The Effect of Lithium Doping on the Sintering and Grain Growth of SPS-Processed, Non-Stoichiometric Magnesium Aluminate Spinel
Abstract
:1. Introduction
2. Materials and Experimental Procedures
3. Results and Discussion
3.1. Phase Composition
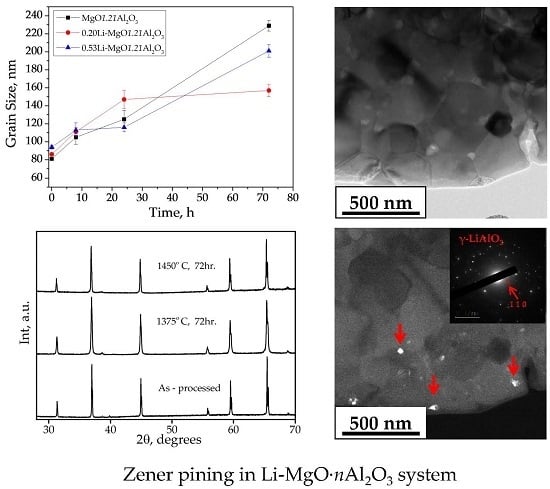
3.2. Grain Growth
4. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SPS | spark plasma sintering |
| HRSEM | high resolution scanning electron microscope |
| HRTEM | high resolution transmission electron microscope |
| HAADF | high angle annular dark field |
| STEM | scanning transmission electron microscope |
| XRD | X-ray powder diffraction |
| MgO s.s. | Mg(Al,Li)O solid solution |
| BF-TEM | bright field transmission electron microscope |
| DF-TEM | dark field transmission electron microscope |
References
- Goldstein, A. Correlation between MgAl2O4-spinel structure, processing factors and functional properties of transparent parts (progress review). J. Eur. Ceram. Soc. 2012, 32, 2869–2886. [Google Scholar] [CrossRef]
- Ganesh, I.; Teja, K.A.; Thiyagarajan, N.; Johnson, R.; Reddy, B.M. Formation and densification behavior of magnesium aluminate spinel: The influence of CaO and moisture in the precursors. J. Am. Ceram. Soc. 2005, 88, 2752–2761. [Google Scholar] [CrossRef]
- Ganesh, I.; Bhattacharjee, S.; Saha, B.P.; Johnson, R.; Mahajan, Y.R. A new sintering aid for magnesium aluminate spinel. Ceram. Int. 2001, 27, 773–779. [Google Scholar] [CrossRef]
- Huang, J.L.; Sun, S.Y.; Chen, C.Y. Investigation of high alumina-spinel: Effects of LiF and CaCO3 addition (part 2). Mater. Sci. Eng. A 1999, 259, 1–7. [Google Scholar] [CrossRef]
- Krell, A.; Hutzler, T.; Klimke, J.; Potthoff, A. Fine-grained transparent spinel windows by the processing of different nanopowders. J. Am. Ceram. Soc. 2010, 93, 2656–2666. [Google Scholar] [CrossRef]
- Du Merac, M.R.; Reimanis, I.E.; Smith, C.; Kleebe, H.-J.; Müller, M.M. Effect of impurities and LiF additive in hot-pressed transparent magnesium aluminate spinel. Int. J. Appl. Ceram. Technol. 2013, 10, E33–E48. [Google Scholar] [CrossRef]
- Chen, S.-K.; Cheng, M.-Y.; Lin, S.-J. Reducing the sintering temperature for MgO-Al2O3 mixtures by addition of cryolite (Na3AlF6). J. Am. Ceram. Soc. 2004, 85, 540–544. [Google Scholar] [CrossRef]
- Bhattacharya, G.; Zhang, S.; Smith, M.E.; Jayaseelan, D.D.; Lee, W.E. Mineralizing magnesium aluminate spinel formation with B2O3. J. Am. Ceram. Soc. 2006, 89, 3034–3042. [Google Scholar] [CrossRef]
- Rozenburg, K.; Reimanis, I.E.; Kleebe, H.-J.; Cook, R.L. Chemical interaction between LiF and MgAl2O4 spinel during sintering. J. Am. Ceram. Soc. 2007, 90, 2038–2042. [Google Scholar] [CrossRef]
- Meir, S.; Kalabukhov, S.; Froumin, N.; Dariel, M.P.; Frage, N. Synthesis and densification of transparent magnesium aluminate spinel by SPS processing. J. Am. Ceram. Soc. 2009, 92, 358–364. [Google Scholar] [CrossRef]
- Rozenburg, K.; Reimanis, I.E.; Kleebe, H.-J.; Cook, R.L. Sintering kinetics of a MgAl2O4 spinel doped with LiF. J. Am. Ceram. Soc. 2008, 91, 444–450. [Google Scholar] [CrossRef]
- Du Merac, M.R.; Kleebe, H.-J.; Müller, M.M.; Reimanis, I.E. Fifty years of research and development coming to fruition; Unraveling the complex interactions during processing of transparent magnesium aluminate (MgAl2O4) spinel. J. Am. Ceram. Soc. 2013, 96, 3341–3365. [Google Scholar] [CrossRef]
- Carnall, E. The densification of MgO in the presence of a liquid phase. Mater. Res. Bull. 1967, 2, 1075–1086. [Google Scholar] [CrossRef]
- Reimanis, I.; Kleebe, H.-J. A review on the sintering and microstructure development of transparent spinel (MgAl2O4). J. Am. Ceram. Soc. 2009, 92, 1472–1480. [Google Scholar] [CrossRef]
- Mordekovitz, Y.; Hayun, S. On the effect of lithium on the energetics and thermal stability of nano-sized nonstoichiometric magnesium aluminate spinel. J. Am. Ceram. Soc. 2016. [Google Scholar] [CrossRef]
- Morita, K.; Kim, B.-N.; Hiraga, K.; Yoshida, H. Fabrication of transparent MgAl2O4 spinel polycrystal by spark plasma sintering processing. Scr. Mater. 2008, 58, 1114–1117. [Google Scholar] [CrossRef]
- Morita, K.; Kim, B.-N.; Yoshida, H.; Hiraga, K. Densification behavior of a fine-grained MgAl2O4 spinel during spark plasma sintering (SPS). Scr. Mater. 2010, 63, 565–568. [Google Scholar] [CrossRef]
- Frage, N.; Cohen, S.; Meir, S.; Kalabukhov, S.; Dariel, M.P. Spark plasma sintering (SPS) of transparent magnesium-aluminate spinel. J. Mater. Sci. 2007, 42, 3273–3275. [Google Scholar] [CrossRef]
- Morita, K.; Kim, B.-N.; Yoshida, H.; Hiraga, K. Spark-plasma-sintering condition optimization for producing transparent MgAl2O4 spinel polycrystal. J. Am. Ceram. Soc. 2009, 92, 1208–1216. [Google Scholar] [CrossRef]
- Rothman, A.; Kalabukhov, S.; Sverdlov, N.; Dariel, M.P.; Frage, N. The effect of grain size on the mechanical and optical properties of spark plasma sintering-processed magnesium aluminate spinel MgAl2O4. Int. J. Appl. Ceram. Technol. 2014, 11, 146–153. [Google Scholar] [CrossRef]
- Bonnefont, G.; Fantozzi, G.; Trombert, S.; Bonneau, L. Fine-grained transparent MgAl2O4 spinel obtained by spark plasma sintering of commercially available nanopowders. Ceram. Int. 2012, 38, 131–140. [Google Scholar] [CrossRef]
- Fu, P.; Lu, W.; Lei, W.; Xu, Y.; Wang, X.; Wu, J. Transparent polycrystalline MgAl2O4 ceramic fabricated by spark plasma sintering: Microwave dielectric and optical properties. Ceram. Int. 2013, 39, 2481–2487. [Google Scholar] [CrossRef]
- Morita, K.; Kim, B.-N.; Yoshida, H.; Zhang, H.; Hiraga, K.; Sakka, Y. Effect of loading schedule on densification of MgAl2O4 spinel during spark plasma sintering (SPS) processing. J. Eur. Ceram. Soc. 2012, 32, 2303–2309. [Google Scholar] [CrossRef]
- Khasanov, O.; Dvilis, E.; Khasanov, A.; Polisadova, E.; Kachaev, A. Optical and mechanical properties of transparent polycrystalline MgAl2O4 spinel depending on SPS conditions. Phys. Status Solidi 2013, 10, 918–920. [Google Scholar] [CrossRef]
- Ianoş, R.; Lazǎu, I.; Pǎcurariu, C.; Barvinschi, P. Solution combustion synthesis of MgAl2O4 using fuel mixtures. Mater. Res. Bull. 2008, 43, 3408–3415. [Google Scholar] [CrossRef]
- Halabi, M.; (Ben-Gurion University of the Negev, Beer-Sheva, Israel). Personal communication, 2016.
- Hayun, S.; Dilman, H.; Dariel, M.P.; Frage, N.; Dub, S. Effect of the carbon source on the microstructure and mechanical properties of reaction bonded boron carbide. In Ceramic Transactions 209 (Advances in Sintering Science and Technology); American Ceramic Society: Westerville, OH, USA, 2010; pp. 29–41. [Google Scholar]
- ASTM B-311. Standard test method for density of powder metallurgy (PM) materials containing less than two percent porosity 1. ASTM Int. 2008. [Google Scholar] [CrossRef]
- C-1327 A. Standard test method for vickers indentation hardness of advanced ceramics 1. ASTM Int. 2008. [Google Scholar] [CrossRef]
- Doman, R.C.; McNally, R.N. Solid solution studies in the MgO-LiAIO system. J. Mater. Sci. 1973, 8, 189–191. [Google Scholar] [CrossRef]
- Izquierdo, G.; West, A.R. Compatibility relations in the system Li2O-MgO-Al2O3. J. Am. Ceram. Soc. 1980. [Google Scholar] [CrossRef]
- Garitaonandia, J.S.; Gorria, P.; Barquín, L.F.; Barandiarán, J.M.; Barqu’in, L.F.; Barandiarán, J.M. Low-temperature magnetic properties of Fe nanograins in an amorphous Fe-Zr-B matrix. Phys. Rev. B 2000, 61, 6150–6155. [Google Scholar] [CrossRef]
- Hassold, G.N.; Holm, E. Effects of particle size on inhibited grain growth. Scr. Met. Mater. 1990, 24, 11–13. [Google Scholar] [CrossRef]
- Grewal, G.; Ankem, S. Modeling matrix grain growth in the presence of growing second phase particles in two phase alloys. Acta Metall. Mater. 1990, 38, 1607–1617. [Google Scholar] [CrossRef]
- Fan, D.; Chen, L.-Q.; Chen, S.-P.P. Numerical simulation of zener pinning with growing second-phase particles. J. Am. Ceram. Soc. 1998, 81, 526–532. [Google Scholar] [CrossRef]
- Rios, P.R. Overview no. 62. A theory for grain boundary pinning by particles. Acta Metall. 1987, 35, 2805–2814. [Google Scholar] [CrossRef]
- Lappalainen, R.; Pannikkat, A.; Raj, R. Superplastic flow in a non-stoichiometric ceramic: Magnesium aluminate spinel. Acta Metall. Mater. 1993, 41, 1229–1235. [Google Scholar] [CrossRef]
- Chaim, R. Activation energy and grain growth in nanocrystalline Y-TZP ceramics. Mater. Sci. Eng. A 2008, 486, 439–446. [Google Scholar] [CrossRef]
- Chiang, Y. Grain boundary mobility and segregation in non-stoichiometric solid solutions of magnesium aluminate spinel. J. Am. Ceram. Soc. 1980. [Google Scholar] [CrossRef]
- Bratton, R.J. Sintering and grain-growth kinetics of something is missing here. J. Eur. Ceram. Soc. 1970, 35, 141–143. [Google Scholar]



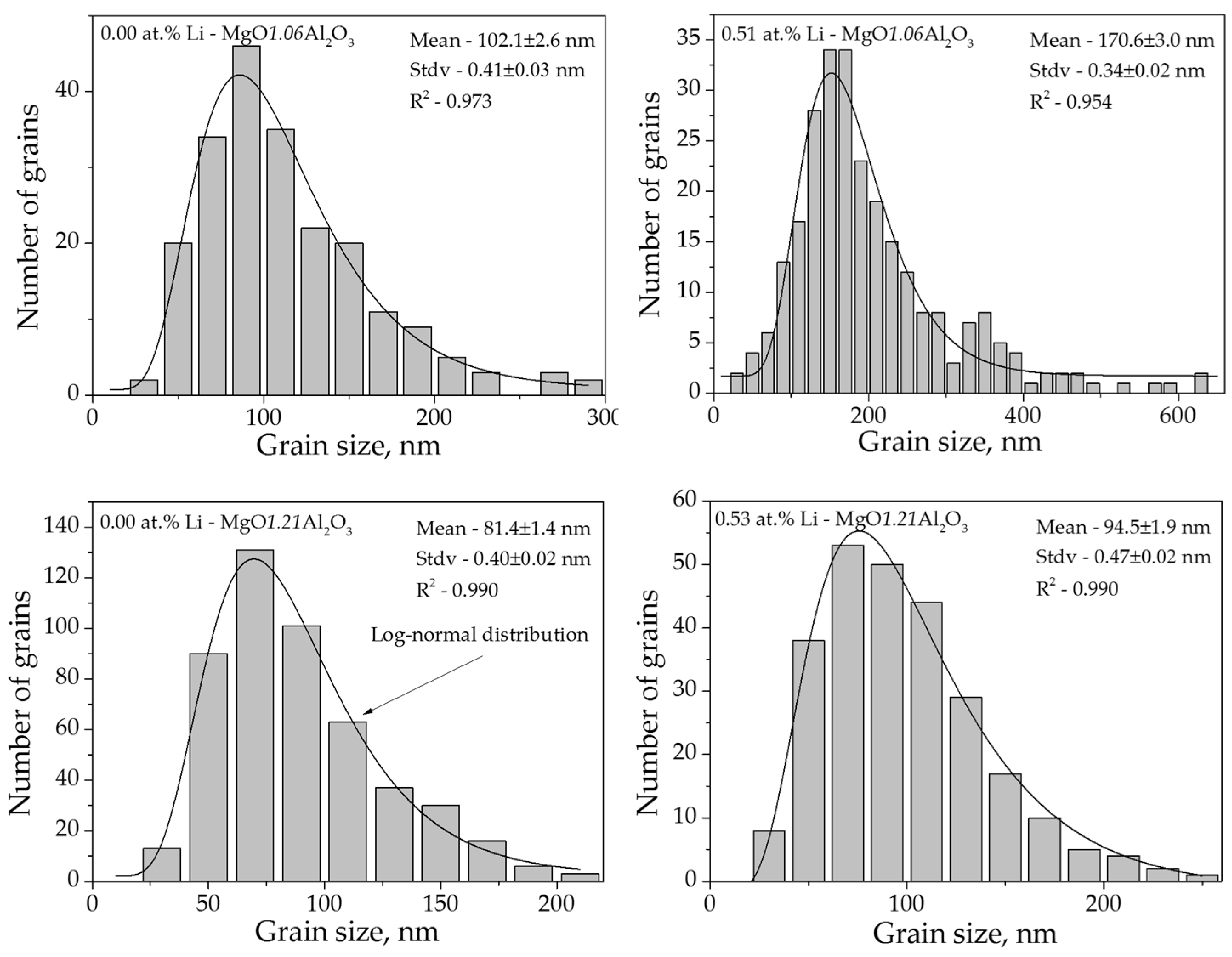



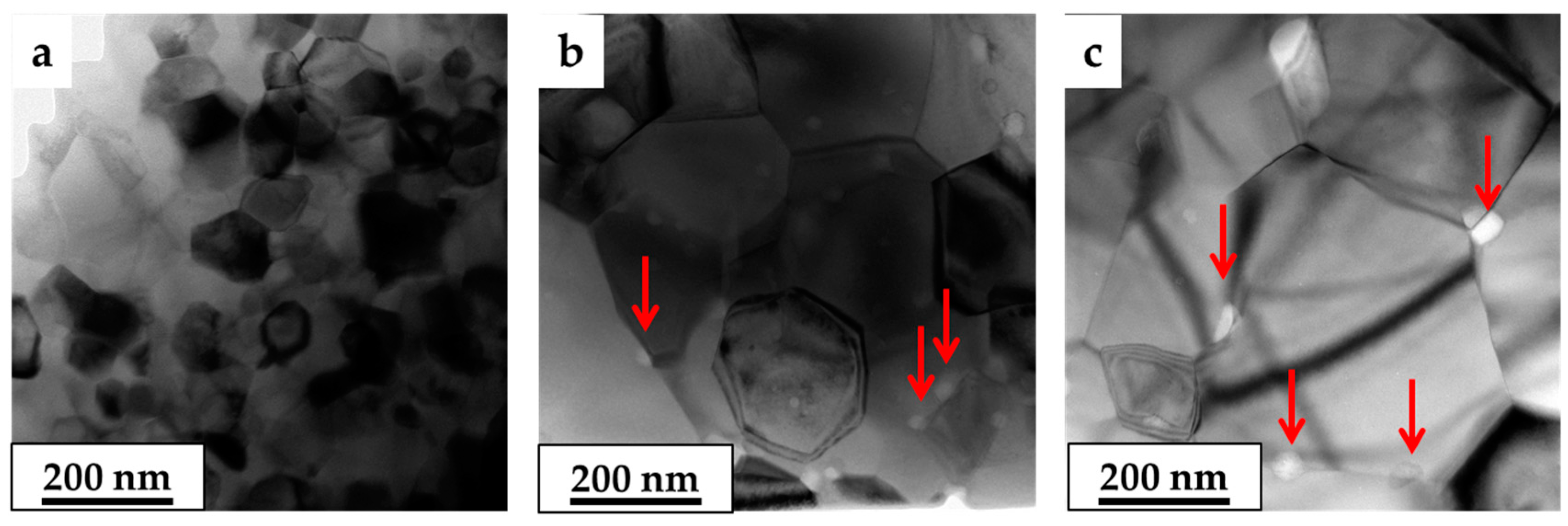


| Li (at. %) | A (Å) | D (nm) | ρ (g/cm3) | Trans. (500 nm) (%) | Trans. (1000 nm) (%) | Hardness (GPa) | Mgx(Al,Li)1–xO | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Wt. % | a (Å) | Composition * | ||||||||
| Mg | (Al, Li) | |||||||||
| Li-MgO·1.06Al2O3 | ||||||||||
| - | 8.0810 (1) | 102 ± 3 | 3.49 ± 0.01 | - | - | 14.3 ± 0.2 | - | - | - | - |
| 0.28 ± 0.02 | 8.0815 (1) | 160 ± 5 | 3.54 ± 0.01 | 3.5 ± 0.1 | 14.4 ± 0.1 | 14.7 ± 0.3 | 0.9 ± 0.1 | 4.180 (9) | 0.86 | 0.14 |
| 0.51 ± 0.05 | 8.0784 (1) | 171 ± 3 | 3.56 ± 0.01 | 25.0 ± 0.1 | 45.3 ± 0.1 | 15.3 ± 0.4 | 1.8 ± 0.1 | 4.141 (9) | 0.68 | 0.32 |
| 1.03 ± 0.10 | 8.0773 (1) | 150 ± 8 | 3.54 ± 0.01 | 7.4 ± 0.1 | 22.9 ± 0.1 | 14.6 ± 0.5 | 2.9 ± 0.2 | 4.127 (9) | 0.63 | 0.37 |
| Li-MgO·1.21Al2O3 | ||||||||||
| - | 8.0647 (1) | 81 ± 1 | 3.48 ± 0.01 | - | - | 14.4 ± 0.5 | - | - | - | - |
| 0.20 ± 0.02 | 8.0654 (1) | 86 ± 2 | 3.49 ± 0.01 | - | - | 14.2 ± 0.2 | - | - | - | - |
| 0.53 ± 0.06 | 8.0656 (4) | 94 ± 2 | 3.48 ± 0.01 | - | - | 14.1 ± 0.4 | - | - | - | - |
| 1.04 ± 0.10 | 8.0779 (2) | 138 ± 4 | 3.61 ± 0.01 | 2.0 ± 0.1 | 10 ± 0.1 | 13.9 ± 0.3 | 2.3 ± 0.3 | 4.117 (9) | 0.6 | 0.4 |
| Temperature (°C)/Time (h) | Grain Size (nm) | ||
|---|---|---|---|
| 8 | 24 | 72 | |
| MgO·1.21Al2O3 | |||
| 1300 | 105 ± 8 | 125 ± 10 | 229 ± 6 |
| 1375 | 131 ± 10 | 200 ± 19 | 272 ± 26 |
| 1450 | 181 ± 12 | 292 ± 26 | 513 ± 22 |
| 0.28-MgO·1.21Al2O3 | |||
| 1300 | 111 ± 5 | 147 ± 10 | 157 ± 7 |
| 1375 | 154 ± 5 | 242 ± 18 | 249 ± 18 |
| 1450 | 306 ± 11 | 438 ± 9 | 991 ± 115 |
| 0.53-MgO·1.21Al2O3 | |||
| 1300 | 113 ± 8 | 116 ± 5 | 201 ± 7 |
| 1375 | 149 ± 8 | 237 ± 53 | 280 ± 8 |
| 1450 | 197 ± 18 | 300 ± 6 | 948 ± 47 |
| MgO·nAl2O3 | Activation Energy for Grain Growth (kJ/mol) | ln(K0) |
|---|---|---|
| Undoped | ||
| 1.56 (Chiang [39]) | 248 ± 29 | 16.35 |
| 1.21 (This study) | 288 ± 40 | 14.55 |
| 1.013 (Chiang [39]) | 422 ± 10 | 28.23 |
| ~1.00 (Bratton [40]) | 462 | 30.54 |
| at. % Li | Lithium doped n = 1.21 (This study) | |
| 0.20 | 670 ± 45 | 42.67 |
| 0.53 | 543 ± 40 | 33.56 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mordekovitz, Y.; Shelly, L.; Halabi, M.; Kalabukhov, S.; Hayun, S. The Effect of Lithium Doping on the Sintering and Grain Growth of SPS-Processed, Non-Stoichiometric Magnesium Aluminate Spinel. Materials 2016, 9, 481. https://doi.org/10.3390/ma9060481
Mordekovitz Y, Shelly L, Halabi M, Kalabukhov S, Hayun S. The Effect of Lithium Doping on the Sintering and Grain Growth of SPS-Processed, Non-Stoichiometric Magnesium Aluminate Spinel. Materials. 2016; 9(6):481. https://doi.org/10.3390/ma9060481
Chicago/Turabian StyleMordekovitz, Yuval, Lee Shelly, Mahdi Halabi, Sergey Kalabukhov, and Shmuel Hayun. 2016. "The Effect of Lithium Doping on the Sintering and Grain Growth of SPS-Processed, Non-Stoichiometric Magnesium Aluminate Spinel" Materials 9, no. 6: 481. https://doi.org/10.3390/ma9060481