Effect of A-Site Cation Ordering on Chemical Stability, Oxygen Stoichiometry and Electrical Conductivity in Layered LaBaCo2O5+δ Double Perovskite
Abstract
:1. Introduction
2. Results
3. Discussion
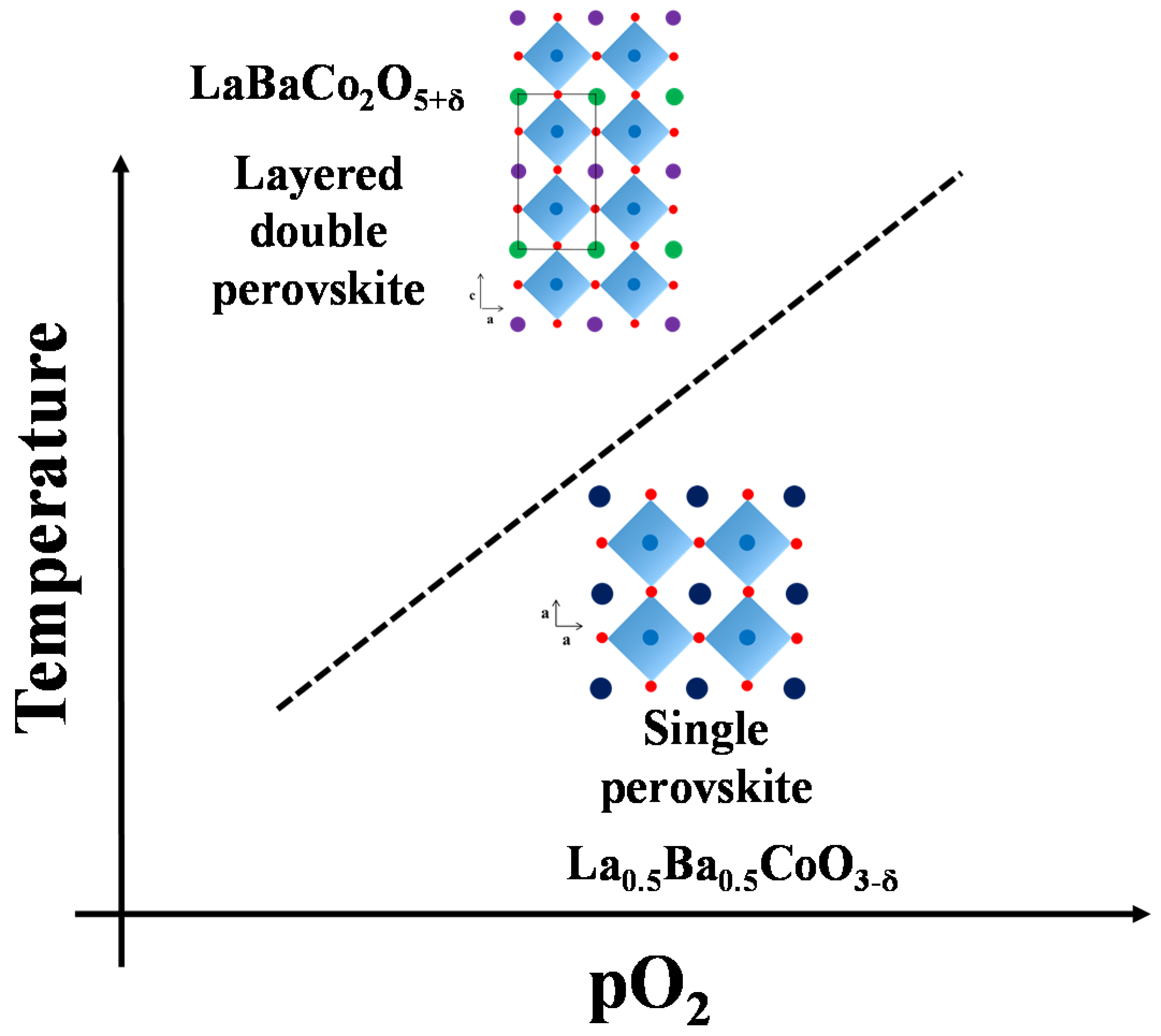
3.1. Crystal Structure: Ordering Effects
3.2. Thermal/Chemical Expansion and Electrical Conductivity
4. Materials and Methods
4.1. Synthesis of the Materials
4.2. Structural Characterization at Room and High Temperature
4.3. Thermogravimetrical Analysis
4.4. Electrical Conductivity
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RT | Room Temperature |
| LDP | Layered double perovskite |
| SOFC | Solid oxide fuel cells |
| XRD | X-Ray Diffraction data |
| YSZ | Yttria-stabilised zirconia |
| SEM | Scanning electron microscopy |
| HT-XRD | High temperature x-ray diffraction |
| PV-TCHZ | Thomson Cox-Hastings pseudo-Voigt |
| TGA | Thermogravimetric analysis |
| TEC | Thermal expansion coefficient |
References
- Sengodan, S.; Choi, S.; Jun, A.; Shin, T.H.; Ju, Y.W.; Jeong, H.Y.; Shin, J.; Irvine, J.T.S.; Kim, G. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 2015, 14, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Broux, T.; Bahout, M.; Hanlon, J.M.; Hernandez, O.; Paofai, S.; Berenov, A.; Skinner, S.J. High temperature structural stability, electrical properties and chemical reactivity of NdBaCo2-xMnxO5+δ (0 ≤ x ≤ 2) for use as cathodes in solid oxide fuel cells. J. Mater. Chem. A 2014, 2, 17015–17023. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Reimus, L.; Brodersen, P.; Mims, C.A. Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+x with a perovskite related structure and ordered a cations. J. Mater. Chem. 2007, 17, 2500–2505. [Google Scholar] [CrossRef]
- Tarancón, A.; Burriel, M.; Santiso, J.; Skinner, S.J.; Kilner, J.A. Advances in layered oxide cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 2010, 20, 3799–3813. [Google Scholar] [CrossRef]
- Tarancón, A.; Skinner, S.J.; Chater, R.J.; Hernández-Ramírez, F.; Kilner, J.A. Layered perovskites as promising cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 2007, 17, 3175–3181. [Google Scholar] [CrossRef]
- Pelosato, R.; Cordaro, G.; Stucchi, D.; Cristiani, C.; Dotelli, G. Cobalt based layered perovskites as cathode material for intermediate temperature Solid Oxide Fuel Cells: A brief review. J. Power Sources 2015, 298, 46–67. [Google Scholar] [CrossRef]
- Kim, J.H.; Manthiram, A. LnBaCo2O5+δ oxides as cathodes for intermediate-temperature solid oxide fuel cells. J. Electrochem. Soc. 2008, 155, B385–B390. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. Layered LnBaCo2O5+δ perovskite cathodes for solid oxide fuel cells: An overview and perspective. J. Mater. Chem. A 2015, 3, 24195–24210. [Google Scholar] [CrossRef]
- Kim, J.P.; Pyo, D.W.; Magnone, E.; Park, J.H. Preparation and oxygen permeability of ReBaCo2O5+δ (Re = Pr, Nd, Y) ceramic membranes. Adv. Mater. Res. 2012, 560–561, 959–964. [Google Scholar] [CrossRef]
- Zhang, K.; Ge, L.; Ran, R.; Shao, Z.; Liu, S. Synthesis, characterization and evaluation of cation-ordered LnBaCo2O5+δ as materials of oxygen permeation membranes and cathodes of SOFCs. Acta Mater. 2008, 56, 4876–4889. [Google Scholar] [CrossRef]
- King, G.; Woodward, P.M. Cation ordering in perovskites. J. Mater. Chem. 2010, 20, 5785–5796. [Google Scholar] [CrossRef]
- Troyanchuk, I.O.; Kasper, N.V.; Khalyavin, D.D.; Szymczak, H.; Szymczak, R.; Baran, M. Magnetic and electrical transport properties of orthocobaltites R0.5Ba0.5CoO3 (R = La, Pr, Nd, Sm, Eu, Gd, Tb, Dy). Phys. Rev. B 1998, 58, 2418–2421. [Google Scholar] [CrossRef]
- Maignan, A.; Martin, C.; Pelloquin, D.; Nguyen, N.; Raveau, B. Structural and Magnetic Studies of Ordered Oxygen-Deficient Perovskites LnBaCo2O5+δ, Closely Related to the “112” Structure. J. Solid State Chem. 1999, 142, 247–260. [Google Scholar] [CrossRef]
- Muñoz-Gil, D.; Ávila-Brande, D.; Urones-Garrote, E.; García-Martín, S. Ordering effects in the crystal structure and electrochemical properties of the Gd0.5Ba0.5Mn0.5Fe0.5O3-δ perovskite. Dalton Trans. 2015, 44, 10867–10874. [Google Scholar] [CrossRef] [PubMed]
- Rautama, E.L.; Caignaert, V.; Boullay, P.; Kundu, A.K.; Pralong, V.; Karppinen, M.; Ritter, C.; Raveau, B. New Member of the “112” Family, LaBaCo2O5.5: Synthesis, Structure, and Magnetism. Chem. Mater. 2008, 21, 102–109. [Google Scholar] [CrossRef]
- Streule, S.; Podlesnyak, A.; Mesot, J.; Medarde, M.; Conder, K.; Pomjakushina, E.; Mitberg, E.; Kozhevnikov, V. Effect of oxygen ordering on the structural and magnetic properties of the layered perovskites PrBaCo2O5+δ. J. Phys. Condens. Matter 2005, 17, 3317–3324. [Google Scholar] [CrossRef]
- Frontera, C.; Caneiro, A.; Carrillo, A.E.; Oró-Solé, J.; García-Muñoz, J.L. Tailoring oxygen content on PrBaCo2O5+δ layered cobaltites. Chem. Mater. 2005, 17, 5439–5445. [Google Scholar] [CrossRef]
- Burley, J.C.; Mitchell, J.F.; Short, S.; Miller, D.; Tang, Y. Structural and Magnetic Chemistry of NdBaCo2O5+δ. J. Solid State Chem. 2003, 170, 339–350. [Google Scholar] [CrossRef]
- Akahoshi, D.; Ueda, Y. Oxygen nonstoichiometry, structures, and physical properties of YBaCo2O5+x (0.00 ≤ x ≤ 0.52). J. Solid State Chem. 2001, 156, 355–363. [Google Scholar] [CrossRef]
- Kuang, X.; Allix, M.; Ibberson, R.M.; Claridge, J.B.; Niu, H.; Rosseinsky, M.J. Oxygen Vacancy Ordering Phenomena in the Mixed-Conducting Hexagonal Perovskite Ba7Y2Mn3Ti2O20. Chem. Mater. 2007, 19, 2884–2893. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Ruiz-Diaz, J.E.; Zhen, Y.S. Oxide-ion conduction in Ba2In2O5 and Ba3In2MO8 (M = Ce, Hf, or Zr). Solid State Ion. 1990, 44, 21–31. [Google Scholar] [CrossRef]
- Colville, A.A.; Geller, S. The crystal structure of brownmillerite, CaFeAlO2.5. Acta Crystallogr. B 1971, 27, 2311–2315. [Google Scholar] [CrossRef]
- Setevich, C.; Mogni, L.; Caneiro, A.; Prado, F. Characterization of the La1-xBaxCoO3-δ (0 ≤ x ≤ 1) system as cathode material for it-sofc. J. Electrochem. Soc. 2012, 159, B72–B79. [Google Scholar] [CrossRef]
- Atkinson, A.; Ramos, T.M.G.M. Chemically-induced stresses in ceramic oxygen ion-conducting membranes. Solid State Ion. 2000, 129, 259–269. [Google Scholar] [CrossRef]
- Bishop, S.R.; Duncan, K.; Wachsman, E.D. Thermo-chemical expansion of SOFC materials. ECS Trans. 2006, 1, 13–21. [Google Scholar]
- Sato, K.; Yashiro, K.; Kawada, T.; Yugami, H.; Hashida, T.; Mizusaki, J. Fracture process of nonstoichiometric oxide based solid oxide fuel cell under oxidizing/reducing gradient conditions. J. Power Sources 2010, 195, 5481–5486. [Google Scholar] [CrossRef]
- Tietz, F. Thermal expansion of SOFC materials. Ionics 1999, 5, 129–139. [Google Scholar] [CrossRef]
- Hendriksen, P.V.; Larsen, P.H.; Mogensen, M.; Poulsen, F.W.; Wiik, K. Prospects and problems of dense oxygen permeable membranes. Catal. Today 2000, 56, 283–295. [Google Scholar] [CrossRef]
- Rautama, E.-L.; Boullay, P.; Kundu, A.K.; Caignaert, V.; Pralong, V.; Karppinen, M.; Raveau, B. Cationic Ordering and Microstructural Effects in the Ferromagnetic Perovskite La0.5Ba0.5CoO3: Impact upon Magnetotransport Properties. Chem. Mater. 2008, 20, 2742–2750. [Google Scholar] [CrossRef]
- Amin, R.; Karan, K. Characterization of La0.5Ba0.5CoO3-δ as a SOFC Cathode Material. J. Electrochem. Soc. 2010, 157, B285–B291. [Google Scholar] [CrossRef]
- Pang, S.; Jiang, X.; Li, X.; Su, Z.; Xu, H.; Xu, Q.; Chen, C. Characterization of cation-ordered perovskite oxide LaBaCo2O5+δ as cathode of intermediate-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2012, 37, 6836–6843. [Google Scholar] [CrossRef]
- Kim, J.H.; Mogni, L.; Prado, F.; Caneiro, A.; Alonso, J.A.; Manthiram, A. High temperature crystal chemistry and oxygen permeation properties of the mixed ionic-electronic conductors LnBaCo2O5+δ (Ln = Lanthanide). J. Electrochem. Soc. 2009, 156, B1376–B1382. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J. Relations between the Concentrations of Imperfections in Crystalline Solids. Solid State Phys. 1956, 3, 307–435. [Google Scholar]
- Garces, D.; Setevich, C.F.; Caneiro, A.; Cuello, G.J.; Mogni, L. Effect of cationic order-disorder on the transport properties of LaBaCo2O6-δ and La0.5Ba0.5CoO3-δ perovskites. J. Appl. Crystallogr. 2014, 47, 325–334. [Google Scholar] [CrossRef]
- Rautama, E.-L.; Karppinen, M. R-site varied series of RBaCo2O5.5 (R2Ba2Co4O11) compounds with precisely controlled oxygen content. J. Solid State Chem. 2010, 183, 1102–1107. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Ivanov, I.L.; Malyshkin, D.A.; Zuev, A.Y. Oxygen content, crystal structure and chemical expansion of PrBaCo2-xFexO6-δ double perovskites. Dalton Trans. 2014, 43, 11862–11866. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, N.; Asaka, T.; Kudo, T.; Fukuda, K.; Yasuhara, A.; Abe, N.; Arima, T.H. Structural evolution of GdBaCo2O5+σ (δ = 7/18) at elevated temperatures. Chem. Mater. 2014, 26, 6503–6517. [Google Scholar] [CrossRef]
- McKinlay, A.; Connor, P.; Irvine, J.T.S.; Zhou, W. Structural chemistry and conductivity of a solid solution of YBa1-xSrxCo2O5+δ. J. Phys. Chem. C 2007, 111, 19120–19125. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.N.; Bi, Z.; Manthiram, A.; Paranthaman, M.P.; Huq, A. Overcoming phase instability of RBaCo2O5+δ (R = y and Ho) by Sr substitution for application as cathodes in solid oxide fuel cells. Solid State Ion. 2013, 253, 81–87. [Google Scholar] [CrossRef]
- Kim, J.H.; Prado, F.; Manthiram, A. Characterization of GdBa1-xSrxCo2O5+δ (0 ≤ x ≤ 1.0) Double Perovskites as Cathodes for Solid Oxide Fuel Cells. J. Electrochem. Soc. 2008, 155, B1023–B1028. [Google Scholar] [CrossRef]
- Mogni, L.; Prado, F.; Jiménez, C.; Caneiro, A. Oxygen order–disorder phase transition in layered GdBaCo2O5+δ perovskite: Thermodynamic and transport properties. Solid State Ion. 2013, 240, 19–28. [Google Scholar] [CrossRef]
- Adler, S.B. Chemical Expansivity of Electrochemical Ceramics. J. Am. Ceram. Soc. 2001, 84, 2117–2119. [Google Scholar] [CrossRef]
- Oygarden, V.; Lein, H.L.; Grande, T. Structure, thermal expansion and electrical conductivity of Nb-substituted LaCoO3. J. Solid State Chem. 2012, 192, 246–254. [Google Scholar] [CrossRef]
- Chen, X.; Grande, T. Anisotropic chemical expansion of La1-xSrxCoO3-δ. Chem. Mater. 2013, 25, 927–934. [Google Scholar] [CrossRef]
- Petrov, A.N.; Cherepanov, V.A.; Zuev, A.Y. Thermodynamics, defect structure, and charge transfer in doped lanthanum cobaltites: An overview. J. Solid State Electrochem. 2006, 10, 517–537. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Wærnhus, I.; Vullum, P.E.; Holmestad, R.; Grande, T.; Wiik, K. Electronic properties of polycrystalline LaFeO3. Part I: Experimental results and the qualitative role of Schottky defects. Solid State Ion. 2005, 176, 2783–2790. [Google Scholar] [CrossRef]






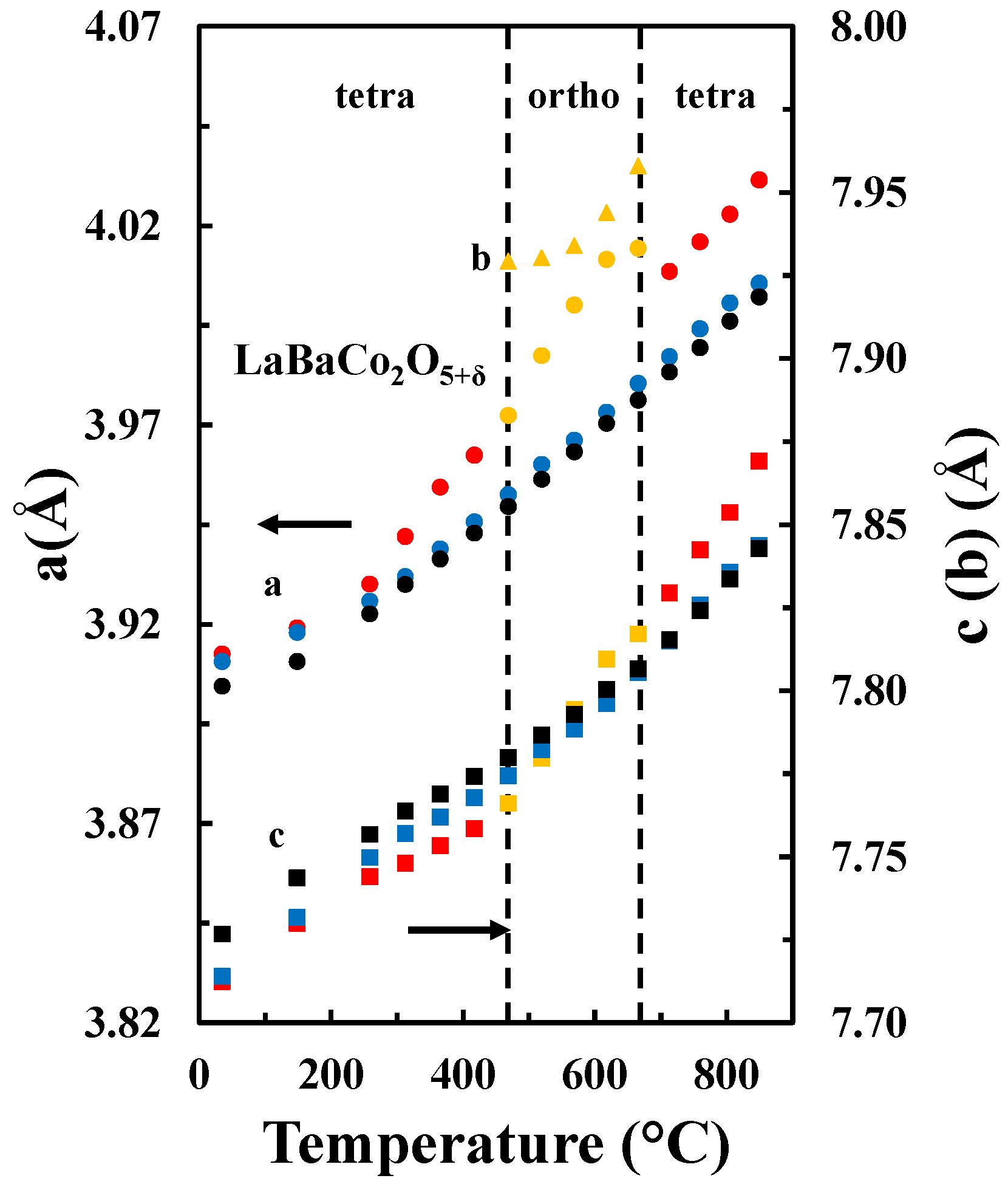






| Space Group | Lattice Par. (Å) | Atom | x | y | z | Occ | Beq | Rwp | Rexp | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| a = 3.9410 (2) | La | 0 | 0 | 0 | 0.5 | 3.8 (1) | 3.2 | 2.72 | 1.18 | |
| Ba | 0 | 0 | 0 | 0.5 | 3.8 (1) | |||||
| Co | 0.5 | 0.5 | 0.5 | 1 | 2.7 (2) | |||||
| O | 0 | 0.5 | 0.5 | 1 | 4.8 (2) | |||||
| P4/mmm | a = 3.9633 (2) | La | 0 | 0 | 0 | 1 | 3.9 (1) | 3.38 | 2.70 | 1.25 |
| c = 7.7928 (3) | Ba | 0 | 0 | 0.5 | 1 | 3.9 (1) | ||||
| Co | 0.5 | 0.5 | 0.253 (6) | 1 | 3.0 (1) | |||||
| O1 | 0.5 | 0.5 | 0 | 1 | 6.7 (3) | |||||
| O2 | 0 | 0.5 | 0.234 (8) | 1 | 6.7 (3) | |||||
| O3 | 0.5 | 0.5 | 0.5 | 1 | 6.7 (3) | |||||
| Pmmm | a = 4.0001 (2) | La | 0.5 | 0.252 (5) | 0 | 1 | 3.3 (2) | 4.18 | 3.12 | 1.61 |
| b = 7.9340 (5) | Ba | 0.5 | 0.251 (4) | 0.5 | 1 | 3.3 (2) | ||||
| c = 7.7942 (5) | Co1 | 0 | 0 | 0.253 (7) | 1 | 2.8 (2) | ||||
| Co2 | 0 | 0.5 | 0.240 (6) | 1 | 2.8 (2) | |||||
| O1 | 0 | 0.244 (20) | 0.204 (5) | 1 | 3.7 (4) | |||||
| O2 | 0.5 | 0 | 0.22 (3) | 1 | 3.7 (4) | |||||
| O3 | 0.5 | 0.5 | 0.22 (3) | 1 | 3.7 (4) | |||||
| O4 | 0 | 0 | 0.5 | 1 | 3.7 (4) | |||||
| O5 | 0 | 0.5 | 0.5 | 1 | 3.7 (4) | |||||
| O6 | 0 | 0.5 | 0 | 1 | 3.7 (4) |
| O2 | ||||
| Material | T range (°C) | αa (10−6 K−1) | αc (10−6 K−1) | αi (10−6 K−1) |
| La0.5Ba0.5CoO3-δ | RT-250 | 19 ± 1 | ||
| 300–800 | 31.3 ± 0.1 | |||
| LaBaCo2O5+δ | RT-200 | 13 ± 1 | 18 ± 1 | 15 ± 1 |
| 250–750 | 34.6 ± 0.2 | 16.1 ± 0.3 | 29.3 ± 0.3 | |
| 800–850 | 36.4 ± 0.2 | 25.2 ± 0.3 | 32.8 ± 0.3 | |
| N2 | ||||
| Material | T range (°C) | αa (10−6 K−1) | αc (10−6 K−1) | αi (10−6 K−1) |
| La0.5Ba0.5CoO3-δ | RT-250 | 15 ± 1 | ||
| 300–800 | 37.4 ± 0.3 | |||
| LaBaCo2O5+δ | RT-200 | 20 ± 1 | 18 ± 1 | 20 ± 1 |
| 250–400 | 50.9 ± 0.5 | 13.6 ± 0.4 | 38.4 ± 7.1 | |
| 450–650 | 53.0 ± 2.6 | 33.2 ± 0.8 | 39.3 ± 8.5 | |
| 700–850 | 41.9 ± 0.5 | 37.1 ± 0.6 | 40.3 ± 5.4 | |
| LaBaCo2O5+δ | La0.5Ba0.5CoO3-δ | |||
|---|---|---|---|---|
| Chemical Strain | Chemical Strain | |||
| T (°C) | (Δa/ao)/Δδ | (Δc/co)/Δδ | ⅓ (ΔV/Vo)/Δδ | (Δa/ao)/Δδ |
| 450 | 0.05 ± 0.01 | -0.02 ± 0.01 | 0.02 ± 0.01 | 0.07 ± 0.01 |
| 550 | 0.07 ± 0.01 | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.07 ± 0.01 |
| 650 | 0.06 ± 0.01 | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.07 ± 0.01 |
| 750 | 0.04 ± 0.01 | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.01 |
| 850 | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.06 ± 0.01 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernuy-Lopez, C.; Høydalsvik, K.; Einarsrud, M.-A.; Grande, T. Effect of A-Site Cation Ordering on Chemical Stability, Oxygen Stoichiometry and Electrical Conductivity in Layered LaBaCo2O5+δ Double Perovskite. Materials 2016, 9, 154. https://doi.org/10.3390/ma9030154
Bernuy-Lopez C, Høydalsvik K, Einarsrud M-A, Grande T. Effect of A-Site Cation Ordering on Chemical Stability, Oxygen Stoichiometry and Electrical Conductivity in Layered LaBaCo2O5+δ Double Perovskite. Materials. 2016; 9(3):154. https://doi.org/10.3390/ma9030154
Chicago/Turabian StyleBernuy-Lopez, Carlos, Kristin Høydalsvik, Mari-Ann Einarsrud, and Tor Grande. 2016. "Effect of A-Site Cation Ordering on Chemical Stability, Oxygen Stoichiometry and Electrical Conductivity in Layered LaBaCo2O5+δ Double Perovskite" Materials 9, no. 3: 154. https://doi.org/10.3390/ma9030154






