Bio-Inspired Supramolecular Chemistry Provides Highly Concentrated Dispersions of Carbon Nanotubes in Polythiophene
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Synthesis of U–NH2
2.1.2. Preparation of U–Grafted Carbon Nanotubes (CNT–U)
2.1.3. Preparation of CNT–U/PAT Nanocomposites
2.2. Characterization
3. Results and Discussion
3.1. Spectroscopic and Thermal Properties of CNT–U
3.2. Effect of Grafting Uracil onto CNTs
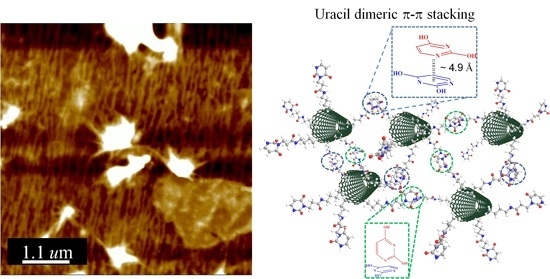
3.3. Supramolecular Interactions in CNT–U/PAT Dispersions: Structure and Morphology
3.4. Mechanism of Interaction and Energy Transfer for CNT–U and PAT
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MWCNTs | Multi-walled Carbon Nanotubes; |
| CNT–U | Uracil-functionalized CNT derivative; |
| CNT | Carbon Nanotube; |
| PAT | Adenine-terminated poly(3-adeninehexyl thiophene); |
| DMSO | Dimethyl sulfoxide; |
| U–U | Uracil–Uracil. |
References
- Fernandes, F.M.; Ruiz-Hitzky, E. Assembling nanotubes and nanofibres: Cooperativeness in sepiolite–carbon nanotube materials. Carbon 2014, 72, 296–303. [Google Scholar] [CrossRef]
- Shih, H.-K.; Hsieh, C.-C.; Mohamed, M.G.; Zhu, C.-Y.; Kuo, S.-W. Ternary polybenzoxazine/poss/swcnt hybrid nanocomposites stabilized through supramolecular interactions. Soft Matter. 2016, 12, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Al-Mahaidi, R.; Sbarski, I.; Mohammadzadeh, E. Surfactant-assisted dispersion of mwcnts in epoxy resin used in cfrp strengthening systems. J. Adhes. 2015, 91, 461–480. [Google Scholar] [CrossRef]
- Primo, E.; Gutierrez, F.; Luque, G.; Dalmasso, P.; Gasnier, A.; Jalit, Y.; Moreno, M.; Bracamonte, M.; Rubio, M.E.; Pedano, M. Comparative study of the electrochemical behavior and analytical applications of (bio) sensing platforms based on the use of multi-walled carbon nanotubes dispersed in different polymers. Anal. Chim. Acta 2013, 805, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Matsushita, M.; Katagiri, Y.; Fukumori, K. Effects of the composition and molecular weight of maleimide polymers on the dispersibility of carbon nanotubes in chloroform. Carbon 2011, 49, 5185–5195. [Google Scholar] [CrossRef]
- Erdem, A.; Papakonstantinou, P.; Murphy, H.; McMullan, M.; Karadeniz, H.; Sharma, S. Streptavidin modified carbon nanotube based graphite electrode for label-free sequence specific DNA detection. Electroanalysis 2010, 22, 611–617. [Google Scholar] [CrossRef]
- Yang, C.-C.; Lin, Y.-C.; Wang, P.-I.; Liaw, D.-J.; Kuo, S.-W. Polybenzoxazine/single-walled carbon nanotube nanocomposites stabilized through noncovalent bonding interactions. Polymer 2014, 55, 2044–2050. [Google Scholar] [CrossRef]
- Yang, J.; Yu, G.; Xia, D.; Huang, F. A pillar [6] arene-based uv-responsive supra-amphiphile: Synthesis, self-assembly, and application in dispersion of multiwalled carbon nanotubes in water. Chem. Commun. 2014, 50, 3993–3995. [Google Scholar] [CrossRef] [PubMed]
- Baykal, B.; Ibrahimova, V.; Er, G.; Bengü, E.; Tuncel, D. Dispersion of multi-walled carbon nanotubes in an aqueous medium by water-dispersible conjugated polymer nanoparticles. Chem. Commun. 2010, 46, 6762–6764. [Google Scholar] [CrossRef] [PubMed]
- Lillehei, P.T.; Kim, J.-W.; Gibbons, L.J.; Park, C. A quantitative assessment of carbon nanotube dispersion in polymer matrices. Nanotechnology 2009, 20, 325708. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Burns, C.L.; Abdelwahed, S.A. Hopping of a single hole in hexakis [4-(1,1,2-triphenyl-ethenyl) phenyl] benzene cation radical through the hexaphenylbenzene propeller. Org. Lett. 2004, 6, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- Gervasio, F.L.; Laio, A.; Parrinello, M.; Boero, M. Charge localization in DNA fibers. Phys. Rev. Lett. 2005, 94, 158103. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-F.; Kuo, S.-W.; Lin, C.-H.; Chen, H.-G.; Liao, C.-S.; Hung, P.-R. Benzoxazine as a reactive noncovalent dispersant for carbon nanotubes. RSC Adv. 2014, 4, 36012–36016. [Google Scholar] [CrossRef]
- Hunter, R.S.; Van Mourik, T. DNA base stacking: The stacked uracil/uracil and thymine/thymine minima. J. Comput. Chem. 2012, 33, 2161–2172. [Google Scholar] [CrossRef] [PubMed]
- Zadorozhnaya, A.A.; Krylov, A.I. Ionization-induced structural changes in uracil dimers and their spectroscopic signatures. J. Chem. Theory Comput. 2010, 6, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Bittner, E.R. Lattice theory of ultrafast excitonic and charge-transfer dynamics in DNA. J. Chem. Phys. 2006, 125, 094909. [Google Scholar] [CrossRef] [PubMed]
- Kratochvíl, M.; Engkvist, O.; Šponer, J.; Jungwirth, P.; Hobza, P. Uracil dimer: Potential energy and free energy surfaces. Ab initio beyond hartree-fock and empirical potential studies. J. Phys. Chem. A 1998, 102, 6921–6926. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Tu, Y.-Y.; Wang, T.-L.; Lin, R.-H.; Yang, C.-H.; Twu, Y.-K. Apparent electrocatalytic activities of composites of self-doped polyaniline, chitosan, and carbon nanotubes. J. Electroanal. Chem. 2013, 704, 190–196. [Google Scholar] [CrossRef]
- Xu, D.; Li, B.; Wei, C.; He, Y.-B.; Du, H.; Chu, X.; Qin, X.; Yang, Q.-H.; Kang, F. Preparation and characterization of mno 2/acid-treated cnt nanocomposites for energy storage with zinc ions. Electrochim. Acta 2014, 133, 254–261. [Google Scholar] [CrossRef]
- Jang, J.; Bae, J.; Choi, M.; Yoon, S.-H. Fabrication and characterization of polyaniline coated carbon nanofiber for supercapacitor. Carbon 2005, 43, 2730–2736. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Chang, F.-C.; Ko, F.-H.; Yu, F.-C.; Lin, Y.-T.; Shieh, Y.-T.; Chen, J.-K.; Lee, D.-J. Supramolecular polymeric micelles as high performance electrochemical materials. J. Mater. Chem. C 2015, 3, 9528–9533. [Google Scholar] [CrossRef]
- Prevoteau, A.; Soulié-Ziakovic, C.; Leibler, L. Universally dispersible carbon nanotubes. J. Am. Chem. Soc. 2012, 134, 19961–19964. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Choudhury, A. Carboxylic acid functionalized multi-walled carbon nanotube doped polyaniline for chloroform sensors. Sens. Actuators B Chem. 2013, 183, 25–33. [Google Scholar] [CrossRef]
- Park, S.-N.; Park, J.-C.; Kim, H.O.; Song, M.J.; Suh, H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide cross-linking. Biomaterials 2002, 23, 1205–1212. [Google Scholar] [CrossRef]
- Seneewong-Na-Ayutthaya, M.; Pongprayoon, T. Water-dispersible carbon nanotube prepared by non-destructive functionalization technique of admicellar polymerization. Diam. Relat. Mater. 2015, 60, 111–116. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Naebe, M. One-pot synthesis of aminated multi-walled carbon nanotube using thiol-ene click chemistry for improvement of epoxy nanocomposites properties. RSC Adv. 2015, 5, 98692–98699. [Google Scholar] [CrossRef]
- Wu, T.-M.; Lin, Y.-W. Doped polyaniline/multi-walled carbon nanotube composites: Preparation, characterization and properties. Polymer 2006, 47, 3576–3582. [Google Scholar] [CrossRef]
- Mahanandia, P.; Schneider, J.J.; Engel, M.; Stühn, B.; Subramanyam, S.V.; Nanda, K.K. Studies towards synthesis, evolution and alignment characteristics of dense, millimeter long multiwalled carbon nanotube arrays. Beilstein J. Nanotechnol. 2011, 2, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, S.; Nishino, H.; Futaba, D.N.; Yasuda, S.; Yamada, T.; Maigne, A.; Matsuo, Y.; Nakamura, E.; Yumura, M.; Hata, K. Role of subsurface diffusion and ostwald ripening in catalyst formation for single-walled carbon nanotube forest growth. J. Am. Chem. Soc. 2012, 134, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Head-Gordon, M. A fast correlated electronic structure method for computing interaction energies of large van der waals complexes applied to the fullerene–porphyrin dimer. Phys. Chem. Chem. Phys. 2006, 8, 2831–2840. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.W.; Wu, Y.R.; Jeong, K.U.; Kuo, S.W. From random coil polymers to helical structures induced by carbon nanotubes and supramolecular interactions. Macromol. Rapid Commun. 2013, 34, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Šponer, J.; Leszczynski, J.; Hobza, P. Electronic properties, hydrogen bonding, stacking, and cation binding of DNA and rna bases. Biopolymers 2001, 61, 3–31. [Google Scholar] [CrossRef]
- Lizundia, E.; Oleaga, A.; Salazar, A.; Sarasua, J. Nano-and microstructural effects on thermal properties of poly (l-lactide)/multi-wall carbon nanotube composites. Polymer 2012, 53, 2412–2421. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Guo, W.; Di, H.; Fang, C.; Yang, B. Electrochemical application of titanium dioxide nanoparticle/gold nanoparticle/multiwalled carbon nanotube nanocomposites for nonenzymatic detection of ascorbic acid. J. Solid State Electrochem. 2014, 18, 477–485. [Google Scholar] [CrossRef]
- De, P.K.; Neckers, D.C. Polymer-quantum dot-carbon nanotube composites formation under visible light irradiation. J. Photochem. Photobiol. A Chem. 2013, 252, 8–13. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Liu, S.; Wang, G.; Tian, J.; Jiang, C.; Zhu, S.; Wang, R. Remarkable activity of pdir nanoparticles supported on the surface of carbon nanotubes pretreated via a sonochemical process for formic acid electro-oxidation. Appl. Surf. Sci. 2013, 287, 457–460. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Hassan, S.H.; Oh, S.-E.; Ismail, M.; Rahimnejad, M.; Jahim, J.M. Nano-structured carbon as electrode material in microbial fuel cells: A comprehensive review. J. Alloy. Compd. 2013, 580, 245–255. [Google Scholar] [CrossRef]
- Yu, G.; Liu, X.; Xing, G.; Chen, S.; Ng, C.F.; Wu, X.; Yeow, E.K.L.; Lew, W.S.; Sum, T.C. Spatially-resolved ultrafast optical spectroscopy of polymer-grafted residues on cvd graphene. J. Phys. Chem. C 2013, 118, 708–713. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Hsu, K.-C.; Kuo, S.-W. Bifunctional polybenzoxazine nanocomposites containing photo-crosslinkable coumarin units and pyrene units capable of dispersing single-walled carbon nanotubes. Polym. Chem. 2015, 6, 2423–2433. [Google Scholar] [CrossRef]
- Yan, H.; Li, D.; Zhang, Y.; Yang, Y.; Wei, Z. Rational design of ternary-phase polymer solar cells by controlling polymer phase separation. J. Phys. Chem. C 2014, 118, 10552–10559. [Google Scholar] [CrossRef]
- Hu, T.; Han, L.; Xiao, M.; Bao, X.; Wang, T.; Sun, M.; Yang, R. Enhancement of photovoltaic performance by increasing conjugation of the acceptor unit in benzodithiophene and quinoxaline copolymers. J. Mater. Chem. C 2014, 2, 8047–8053. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sahu, M.; Krishnan, K.; Saxena, M.; Natarajan, V.; Godbole, S. Bluish white emitting sr 2 ceo 4 and red emitting sr 2 ceo 4: Eu 3+ nanoparticles: Optimization of synthesis parameters, characterization, energy transfer and photoluminescence. J. Mater. Chem. C 2013, 1, 7054–7063. [Google Scholar] [CrossRef]
- Ogihara, H.; Xie, J.; Saji, T. Spraying carbon nanotube dispersions to prepare superhydrophobic films. J. Mater. Sci. 2014, 49, 3183–3188. [Google Scholar] [CrossRef]











© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Singh, R.; Kuo, S.-W.; Ko, F.-H. Bio-Inspired Supramolecular Chemistry Provides Highly Concentrated Dispersions of Carbon Nanotubes in Polythiophene. Materials 2016, 9, 438. https://doi.org/10.3390/ma9060438
Lin Y-T, Singh R, Kuo S-W, Ko F-H. Bio-Inspired Supramolecular Chemistry Provides Highly Concentrated Dispersions of Carbon Nanotubes in Polythiophene. Materials. 2016; 9(6):438. https://doi.org/10.3390/ma9060438
Chicago/Turabian StyleLin, Yen-Ting, Ranjodh Singh, Shiao-Wei Kuo, and Fu-Hsiang Ko. 2016. "Bio-Inspired Supramolecular Chemistry Provides Highly Concentrated Dispersions of Carbon Nanotubes in Polythiophene" Materials 9, no. 6: 438. https://doi.org/10.3390/ma9060438