The Effect of Curing Temperature on the Properties of Cement Pastes Modified with TiO2 Nanoparticles
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Specimen Preparation
2.2. Degree of Hydration
2.3. Compressive Strength Test
2.4. Electrical Resistivity Test
2.5. Porosity Measurement
2.6. X-ray Diffraction (XRD)
2.7. Microscopic Examination and Elemental Analysis
3. Results and Discussion
3.1. Degree of Hydration
3.2. Compressive Strength
3.3. Electrical Resistivity
3.4. Porosity Measurement
3.5. XRD Analysis
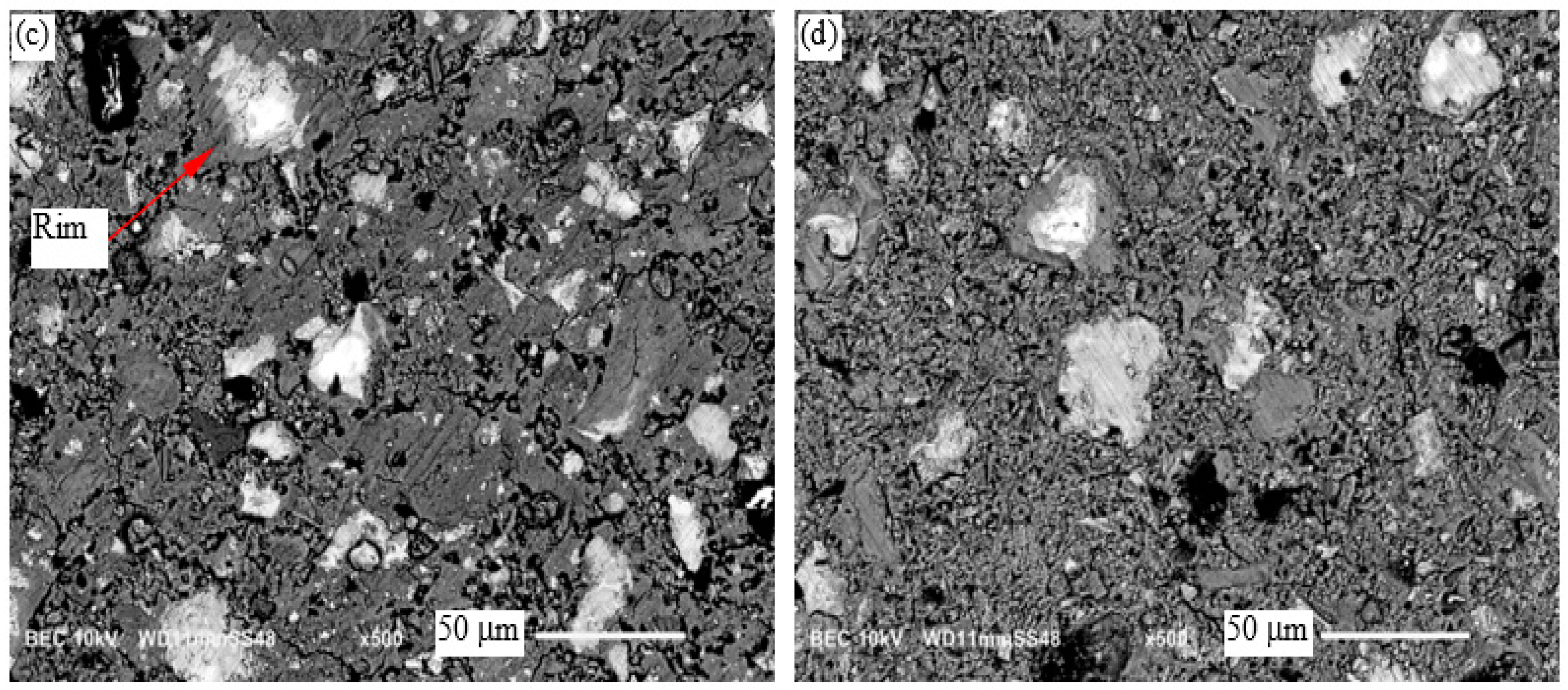
3.6. Microscopic Examination
4. Conclusions
- Temperature generally tends to increase the early hydration of the cement paste and cement pastes with TiO2 nanoparticles.
- It was observed that the cement pastes cured at elevated temperatures exhibited a rise in compressive strength at early age, but the rate of strength gain decreased at later ages more noticeably at a curing temperature of 60 °C than at 40 °C.
- The electrical resistivity of the cement pastes cured at elevated temperatures was shown to exhibit insignificant change after 14 days of curing. At 28 days, the electrical resistivity of these cement pastes was decreased compared to that of the cement paste cured at room temperature. It was suggested that a competition between improved hydration and non-uniform distribution of the hydration product in the microstructure appeared to determine the electrical resistivity of the cement pastes cured at elevated temperatures.
- The porosity of the cement pastes cured at elevated temperatures was shown to be generally lower than that of the cement paste cured at room temperature. The contrast between the porosity and electrical resistivity results is explained due to the dominant effect of pore structure and non-uniform distribution of hydration product on the transport behavior of cement pastes cured at elevated temperatures.
- Microstructural examination indicated a more uniformly distributed hydration product in the cement pastes cured at room temperature than in the cement pastes cured at 60 °C.
- It was observed that the effect of high temperature curing on reducing the compressive strength and electrical resistivity of the cement pastes at late ages was more pronounced with higher levels of added TiO2 nanoparticles.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.S. Photocatalytic activity of titanium dioxide modified concrete materials—Influence of utilizing recycled glass cullets as aggregates. J. Environ. Manag. 2009, 90, 3436–3442. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kou, S.; Poon, C. Photocatalytic cement-based materials: Comparison of nitrogen oxides and toluene removal potentials and evaluation of self-cleaning performance. Build. Environ. 2011, 46, 1827–1833. [Google Scholar] [CrossRef]
- Aly, M.; Hashmi, M.S.J.; Olabi, A.G.; Messeiry, M.; Hussain, A.I. Effect of nano clay particles on mechanical, thermal and physical behaviours of waste-glass cement mortars. Mater. Sci. Eng. A 2011, 528, 7991–7998. [Google Scholar] [CrossRef]
- Senff, L.; Labrincha, J.A.; Ferreira, V.M.; Hotza, D.; Repette, W.L. Effect of nano-silica on rheology and fresh properties of cement pastes and mortars. Constr. Build. Mater. 2009, 23, 2487–2491. [Google Scholar] [CrossRef]
- Gao, K.; Lin, K.L.; Wang, D.; Hwang, C.L.; Tuan, B.L.; Shiu, H.S.; Cheng, T.W. Effect of nano-SiO2 on the alkali-activated characteristics of metakaolin-based geopolymers. Constr. Build. Mater. 2013, 48, 441–447. [Google Scholar] [CrossRef]
- Hou, P.K.; Kawashima, S.; Wang, K.J.; Corr, D.J.; Qian, J.S.; Shah, S.P. Effects of colloidal nanosilica on rheological and mechanical properties of fly ash-cement mortar. Cem. Concr. Compos. 2013, 35, 12–22. [Google Scholar] [CrossRef]
- Singh, L.P.; Goel, A.; Bhattacharyya, S.K.; Sharma, U.; Mishra, G.; Singh, L.P.; Goel, A.; Bhattacharyya, S.K.; Sharma, U.; Mishra, G. Hydration studies of cementitious material using silica nanoparticles. J. Adv. Concr. Technol. 2015, 13, 345–354. [Google Scholar] [CrossRef]
- Singh, L.P.; Goel, A.; Bhattachharyya, S.K.; Ahalawat, S. Effect of morphology and dispersibility of silica nanoparticles on the mechanical behaviour of cement mortar. Int. J. Concr. Struct. Mater. 2015, 9, 207–217. [Google Scholar] [CrossRef]
- Kawashima, S.; Hou, P.; Corr, D.J.; Shah, S.P. Modification of cement-based materials with nanoparticles. Cem. Concr. Compos. 2013, 36, 8–15. [Google Scholar] [CrossRef]
- Li, G.Y.; Wang, P.M.; Zhao, X. Mechanical behavior and microstructure of cement composites incorporating surface-treated multi-walled carbon nanotubes. Carbon 2005, 43, 1239–1245. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S.; Metaxa, Z.S.; Shah, S.P. Multi-scale mechanical and fracture characteristics and early-age strain capacity of high performance carbon nanotube/cement nanocomposites. Cem. Concr. Compos. 2010, 32, 110–115. [Google Scholar] [CrossRef]
- De Ibarra, Y.S.; Gaitero, J.J.; Erkizia, E.; Campillo, I. Atomic force microscopy and nanoindentation of cement pastes with nanotube dispersions. Phys. Status Solidi 2006, 203, 1076–1081. [Google Scholar] [CrossRef]
- Lackhoff, M.; Prieto, X.; Nestle, N.; Dehn, F.; Niessner, R. Photocatalytic activity of semiconductor-modified cement—Influence of semiconductor type and cement ageing. Appl. Catal. B Environ. 2003, 43, 205–216. [Google Scholar] [CrossRef]
- Jayapalan, A.R.; Lee, B.Y.; Fredrich, S.M.; Kurtis, K.E. Influence of Additions of Anatase TiO2 Nanoparticles on Early-Age Properties of Cement-Based Materials. Transp. Res. Rec. J. Transp. Res. Board. 2010, 2141, 41–46. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kurtis, K.E. Influence of TiO2 nanoparticles on early C3S hydration. J. Am. Ceram. Soc. 2010, 93, 3399–3405. [Google Scholar] [CrossRef]
- Chen, J.; Kou, S.C.; Poon, C.S. Hydration and properties of nano-TiO2 blended cement composites. Cem. Concr. Compos. 2012, 34, 642–649. [Google Scholar] [CrossRef]
- Lee, B.Y.; Jayapalan, A.R.; Kurtis, K.E. Effects of nano-TiO2 on properties of cement-based materials. Mag. Concr. Res. 2013, 65, 1293–1302. [Google Scholar] [CrossRef]
- Zhang, R.; Cheng, X.; Hou, P.; Ye, Z. Influences of nano-TiO2 on the properties of cement-based materials: Hydration and drying shrinkage. Constr. Build. Mater. 2015, 81, 35–41. [Google Scholar] [CrossRef]
- Ma, B.; Li, H.; Mei, J.; Li, X.; Chen, F. Effects of nano-TiO2 on the toughness and durability of cement-based material. Adv. Mater. Sci. Eng. 2015, 2015, 583106. [Google Scholar] [CrossRef]
- Martins, T.; Torgal, F.P.; Miraldo, S.; Aguiar, J.B.; Carlos, J. An experimental investigation on nano-TiO2 and fly ash based high performance concrete. Indian Concr. J. 2016, 90, 1–9. [Google Scholar]
- Bost, P.; Regnier, M.; Horgnies, M. Comparison of the accelerating effect of various additions on the early hydration of Portland cement. Constr. Build. Mater. 2016, 113, 290–296. [Google Scholar] [CrossRef]
- Nazari, A. The effects of curing medium on flexural strength and water permeability of concrete incorporating TiO2 nanoparticles. Mater. Struct. 2011, 44, 773–786. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Xu, S. Influence of nanoparticles on fluidity and mechanical properties of cement mortar. Constr. Build. Mater. 2015, 101, 892–901. [Google Scholar] [CrossRef]
- Feng, D.; Xie, N.; Gong, C.; Leng, Z.; Xiao, H.; Li, H. Portland cement paste modified by TiO2 nanoparticles: A microstructure perspective. Ind. Eng. Chem. Res. 2013, 52, 11575–11582. [Google Scholar] [CrossRef]
- Strawhecker, K.E.; Manias, E. Structure and properties of poly (vinyl alcohol )/Na+ montmorillonite nanocomposites. Chem. Mater. 2000, 12, 2943–2949. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Kaushik, A.K.; Arruda, E.M.; Waas, A.M.; Shim, B.S.; Xu, J.; Nandivada, H.; Pumplin, B.G.; Lahann, J.; Ramamoorthy, A.; et al. Ultrastrong and stiff layered polymer nanocomposites. Science 2007, 318, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, R.; Shubha, N.; Hng, H.H.; Srinivasan, M. Effect of nano-clay on ionic conductivity and electrochemical properties of poly(vinylidene fluoride) based nanocomposite porous polymer membranes and their application as polymer electrolyte in lithium ion batteries. Eur. Polym. J. 2013, 49, 307–318. [Google Scholar] [CrossRef]
- Hakamy, A.; Shaikh, F.U.A.; Low, I.M. Effect of calcined nanoclay on microstructural and mechanical properties of chemically treated hemp fabric-reinforced cement nanocomposites. Constr. Build. Mater. 2015, 95, 882–891. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Hüsken, G.; Hunger, M.; Brouwers, H.J.H. Experimental study of photocatalytic concrete products for air purification. Build. Environ. 2009, 44, 2463–2474. [Google Scholar] [CrossRef]
- Yang, L.Y.; Jia, Z.J.; Zhang, Y.M.; Dai, J.G. Effects of nano-TiO2 on strength, shrinkage and microstructure of alkali activated slag pastes. Cem. Concr. Compos. 2015, 57, 1–7. [Google Scholar] [CrossRef]
- Mohseni, E.; Naseri, F.; Amjadi, R.; Khotbehsara, M.M.; Ranjbar, M.M. Microstructure and durability properties of cement mortars containing. Constr. Build. Mater. 2016, 114, 656–664. [Google Scholar] [CrossRef]
- Elkhadiri, I.; Palacios, M.; Puertas, F. Effect of curing temperature on cement hydration. Ceram. Silik. 2009, 53, 65–75. [Google Scholar]
- Price, W. Factors influencing concrete strength. ACI J. 1951, 47, 417–432. [Google Scholar]
- Escalante-García, J.I.; Sharp, J.H. The microstructure and mechanical properties of blended cements hydrated at various temperatures. Cem. Concr. Res. 2001, 31, 695–702. [Google Scholar] [CrossRef]
- Lothenbach, B.; Winnefeld, F.; Alder, C.; Wieland, E.; Lunk, P. Effect of temperature on the pore solution, microstructure and hydration products of Portland cement pastes. Cem. Concr. Res. 2007, 37, 483–491. [Google Scholar] [CrossRef]
- De Weerdt, K.; Haha, M.B.; le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Ballester, P.; Hidalgo, A.; Mármol, I.; Morales, J.; Sánchez, L. Effect of brief heat-curing on microstructure and mechanical properties in fresh cement based mortars. Cem. Concr. Res. 2009, 39, 573–579. [Google Scholar] [CrossRef]
- Elkhadiri, I.; Puertas, F. The effect of curing temperature on sulphate-resistant cement hydration and strength. Constr. Build. Mater. 2008, 22, 1331–1341. [Google Scholar] [CrossRef]
- Kjellsen, K.O.; Detwiler, R.J.; Gjørv, O.E. Development of microstructures in plain cement pastes hydrated at different temperatures. Cem. Concr. Res. 1991, 21, 179–189. [Google Scholar] [CrossRef]
- Kjellsen, K.O.; Detwiler, R.J. Reaction kinetics of Portland cement mortars hydrated at different temperatures. Cem. Concr. Res. 1992, 22, 112–120. [Google Scholar] [CrossRef]
- Jayapalan, A.R.; Jue, M.L.; Kurtis, K.E. Nanoparticles and apparent activation energy of Portland cement. J. Am. Ceram. Soc. 2014, 97, 1534–1542. [Google Scholar] [CrossRef]
- Hou, P.; Kawashima, S.; Kong, D.; Corr, D.J.; Qian, J.; Shah, S.P. Modification effects of colloidal nanoSiO2 on cement hydration and its gel property. Compos. Part B Eng. 2013, 45, 440–448. [Google Scholar] [CrossRef]
- Schwarz, N.; DuBois, M.; Neithalath, N. Electrical conductivity based characterization of plain and coarse glass powder modified cement pastes. Cem. Concr. Compos. 2007, 29, 656–666. [Google Scholar] [CrossRef]
- Neithalath, N.; Persun, J.; Hossain, A. Hydration in high-performance cementitious systems containing vitreous calcium aluminosilicate or silica fume. Cem. Concr. Res. 2009, 39, 473–481. [Google Scholar] [CrossRef]
- Bu, Y.; Weiss, J. The influence of alkali content on the electrical resistivity and transport properties of cementitious materials. Cem. Concr. Compos. 2014, 51, 49–58. [Google Scholar] [CrossRef]
- Feng, X.; Garboczi, E.J.; Bentz, D.P.; Stutzman, P.E.; Mason, T.O. Estimation of the degree of hydration of blended cement pastes by a scanning electron microscope point-counting procedure. Cem. Concr. Res. 2004, 34, 1787–1793. [Google Scholar] [CrossRef]
- Jain, J.A.; Neithalath, N. Chloride transport in fly ash and glass powder modified concretes—Influence of test methods on microstructure. Cem. Concr. Compos. 2010, 32, 148–156. [Google Scholar] [CrossRef]
- Neithalath, N.; Weiss, J.; Olek, J. Characterizing Enhanced Porosity concrete using electrical impedance to predict acoustic and hydraulic performance. Cem. Concr. Res. 2006, 36, 2074–2085. [Google Scholar] [CrossRef]
- Snyder, K.A.; Ferraris, C.; Martys, N.S.; Garboczi, E.J. Using impedance spectroscopy to assess the viability of the rapid chloride test for determining concrete conductivity. J. Res. Natl. Inst. Stand. Technol. 2000, 105, 497. [Google Scholar] [CrossRef] [PubMed]
- Chindaprasirt, P.; Jaturapitakkul, C.; Sinsiri, T. Effect of fly ash fineness on microstructure of blended cement paste. Constr. Build. Mater. 2007, 21, 1534–1541. [Google Scholar] [CrossRef]
- Aly, M.; Hashmi, M.S.; Olabi, A.G.; Messeiry, M.; Abadir, E.F.; Hussain, A.I. Effect of colloidal nano-silica on the mechanical and physical behaviour of waste-glass cement mortar. Mater. Des. 2012, 33, 127–135. [Google Scholar] [CrossRef]
- Patel, H.H.; Bland, C.H.; Poole, A.B. The microstructure of concrete cured at elevated temperatures. Cem. Concr. Res. 1995, 25, 485–490. [Google Scholar] [CrossRef]
- Joint Committee on Powder Diffraction Standards; JCPDS-International Center for Diffraction Data: Newtown Square, PA, USA, 2000.
- Famy, C.; Scrivener, K.L.; Crumbie, A.K. What causes differences of C-S-H gel grey levels in backscattered electron images? Cem. Concr. Res. 2002, 32, 1465–1471. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Nanostructure of C-S-H: Current status. Adv. Cem. Based Mater. 1993, 1, 38–46. [Google Scholar] [CrossRef]








| Composition (% by Mass) | |
|---|---|
| Silica | 20.82 |
| Alumina | 4.98 |
| Iron oxide | 3.68 |
| Calcium oxide | 64.34 |
| Magnesium oxide | 0.91 |
| Sodium oxide | 0.19 |
| Potassium oxide | 0.41 |
| Sulfur trioxide | 2.79 |
| Titanium dioxide | 0.24 |
| Loss on ignition (LOI) | 2.03 |
| Sample | Rim | |||
|---|---|---|---|---|
| Ca/Si | Al/Ca | Ca/Si | Al/Ca | |
| CP (RT) | 1.90 | 0.06 | - | - |
| 0.8% TiO2 (RT) | 1.81 | 0.08 | - | - |
| CP (60 °C) | 1.99 | 0.04 | 1.95 | 0.04 |
| 0.8% TiO2 (60 °C) | 1.97 | 0.05 | 1.89 | 0.04 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimenta Teixeira, K.; Perdigão Rocha, I.; De Sá Carneiro, L.; Flores, J.; Dauer, E.A.; Ghahremaninezhad, A. The Effect of Curing Temperature on the Properties of Cement Pastes Modified with TiO2 Nanoparticles. Materials 2016, 9, 952. https://doi.org/10.3390/ma9110952
Pimenta Teixeira K, Perdigão Rocha I, De Sá Carneiro L, Flores J, Dauer EA, Ghahremaninezhad A. The Effect of Curing Temperature on the Properties of Cement Pastes Modified with TiO2 Nanoparticles. Materials. 2016; 9(11):952. https://doi.org/10.3390/ma9110952
Chicago/Turabian StylePimenta Teixeira, Karine, Isadora Perdigão Rocha, Leticia De Sá Carneiro, Jessica Flores, Edward A. Dauer, and Ali Ghahremaninezhad. 2016. "The Effect of Curing Temperature on the Properties of Cement Pastes Modified with TiO2 Nanoparticles" Materials 9, no. 11: 952. https://doi.org/10.3390/ma9110952





