Optimising Ambient Setting Bayer Derived Fly Ash Geopolymers
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis
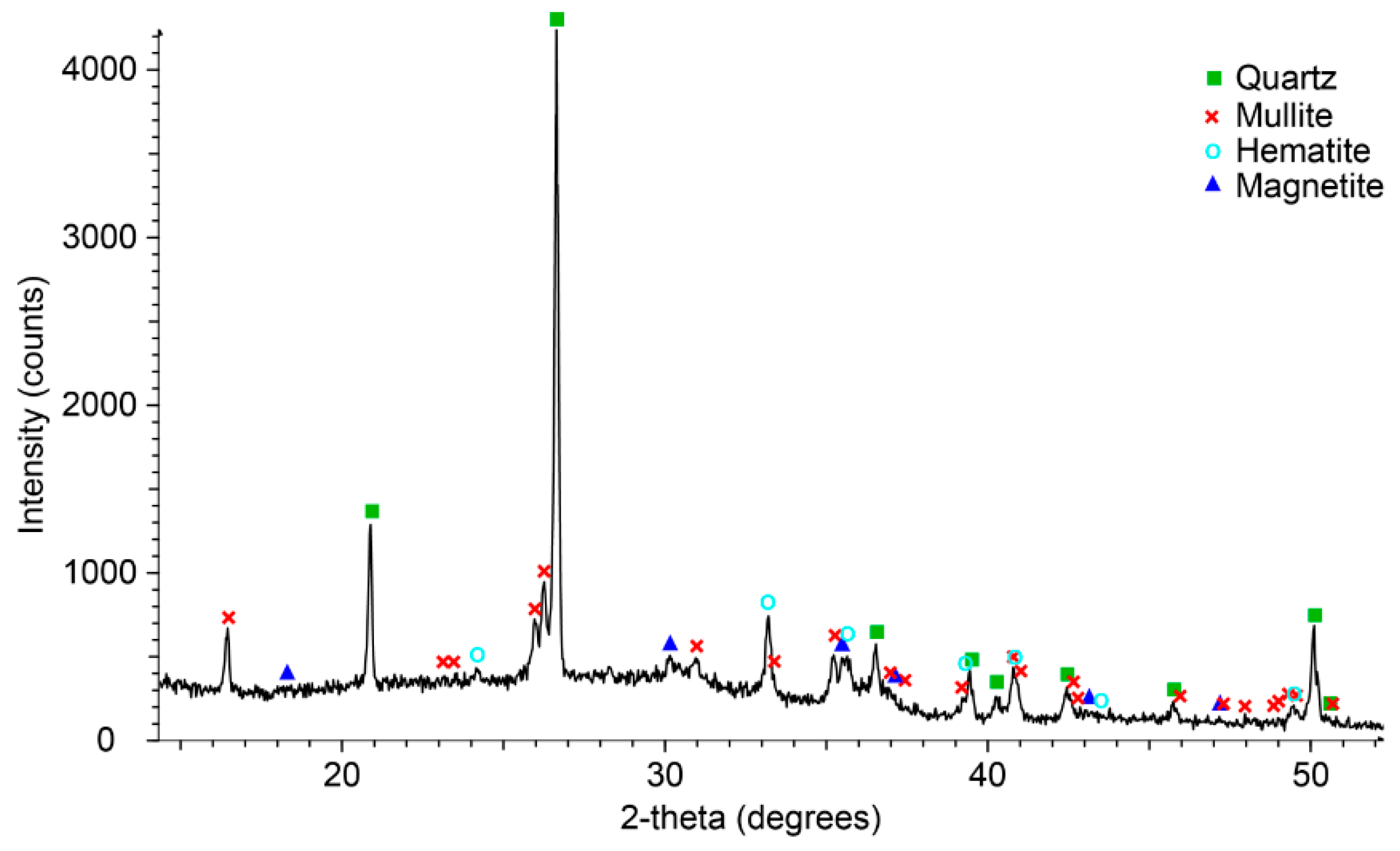
2.3. Characterisation
3. Results
3.1. Curing Temperature
3.2. Silica Fume Content
3.3. Ambient Curing
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rangan, B.V. Studies on Fly Ash-Based Geopolymer Concrete. Malays. Constr. Res. J. 2008, 3, 1–20. [Google Scholar]
- Rangan, B.V. Low-Calcium, Fly-Ash-Based Geopolymer Concrete. In Concrete Construction Engineering Handbook; Taylor and Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Rickard, W.; van Riessen, A.; Walls, P. Thermal Character of Geopolymers Synthesized from Class F Fly Ash Containing High Concentrations of Iron and a-Quartz. Int. J. Appl. Ceram. Technol. 2010, 7, 81–88. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Lee, M.; Chen-Tan, N.; van Riessen, A. Characterisation of class F fly ash geopolymer pastes immersed in acid and alkaline solutions. Cem. Concr. Compos. 2011, 33, 1086–1091. [Google Scholar] [CrossRef]
- McLellan, B.; Williams, R.; Lay, J.; van Riessen, A.; Corder, G. Costs and carbon emissions for geopolymer pastes in comparison to ordinary Portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, E.; McLellan, B.; van Riessen, A.; Nikraz, H. Embodied energy of Bayer derived Geopolymers. J. Clean. Prod. 2015, 99, 112–118. [Google Scholar] [CrossRef]
- Davidovits, J. Global Warning Impact on the cement and aggregate industries. World Resour. Rev. 1994, 6, 263–278. [Google Scholar]
- Davidovits, J. Environmentally driven geopolymer cement applications. In Proceedings of the Geopolymer 3rd International Conference: Turning Potential into Profit, Melbourne, Australia, 28–29 October 2002; Williams, J.R., Ed.; Geopolymer Institute: Melbourne, Australia, 2002. [Google Scholar]
- Tavor, D.; Wolfson, A.; Shamaev, A.; Shvarzman, A. Recycling of industrial waste water by its immobilization in geopolymer cement. Ind. Eng. Chem. Res. 2007, 46, 6801–6805. [Google Scholar] [CrossRef]
- Jamieson, E. Method for Management of Contaminants in Alkaline Process Liquors. International Patent WO 2008/017109 A1, 14 February 2008. [Google Scholar]
- Gourley, J. Geopolymers; opportunities for environmentally friendly construction materials. In Proceedings of the Materials 2003 Conference: Adaptive Materials for a Modern Society, Sydney, Australia, 1–3 October 2003.
- Gourley, J.; Johnson, G. Developments in Geopolymer Precast Concrete. In Proceedings of the International Workshop on Geopolymers and Geopolymer Concrete, Perth, Australia, 28–29 September 2005.
- Southam, D.; Brent, G.; Felipe, F.; Carr, C.; Hart, R.; Wright, K. Towards More Sustainable Mine Fills—Replacement of Ordinary Portland Cement with Geopolymer Cements. In Proceedings of the World Gold Conference, Cairns, Australia, 22–24 October 2007; pp. 22–24.
- Hart, R.; Lowe, J.; Southam, D.; Perera, D.; Walls, P.; Vance, E.; Gourley, T.; Wright, K. Aluminosilicate inorganic polymers from waste materials. In Proceedings of the Green Processing, Third International Conference on Sustainable Processing of Minerals and Metals, Newcastle, Australia, 5–6 June 2006; pp. 93–103.
- Sriwahyuni, A.; Nikraz, H.; Jamieson, E.; Cooling, D. Sustainable use of bauxite residue sand (red sand) in concrete. In Proceedings of the Green Processing, Third International Conference on Sustainable Processing of Minerals and Metals, Newcastle, Australia, 5–6 June 2006; pp. 105–122.
- Jitsangiam, P.; Nikraz, H.; Jamieson, E. Sustainable use of a Bauxite Residue (red sand) in terms of Roadway Materials. In Proceedings of the 2nd International Conference on Sustainable Engineering and Science, Auckland, New Zealand, 20–23 February 2007.
- Jitsangiam, P.; Nikraz, H.; Jamieson, E.; Kitanovich, R.; Siripun, K. Sustainable use of a Bauxite Residue (red sand) as Highway Embankment Materials. In Proceedings of the 3rd International Conference on Sustainability Engineering and Science, Auckland, New Zealand, 9–12 December 2008; The New Zealand Society for Sustainability Engineering and Science: Auckland, New Zealand, 2008. [Google Scholar]
- Attiwell, S.; Jamieson, E.; Jones, A.; Cooling, D.; Myers, L.; Lee, F.; Malley, I.; Tate, D.; MacDonald, A.; Ketelsen, T.; et al. Demonstration trials for the use of Readygrit®. In Proceedings of the 10th International Alumina Quality Workshop, Perth, Australia, 19–24 April 2015; Volume 10, pp. 187–196.
- Williams, R.; van Riessen, A. Determination of the Reactive Component of Fly Ashes for Geopolymer Production Using XRF and XRD. Fuel 2010, 89, 3683–3692. [Google Scholar] [CrossRef]
- Williams, R.; Hart, R.; van Riessen, A. Quantification of the Extent of Reaction of Metakaolin-Based Geopolymers. J. Am. Ceram. Soc. 2011, 94, 2663–2670. [Google Scholar] [CrossRef]
- Chen-Tan, N.; van Riessen, A.; Ly, C.; Southam, D. Determining the reactivity of a fly ash for production of Geopolymer. J. Am. Ceram. Soc. 2009, 92, 881–887. [Google Scholar] [CrossRef]
- Rickard, W.; Williams, R.; Temuujin, J.; van Riessen, A. Assessing the suitability of three Australian fly ashes as an aluminosilicate source for geopolymers in high temperature applications. Mater. Sci. Eng. A 2011, 528, 3390–3397. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.; Sumajouw, D.; Rangan, B. On the Development of Fly Ash-Based Geopolymer Concrete. ACI Mater. J. 2004, 101, 467–472. [Google Scholar]
- Davidovits, J. Geopolymer Chemistry & Applications, 2nd ed.; Institut Géopolymère: Saint-Quentin, France, 2008; Chapter 25; pp. 526–527. [Google Scholar]
- Xu, H.; van Deventer, J. Geopolymerisation of multiple minerals. Miner. Eng. 2002, 15, 1131–1139. [Google Scholar] [CrossRef]
- Khater, H. Effect of Calcium on Geopolymerization of Aluminosilicate Wastes. J. Mater. Civ. Eng. 2012, 24, 92–101. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Messina, F.; Santoro, L.; Cioffi, R. Recycling of pre-washed municipal solid waste incinerator fly ash in the manufacturing of low temperature setting geopolymer materials. Materials 2013, 6, 3420–3437. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, K. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Brew, D.; MacKenzie, K. Geopolymer synthesis using silica fume and sodium aluminate. J. Mater. Sci. 2007, 42, 3990–3993. [Google Scholar] [CrossRef]
- Phair, J.; van Deventer, J. Characterization of fly-ash-based geopolymeric binders activated with sodium aluminate. Ind. Eng. Chem. Res. 2002, 41, 4242–4251. [Google Scholar] [CrossRef]
- Masi, G.; Rickard, W.D.A.; Bignozzi, M.C.; van Riessen, A. The effect of organic and inorganic fibres on the mechanical and thermal properties of aluminate activated geopolymers. Compos. Part B 2015, 76, 218–228. [Google Scholar] [CrossRef]
- Masi, G.; Rickard, W.D.A.; Vickers, L.; Bignozzi, M.C.; van Riessen, A. A comparison between different foaming methods for the synthesis of light weight geopolymers. Ceram. Int. 2014, 40, 13891–13902. [Google Scholar] [CrossRef]
- Van Riessen, A.; Jamieson, E.; Kealley, C.; Hart, R.; Williams, R. Bayer-Geopolymers, an exploration of synergy between the alumina and geopolymer industries. Cem. Concr. Compos. 2013, 41, 21–33. [Google Scholar] [CrossRef]
- Jamieson, E.; van Riessen, A.; Kealley, C.; Hart, R. Development of Bayer Geopolymer Paste and use as Concrete. In Proceedings of the 9th International Alumina Quality Workshop, Perth, Australia, 18–22 March 2012; Volume 9, pp. 296–299.
- Jamieson, E. Development and Utilisation of Bayer Process by-Products. Ph.D. Thesis, Curtin University, Perth, Australia, October 2013. [Google Scholar]
- Jamieson, E.; Penna, B.; van Riessen, A.; Nikraz, H. Bayer—Fly ash geopolymers: Development and application. In Proceedings of the 10th International Alumina Quality Workshop, Perth, Australia, 19–24 April 2015; Volume 10, pp. 197–204.
- Van Deventer, J.; Provis, J.; Duxson, P.; Brice, D. Chemical research and climate change as drivers in the commercial adoption of alkali activated materials. Waste Biomass Valoriz. 2010, 1, 145–155. [Google Scholar] [CrossRef]
- Guide to Residential Pavements; Australian Standard 3727; Standards Australia: Homebush, Australia, 1993.
- Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens; ASTM C39; ASTM: West Conshohocken, PA, USA, 2012.
- Hardjito, D.; Cheak, C.C.; Ing, C.H.L. Strength and Setting Times of Low Calcium Fly Ash-based Geopolymer Mortar. Mod. Appl. Sci. 2008, 2, 3–11. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef] [PubMed]







| Source of Bayer Liquor | Al2O3 (g/L) | NaOH (g/L) |
|---|---|---|
| Kwinana | 252.7 | 438.5 |
| Wagerup | 228.4 | 449.1 |
| Oxide | Collie | Muja |
|---|---|---|
| wt % | wt % | |
| SiO2 | 49.9 (2) | 44.9 (2) |
| Al2O3 | 24.8 (2) | 32.5 (2) |
| Fe2O3 | 16.60 (4) | 10.6 (4) |
| CaO | 1.8 (1) | 2.0 (1) |
| K2O | 0.61 (8) | 0.69 (8) |
| TiO2 | 1.36 (2) | 1.67 (3) |
| MgO | 1.31 (6) | 1.44 (6) |
| Na2O | 0.4 (1) | 0.6 (1) |
| P2O5 | 1.52 (4) | 1.52 (4) |
| SrO | 0.33 (1) | 0.22 (1) |
| BaO | 0.45 (1) | 0.53 (1) |
| Other (includes LOI) | 0.7 (1) | 3.33 (3) |
| Si/Al (molar ratio) | 1.71 (1) | 1.17 (1) |
| Mineral/Phase | Collie | Muja |
|---|---|---|
| wt % | wt % | |
| Primary Quartz | 13 (1) | 5.9 (6) |
| Secondary Quartz | 11.0 (2) | 4.9 (3) |
| Hematite | 2.42 (8) | 1.13 (7) |
| Magnetite | 2.0 (1) | 3.4 (3) |
| Mullite | 14 (1) | 28 (1) |
| Maghemite C | 6.6 (3) | – |
| Amorphous | 51 (1) | 57 (1) |
| Oxide | Collie | Muja |
|---|---|---|
| wt % | wt % | |
| SiO2 | 21 (2) | 26 (2) |
| Al2O3 | 15 (1) | 12 (1) |
| sum of aluminosilicates | 36 (3) | 38.1 (4) |
| Si/Al (molar ratio) | 1.2 | 1.8 |
| Fly Ash/Fume | Surface Area (m2/cc) | d (10) (µm) | d (50) (µm) | d (80) (µm) | d (90) (µm) |
|---|---|---|---|---|---|
| Collie | 1.01 | 2.7 | 16.0 | 49.3 | 81.9 |
| Muja | 2.24 | 1.1 | 4.4 | 13.2 | 24.6 |
| Silica Fume (SF98) | 18 | 0.3 | 0.5 | – | 0.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamieson, E.; Kealley, C.S.; Van Riessen, A.; Hart, R.D. Optimising Ambient Setting Bayer Derived Fly Ash Geopolymers. Materials 2016, 9, 392. https://doi.org/10.3390/ma9050392
Jamieson E, Kealley CS, Van Riessen A, Hart RD. Optimising Ambient Setting Bayer Derived Fly Ash Geopolymers. Materials. 2016; 9(5):392. https://doi.org/10.3390/ma9050392
Chicago/Turabian StyleJamieson, Evan, Catherine S. Kealley, Arie Van Riessen, and Robert D. Hart. 2016. "Optimising Ambient Setting Bayer Derived Fly Ash Geopolymers" Materials 9, no. 5: 392. https://doi.org/10.3390/ma9050392





