Temperature-Driven Structural and Morphological Evolution of Zinc Oxide Nano-Coalesced Microstructures and Its Defect-Related Photoluminescence Properties
Abstract
:1. Introduction
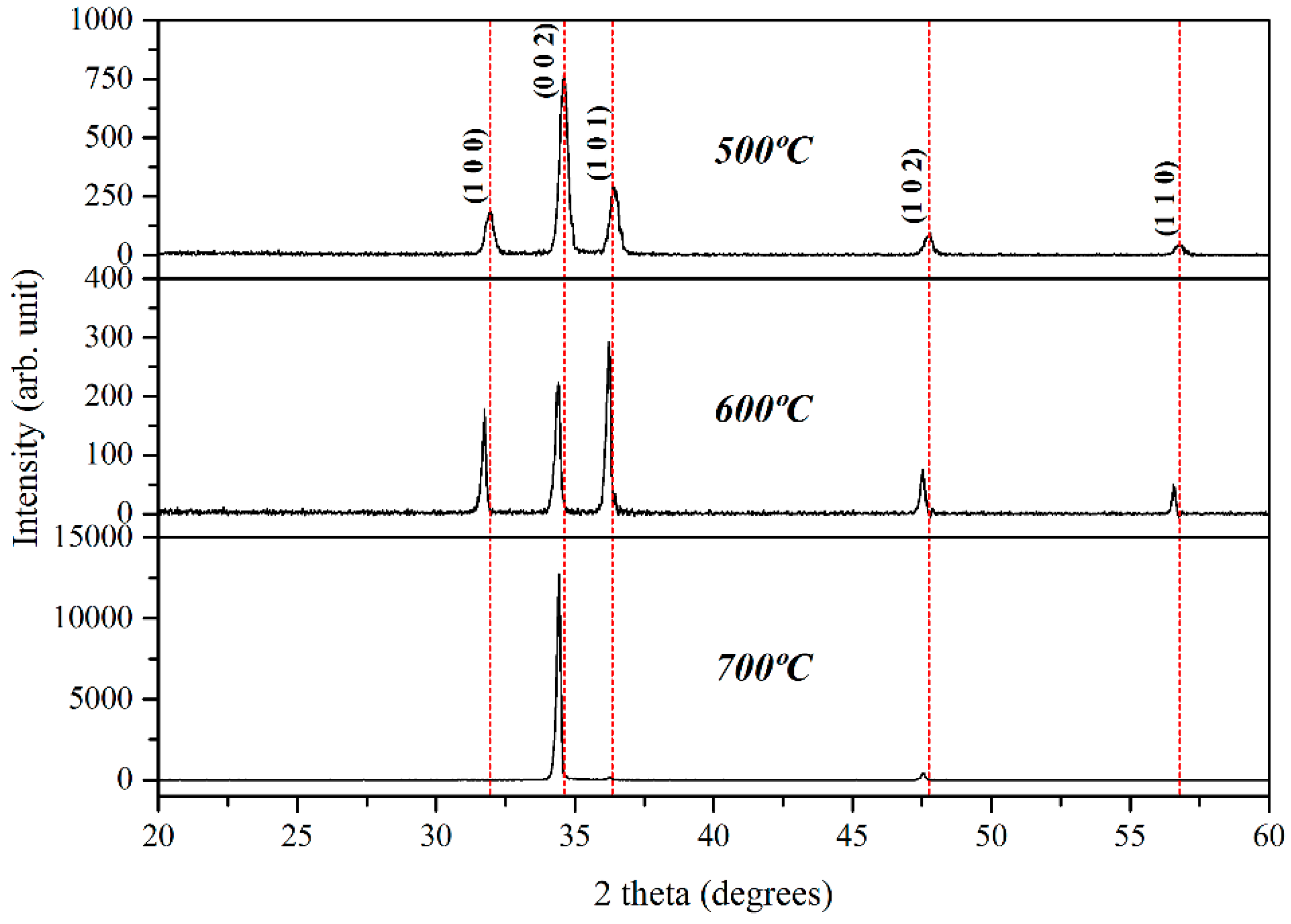
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bunn, C.W. The lattice dimensions of zinc oxide. Proc. Phys. Soc. 1935, 47, 835–842. [Google Scholar] [CrossRef]
- Jagadish, C.; Pearton, S.J. Zinc Oxide Bulk, Thin Films and Nanostructures: Processing, Properties and Applications; Elsevier Ltd.: Hong Kong, China, 2006; pp. 1–21. [Google Scholar]
- Aneesh, P.M.; Kalarikkal, N. ZnO nanostructures and its luminescent properties. In Advanced Nanomaterials: Synthesis, Properties and Applications; Thomas, S., Kalarikkal, N., Stephen, A.M., Raneesh, B., Haghi, A.K., Eds.; Apple Academic Press Inc.: Oakville, ON, Canada, 2014; pp. 117–148. [Google Scholar]
- Abdulgafour, H.I.; Hassan, Z.; Al-Hardan, N.; Yan, F.K. Growth of zinc oxide nanoflowers by thermal evaporation method. Phys. B Condens. Matter. 2010, 405, 2570–2572. [Google Scholar] [CrossRef]
- Umar, A.; Suh, E.K.; Hahn, Y.B. Non-catalytic growth of high-aspect ratio ZnO nanowires by thermal evaporation. Solid State Commun. 2006, 139, 447–451. [Google Scholar] [CrossRef]
- Lim, Y.S.; Park, J.W.; Kim, M.S.; Kim, J. Effect of carbon source on the carbothermal reduction for the fabrication of ZnO nanostructure. Appl. Surf. Sci. 2006, 253, 1601–1605. [Google Scholar] [CrossRef]
- Ellmer, K. Magnetron sputtering of transparent conductive zinc oxide: Relation between the sputtering parameters and the electronic properties. J. Phys. D Appl. Phys. 2000, 33, 17–32. [Google Scholar] [CrossRef]
- Yoshida, T.; Komatsu, D.; Shimokawa, N.; Minoura, H. Mechanism of cathodic electrodeposition of zinc oxide thin films from aqueous zinc nitrate baths. Thin Solid Films 2004, 451, 166–169. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Abdul Hamid, M.A.; Jalar, A.; Lim, K.K.; Shamsudin, R.; Majlis, B.Y. Bi-structure ZnO prepared via cathodic electrodeposition method. Int. J. Electrochem. Sci. 2013, 8, 2430–2439. [Google Scholar]
- Barush, S.; Dutta, H. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 13001–13018. [Google Scholar] [CrossRef]
- Djurisic, A.B.; Chen, X.Y.; Leung, Y.H. Recent progress in hydrothermal synthesis of zinc oxide nanomaterials. Recent Pat. Nanotechnol. 2012, 6, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Wang, L.Y.; Liu, H.; Yang, C.H.; Xu, H.Y.; Li, X.; Cui, H.M. Sol-gel preparation of transparent zinc oxide films with highly preferential crystal orientation. Vacuum 2004, 77, 57–62. [Google Scholar] [CrossRef]
- Znaidi, L. Sol-gel-deposited ZnO thin films: A review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Amin, G.; Asif, M.H.; Zainelabdin, A.; Zaman, S.; Nur, O.; Willander, M. Influence of pH, precursor concentration, growth time, and temperature on the morphology of ZnO nanostructures grown by the hydrothermal method. J. Nanomater. 2011, 5, 1–5. [Google Scholar] [CrossRef]
- Rusli, N.I.; Tanikawa, M.; Mahmood, M.R.; Yasui, K.; Hashim, A.M. Growth of high-density zinc oxide nanorods on porous silicon by thermal evaporation. Materials 2012, 9, 2817–2832. [Google Scholar] [CrossRef]
- Chin, H.S.; Chao, L.S. The effect of thermal annealing processes on structural and photoluminescence of Zinc oxide thin film. J. Nanomater. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Fang, F.; Zhao, D.X.; Zhang, J.Y.; Shen, D.Z.; Lu, Y.M.; Fan, X.W.; Li, B.H.; Wang, X.H. The influence of growth temperature on ZnO nanowires. Mater. Lett. 2008, 62, 1092–1095. [Google Scholar] [CrossRef]
- Al-Azri, K.; Nor, R.M.; Amin, Y.M.; Al-Ruqeishi, M.S. Effect of source temperature on the morphology and photoluminescence properties of ZnO nanostructures. Appl. Surf. Sci. 2010, 256, 5957–5960. [Google Scholar] [CrossRef]
- Yousefi, R.; Muhamad, M.R.; Zak, A.K. The effect of source temperature on morphological and optical properties of ZnO nanowires grown using a modified thermal evaporation set-up. Curr. Appl. Phys. 2011, 11, 767–770. [Google Scholar] [CrossRef]
- Liu, F.; Cao, P.J.; Zhang, H.R.; Sheng, C.M.; Wang, Z.; Li, J.Q.; Gao, H.J. Well-aligned zinc oxide nanorods and nanowires prepared without catalyst. J. Cryst. Growth 2005, 274, 126–131. [Google Scholar] [CrossRef]
- Calestani, D.; Zha, M.Z.; Zanotti, L.; Villani, M.; Zappettini, A. Low temperature thermal evaporation growth of aligned ZnO nanorods on ZnO film: A growth mechanism promoted by Zn nanoclusters on polar surfaces. CrystEngComm 2011, 13, 1707–1712. [Google Scholar] [CrossRef]
- Lee, G.H. Relationship between crystal structure and photoluminescence properties of ZnO films formed by oxidation of metallic zinc. Electron. Mater. Lett. 2010, 6, 155–159. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, C.; Cai, R.S.; Wang, Y.Q.; Zhou, G.W. Temperature-dependent growth mechanism and microstructure of ZnO nanostructures grown from the thermal oxidation of zinc. J. Cryst. Growth 2014, 390, 101–108. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H. Optical properties of ZnO nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.Y.; Andelman, T.; Neumark, G.F.; O’Brien, S.; Kuskovsky, L.L. Origin of defect-related green emission from ZnO nanoparticles: Effect of surface modification. Nanoscale Res. Lett. 2007, 2, 297–302. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Hse, Y.F.; Ding, L.; Ge, W.K.; Zhong, Y.C.; Wong, K.S.; Chan, W.K.; Tam, H.L. Defect emissions in ZnO nanostructures. Nanotechnology 2007, 18, 95702–95710. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Jalar, A.; Abdul Hamid, M.A.; Lim, K.K.; Ahmed, N.M.; Shamsudin, R. A wide-band UV photodiode based on n-ZnO/p-Si heterojunctions. Sens. Actuators A Phys. 2014, 207, 61–66. [Google Scholar] [CrossRef]
- Xu, C.K.; Kim, D.G.; Chun, J.H.; Rho, K.H.; Chon, B.H.; Hong, S.S.; Joo, T.H. Temperature-controlled growth of ZnO nanowires and nanoplates in the temperature range 250 °C–300 °C. J. Phys. Chem. B 2006, 110, 21741–21746. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, Z.L. Scanning Microscopy for Nanotechnology: Techniques and Applications; Springer: New York, NY, USA, 2007. [Google Scholar]
- Dalal, S.H.; Baptista, D.L.; Teo, K.B.K.; Lacerda, R.G.; Jafferson, D.A.; Wilne, W.I. Controllable growth of vertically aligned zinc oxide nanowires using vapour deposition. Nanotechnology 2006, 17, 4811–4818. [Google Scholar] [CrossRef]
- Dai, Z.R.; Pan, Z.W.; Wang, Z.L. Novel nanostructures of functional oxides synthesized by thermal evaporation. Adv. Funct. Mater. 2003, 13, 9–24. [Google Scholar] [CrossRef]
- Kup, S.Y.; Lin, H.I. Field emission characteristics of zinc oxide nanowires synthesized by vapor-solid process. Nanoscale Res. Lett. 2014, 9, 1–5. [Google Scholar]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation, Physical Electronic Division: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Chen, M.; Wang, X.; Yu, Y.H.; Pei, Z.L.; Bai, X.D.; Sun, C.; Huang, R.F.; Wen, L.S. X-ray photoelectron spectroscopy and auger electron spectroscopy studies of Al-doped ZnO films. Appl. Surf. Sci. 2000, 158, 134–140. [Google Scholar] [CrossRef]
- Wang, Z.G.; Zu, X.T.; Zhu, S.; Wang, L.M. Green luminescence originates from surface defects in ZnO nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2006, 35, 199–202. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chung, J.Y.; Lim, S.W. Improvement of optical properties of post-annealed ZnO nanorods. Phys. E Low Dimens. Syst. Nanostruct. 2010, 42, 2143–2146. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Qin, J.Q.; Xue, Y.N.; Yu, P.F.; Zhang, B.; Wang, L.M.; Liu, R.P. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H. A study of transparent conductive aluminum-doped zinc oxide fabricated on a flexible polyethersuplhone (PES) substrate. J. Korean Phys. Soc. 2007, 51, 1968–1972. [Google Scholar] [CrossRef]
- Tay, Y.Y.; Li, S.; Sun, C.Q.; Chen, P. Size dependence of Zn 2p3/2 binding energy in nanocrystalline ZnO. Appl. Phys. Lett. 2006, 88, 1–3. [Google Scholar] [CrossRef]
- Shtepliuk, I.; Khyzhun, O.; Lashkarev, G.; Khomyak, V.; Lazorenko, V. XPS and raman characterizations of Zn1−xCdxO films grown at the different growth conditions. Acta Phys. Pol. A 2012, 122, 1034–1038. [Google Scholar] [CrossRef]
- Wang, Q.P.; Zhang, D.H.; Ma, H.L.; Zhang, X.H.; Zhang, X.J. Photoluminescence of ZnO films prepared by r.f. sputtering on different substrates. Appl. Surf. Sci. 2003, 220, 12–18. [Google Scholar] [CrossRef]
- Wang, Q.P.; Zhang, X.J.; Wang, G.Q.; Chen, S.H.; Wu, X.H.; Ma, H.L. Influence of excitation light wavelength on the photoluminescence properties for ZnO films prepared by magnetron sputtering. Appl. Surf. Sci. 2008, 254, 5100–5104. [Google Scholar] [CrossRef]
- Behera, D.; Acharya, B.S. Nano-star formation in Al-doped ZnO thin film deposited by dip-dry method and its characterization using atomic force microscopy, electron probe microscopy, photoluminescence and laser raman spectroscopy. J. Lumin. 2008, 128, 1577–1586. [Google Scholar] [CrossRef]
- Heiland, G.; Mollwo, E.; Stockmann, F. Electronic processes in zinc oxide. In Solid State Physics; Seitz, F., Turnbull, D., Eds.; Academic Press Inc.: New York, NY, USA, 1959; pp. 1577–1587. [Google Scholar]
- Dutta, S.; Chattopadhyay, S.; Sarkar, A.; Chakrabarti, M.; Sanyal, D.; Jana, D. Role of defects in tailoring structural, electrical and optical properties of ZnO. Prog. Mater. Sci. 2009, 54, 89–136. [Google Scholar] [CrossRef]
- Jin, B.J.; Im, S.; Lee, S.Y. Violet and UV luminescence emitted from ZnO thin films grown on sapphine by pulsed laser deposition. Thin Solid Films 2000, 366, 107–110. [Google Scholar] [CrossRef]
- Mishra, S.K.; Srivastava, R.K.; Prakash, S.G. ZnO nanoparticles: Structural, optical and photoconductivity characteristics. J. Alloys Compd. 2012, 539, 1–6. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanism behind green photoluminescence in ZnO phosphor powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Chandrinou, C.; Boukos, N.; Stogios, C.; Travlos, A. PL study of oxygen defect formation in ZnO nanorods. Microelectr. J. 2009, 40, 296–298. [Google Scholar] [CrossRef]
- Alvi, N.H.; Riza, M.; Tzamalis, G.; Nur, O.; Willander, M. Fabrication and characterization of high-brightness light emitting diodes based on n-ZnO nanorods grown by a low-temperature chemical method on p-4H-SiC and p-GaN. Semicond. Sci. Technol. 2010, 25, 1–5. [Google Scholar] [CrossRef]










| Sample | Component | Raw Area (cps eV) | Atomic Concentration (%) |
|---|---|---|---|
| 500 °C | Oxygen | 4871.236 | 58.18 |
| Zinc | 25,094.375 | 41.82 | |
| Total | 29,965.611 | 100.00 | |
| 600 °C | Oxygen | 1322.913 | 52.39 |
| Zinc | 8614.431 | 47.61 | |
| Total | 9937.344 | 100.00 | |
| 700 °C | Oxygen | 764.228 | 56.12 |
| Zinc | 4281.111 | 43.88 | |
| Total | 5045.339 | 100.00 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, K.; Abdul Hamid, M.A.; Shamsudin, R.; Al-Hardan, N.H.; Mansor, I.; Chiu, W. Temperature-Driven Structural and Morphological Evolution of Zinc Oxide Nano-Coalesced Microstructures and Its Defect-Related Photoluminescence Properties. Materials 2016, 9, 300. https://doi.org/10.3390/ma9040300
Lim K, Abdul Hamid MA, Shamsudin R, Al-Hardan NH, Mansor I, Chiu W. Temperature-Driven Structural and Morphological Evolution of Zinc Oxide Nano-Coalesced Microstructures and Its Defect-Related Photoluminescence Properties. Materials. 2016; 9(4):300. https://doi.org/10.3390/ma9040300
Chicago/Turabian StyleLim, Karkeng, Muhammad Azmi Abdul Hamid, Roslinda Shamsudin, N.H. Al-Hardan, Ishak Mansor, and Weesiong Chiu. 2016. "Temperature-Driven Structural and Morphological Evolution of Zinc Oxide Nano-Coalesced Microstructures and Its Defect-Related Photoluminescence Properties" Materials 9, no. 4: 300. https://doi.org/10.3390/ma9040300





