z. High-throughput synthesis of single-layer MoS2 nanosheets as a near-infrared photothermal-triggered drug delivery for effective cancer therapy. ACS Nano 2014, 8, 6922–6933. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.-C.; Lu, F.-I. Biopolymer green lubricant for sustainable manufacturing. Materials 2016, 9, 338. [Google Scholar] [CrossRef]
- Rosentsveig, R.; Margolin, A.; Gorodnev, A.; Popovitz-Biro, R.; Feldman, Y.; Rapoport, L.; Novema, Y.; Naveh, G.; Tenne, R. Synthesis of fullerene-like MoS2 nanoparticles and their tribological behavior. J. Mater. Chem. 2009, 19, 4368–4374. [Google Scholar] [CrossRef]
- Rosentsveig, R.; Gorodnev, A.; Feuerstein, N.; Friedman, H.; Zak, A.; Fleischer, N.; Tannous, J.; Dassenoy, F.; Tenne, R. Fullerene-like MoS2 nanoparticles and their tribological behavior. Tribol. Lett. 2009, 36, 175–182. [Google Scholar] [CrossRef]
- Shi, S.-C.; Su, C.-C. Corrosion inhibition of high speed steel by biopolymer hpmc derivatives. Materials 2016, 9, 612. [Google Scholar] [CrossRef]
- Shi, S.-C.; Wu, J.-Y.; Huang, T.-F. Raman, FTIR, and XRD study of MoS2 enhanced hydroxypropyl methylcellulose green lubricant. Opt. Quantum Electron. 2016, 48, 474. [Google Scholar] [CrossRef]
- Shi, S.-C.; Wu, J.-Y.; Huang, T.-F.; Peng, Y.-Q. Improving the tribological performance of biopolymer coating with MoS2 additive. Surf. Coat. Technol. 2016, 303, 250–255. [Google Scholar] [CrossRef]
- Huang, H.D.; Tu, J.P.; Zou, T.Z.; Zhang, L.L.; He, D.N. Friction and wear properties of IF-MoS2 as additive in paraffin oil. Tribol. Lett. 2005, 20, 247–250. [Google Scholar] [CrossRef]
- Hu, K.H.; Hu, X.G.; Xu, Y.F.; Huang, F.; Liu, J.S. The effect of morphology on the tribological properties of MoS2 in liquid paraffin. Tribol. Lett. 2010, 40, 155–165. [Google Scholar] [CrossRef]
- Rapoport, L.; Fleischer, N.; Tenne, R. Applications of WS2 (MoS2) inorganic nanotubes and fullerene-like nanoparticles for solid lubrication and for structural nanocomposites. J. Mater. Chem. 2005, 15, 1782–1788. [Google Scholar] [CrossRef]
- Zou, T.Z.; Tu, J.P.; Huang, H.D.; Lai, D.M.; Zhang, L.L.; He, D.N. Preparation and tribological properties of inorganic fullerene-like MoS2. Adv. Eng. Mater. 2006, 8, 289–293. [Google Scholar] [CrossRef]
- Shi, S.-C.; Huang, T.-F.; Wu, J.-Y. Preparation and tribological study of biodegradable lubrication films on Si substrate. Materials 2015, 8, 1738–1751. [Google Scholar] [CrossRef]
- Windom, B.C.; Sawyer, W.; Hahn, D.W. A raman spectroscopic study of MoS2 and MoO3: Applications to tribological systems. Tribol. Lett. 2011, 42, 301–310. [Google Scholar] [CrossRef]
- Sekine, T.; Uchinokura, K.; Nakashizu, T.; Matsuura, E.; Yoshizaki, R. Dispersive raman mode of layered compound 2H-MoS2 under the resonant condition. J. Phys. Soc. Jpn. 1984, 53, 811–818. [Google Scholar] [CrossRef]
- Tevet, O.; Von-Huth, P.; Popovitz-Biro, R.; Rosentsveig, R.; Wagner, H.D.; Tenne, R. Friction mechanism of individual multilayered nanoparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 19901–19906. [Google Scholar] [CrossRef] [PubMed]
- Tevet, O.; Goldbart, O.; Cohen, S.; Rosentsveig, R.; Popovitz-Biro, R.; Wagner, H.; Tenne, R. Nanocompression of individual multilayered polyhedral nanoparticles. Nanotechnology 2010, 21, 365705. [Google Scholar] [CrossRef] [PubMed]
- Godet, M. Third-bodies in tribology. Wear 1990, 136, 29–45. [Google Scholar] [CrossRef]
- Colas, G.; Saulot, A.; Godeau, C.; Michel, Y.; Berthier, Y. Decrypting third body flows to solve dry lubrication issue—MoS2 case study under ultrahigh vacuum. Wear 2013, 305, 192–204. [Google Scholar] [CrossRef]
- Harris, K.L.; Curry, J.F.; Pitenis, A.A.; Rowe, K.G.; Sidebottom, M.A.; Sawyer, W.G.; Krick, B.A. Wear debris mobility, aligned surface roughness, and the low wear behavior of filled polytetrafluoroethylene. Tribol. Lett. 2015, 60, 1–8. [Google Scholar]
- Rapoport, L.; Bilik, Y.; Feldman, Y.; Homyonfer, M.; Cohen, S.; Tenne, R. Hollow nanoparticles of WS2 as potential solid-state lubricants. Nature 1997, 387, 791–793. [Google Scholar]
- Tannous, J.; Dassenoy, F.; Lahouij, I.; Le Mogne, T.; Vacher, B.; Bruhács, A.; Tremel, W. Understanding the tribochemical mechanisms of IF-MoS2 nanoparticles under boundary lubrication. Tribol. Lett. 2010, 41, 55–64. [Google Scholar] [CrossRef]
- Gulbiński, W.; Suszko, T.; Sienicki, W.; Warcholiński, B. Tribological properties of silver-and copper-doped transition metal oxide coatings. Wear 2003, 254, 129–135. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hagenmuller, P.; Casalot, A. Les Oxydes des Métaux de Transition; Gauthier-Villars: Chichester, NY, USA, 1973. [Google Scholar]
- Kanakia, M.; Peterson, M. Literature Review of Solid Lubrication Mechanisms; DTIC Document; Defense Technical Information Center: Fort Belvoir, VA, USA, 1987. [Google Scholar]
- Sheehan, P.E.; Lieber, C.M. Nanotribology and nanofabrication of MoO3 structures by atomic force microscopy. Science 1996, 272, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, P.; Chromik, R.R.; Gupta, S.; Lince, J.R. Micro-scale sliding contacts on Au and Au-MoS2 coatings. Surf. Coat. Technol. 2010, 205, 1449–1454. [Google Scholar] [CrossRef]
- Stoyanov, P.; Chromik, R.R.; Goldbaum, D.; Lince, J.R.; Zhang, X. Microtribological performance of Au–MoS2 and Ti–MoS2 coatings with varying contact pressure. Tribol. Lett. 2010, 40, 199–211. [Google Scholar] [CrossRef]
- Wahl, K.; Dunn, D.; Singer, I. Wear behavior of Pb–Mo–S solid lubricating coatings. Wear 1999, 230, 175–183. [Google Scholar] [CrossRef]





| Molecular Weight Mn | Methyl (CH3) Substitution (%) | Hydroxypropyl (CH2CHOHCH3) Substitution (%) | Viscosity (2 wt % Aqueous Solutions at 20 °C) |
|---|---|---|---|
| 35,600 | 28.8 | 9 | 5.94 mPa·s |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.-C. Tribological Performance of Green Lubricant Enhanced by Sulfidation IF-MoS2. Materials 2016, 9, 856. https://doi.org/10.3390/ma9100856
Shi S-C. Tribological Performance of Green Lubricant Enhanced by Sulfidation IF-MoS2. Materials. 2016; 9(10):856. https://doi.org/10.3390/ma9100856
Chicago/Turabian StyleShi, Shih-Chen. 2016. "Tribological Performance of Green Lubricant Enhanced by Sulfidation IF-MoS2" Materials 9, no. 10: 856. https://doi.org/10.3390/ma9100856






