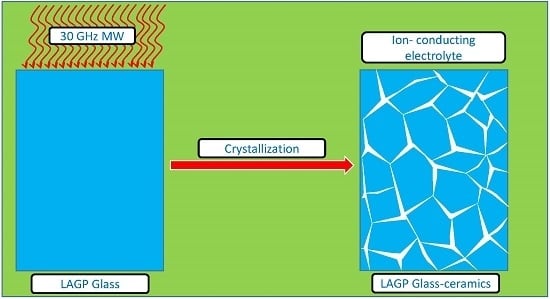
Microwave Crystallization of Lithium Aluminum Germanium Phosphate Solid-State Electrolyte
Abstract
:1. Introduction
2. Experimental Work
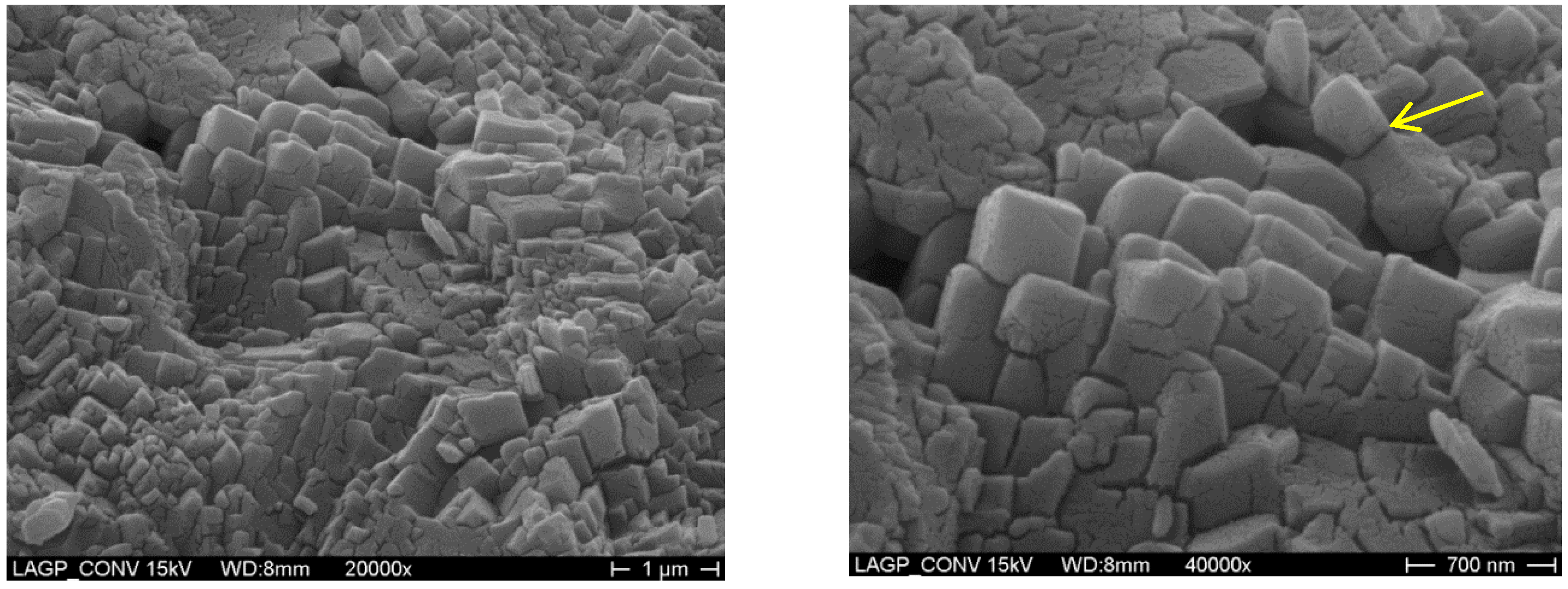
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maleki, H.; Deng, G.; Anani, A.; Howard, J. Thermal Stability Studies of Li-Ion. Cells and Components. J. Electrochem. Soc. 1999, 146, 3224–3229. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Mariappan, C.R.; Yada, C.; Rosciano, F.; Roling, B. Correlation between micro-structural properties and ionic conductivity of Li1.5Al0.5Ge1.5(PO4)3 ceramics. J. Power Sources 2011, 196, 6456–6464. [Google Scholar] [CrossRef]
- Key, B.; Schroeder, D.J.; Ingram, B.J.; Vaughey, J.T. Solution-Based Synthesis and Characterization of Lithium-Ion. Conducting Phosphate Ceramics for Lithium Metal Batteries. Chem. Mater. 2012, 24, 287–293. [Google Scholar] [CrossRef]
- París, M.A.; Sanz, J. Structural changes in the compounds (PO4)3 (MIV = Ge, Ti, Sn, and Hf) as followed by 31P and 7Li NMR. Phys. Rev. B 1997, 55, 14270–14278. [Google Scholar] [CrossRef]
- Leo, C.J.; Chowdari, B.V.R.; Rao, G.V.S.; Souquet, J.L. Lithium conducting glass ceramic with Nasicon structure. Mater. Res. Bull. 2002, 37, 1419–1430. [Google Scholar] [CrossRef]
- Nuspl, G.; Takeuchi, T.; Weiß, A.; Kageyama, H.; Yoshizawa, K.; Yamabe, T. Lithium ion migration pathways in LiTi2(PO4)3 and related materials. J. Appl. Phys. 1999, 86, 5484–5491. [Google Scholar] [CrossRef]
- Schroeder, M.; Glatthaar, S.; Binder, J.R. Influence of spray granulation on the properties of wet chemically synthesized Li1.3Ti1.7Al0.3(PO4)3 (LATP) powders. Solid State Ionics 2011, 201, 49–53. [Google Scholar] [CrossRef]
- Thokchom, J.S.; Gupta, N.; Kumar, B. Superionic Conductivity in a Lithium Aluminum Germanium Phosphate Glass-Ceramic. J. Electrochem. Soc. 2008, 155, A915–A920. [Google Scholar] [CrossRef]
- Fergus, J.W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- Fu, J. Fast Li+ ion conducting glass-ceramics in the system Li2O–Al2O3–GeO2–P2O5. Solid State Ionics 1997, 104, 191–194. [Google Scholar] [CrossRef]
- Robertson, A.D.; West, A.R.; Ritchie, A.G. Review of crystalline lithium-ion conductors suitable for high temperature battery applications. Solid State Ionics 1997, 104, 1–11. [Google Scholar] [CrossRef]
- Gellert, M.; Gries, K.I.; Yada, C.; Rosciano, F.; Volz, K.; Roling, B. Grain Boundaries in a Lithium Aluminum Titanium Phosphate-Type Fast Lithium Ion. Conducting Glass Ceramic: Microstructure and Nonlinear Ion. Transport. Properties. J. Phys. Chem. C 2012, 116, 22675–22678. [Google Scholar] [CrossRef]
- Thokchom, J.S.; Kumar, B. The effects of crystallization parameters on the ionic conductivity of a lithium aluminum germanium phosphate glass-ceramic. J. Power Sources 2010, 195, 2870–2876. [Google Scholar] [CrossRef]
- Kubanska, A.; Castro, L.; Tortet, L.; Schäf, O.; Dollé, M.; Bouchet, R. Elaboration of controlled size Li1.5Al0.5Ge1.5(PO4)3 crystallites from glass-ceramics. Solid State Ionics 2014, 266, 44–50. [Google Scholar] [CrossRef]
- Thokchom, J.S.; Kumar, B. Composite effect in superionically conducting lithium aluminium germanium phosphate based glass-ceramic. J. Power Sources 2008, 185, 480–485. [Google Scholar] [CrossRef]
- Cui, Y.; Mahmoud, M.M.; Rohde, M.; Ziebert, C.; Seifert, H.J. Thermal and ionic conductivity studies of lithium aluminum germanium phosphate solid-state electrolyte. Solid State Ionics 2016, 289, 125–132. [Google Scholar] [CrossRef]
- Leo, C.J.; Subba Rao, G.V.; Chowdari, B.V.R. Effect of MgO addition on the ionic conductivity of LiGe2(PO4)3 ceramics. Solid State Ionics 2003, 159, 357–367. [Google Scholar] [CrossRef]
- Kotobuki, M.; Koishi, M. Sol–gel synthesis of Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte. Ceram. Int. 2015, 41, 8562–8567. [Google Scholar] [CrossRef]
- Kichambare, P.D.; Howell, T.; Rodrigues, S. Sol–Gel-Derived Lithium Superionic Conductor Li1.5Al0.5Ge1.5(PO4)3 Electrolyte for Solid-State Lithium-Oxygen Batteries. Energy Technol. 2014, 2, 391–396. [Google Scholar] [CrossRef]
- Arbi, K.; Bucheli, W.; Jiménez, R.; Sanz, J. High lithium ion conducting solid electrolytes based on NASICON Li1+xAlxM2−x(PO4)3 materials (M = Ti, Ge and 0 ≤ x ≤ 0.5). J. Eur. Ceram. Soc. 2015, 35, 1477–1484. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, Z.; Cheng, J.; Yamamoto, O.; Imanishi, N.; Chi, B.; Pu, J.; Li, J. Solid state lithium ionic conducting thin film Li1.4Al0.4Ge1.6(PO4)3 prepared by tape casting. J. Alloys Compd. 2014, 590, 147–152. [Google Scholar] [CrossRef]
- Kunshina, G.B.; Bocharova, I.V.; Lokshin, E.P. Synthesis and conductivity studies of Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte. Inorg. Mater. 2016, 52, 279–284. [Google Scholar] [CrossRef]
- Liu, Z.; Venkatachalam, S.; van Wüllen, L. Structure, phase separation and Li dynamics in sol–gel-derived Li1+xAlxGe2−x(PO4)3. Solid State Ionics 2015, 276, 47–55. [Google Scholar] [CrossRef]
- Clark, D.E.; Folz, D.C.; Folgar, C.E.; Mahmoud, M.M. Microwave Solutions for Ceramic Engineers; The American Ceramic Society: Westerville, OH, USA, 2006. [Google Scholar]
- Clark, D.E.; Sutton, W.H. Microwave processing of materials. Annu. Rev. Mater. Sci. 1996, 26, 299–331. [Google Scholar] [CrossRef]
- Roy, R.; Fang, Y.; Cheng, J.; Agrawal, D.K. Decrystallizing Solid Crystalline Titania, without Melting, Using Microwave Magnetic Fields. J. Am. Ceram. Soc. 2005, 88, 1640–1642. [Google Scholar] [CrossRef]
- Roy, R.; Peelamedu, R.; Hurtt, L.; Cheng, J.; Agrawal, D. Definitive experimental evidence for Microwave Effects: Radically new effects of separated E and H fields, such as decrystallization of oxides in seconds. Mater. Res. Innov. 2002, 6, 128–140. [Google Scholar] [CrossRef]
- Demirskyi, D.; Agrawal, D.; Ragulya, A. Tough ceramics by microwave sintering of nanocrystalline titanium diboride ceramics. Ceram. Int. 2014, 40, 1303–1310. [Google Scholar] [CrossRef]
- Kelvin Chew, W.J.; Amiriyan, M.; Yaghoubi, A.; Ramesh, S.; Purbolaksono, J.; Tolouei, R.; Teng, W.D.; Agrawal, D.K. Sintering properties and thermal depletion of boron in zirconia-zirconium diboride conductive ceramic. Ceram. Int. 2014, 40, 13313–13320. [Google Scholar] [CrossRef]
- Fukushima, J.; Kashimura, K.; Takayama, S.; Sato, M.; Sano, S.; Hayashi, Y.; Takizawa, H. In-situ kinetic study on non-thermal reduction reaction of CuO during microwave heating. Mater. Lett. 2013, 91, 252–254. [Google Scholar] [CrossRef]
- Thridandapani, R.R.; Folz, D.C.; Clark, D.E. Effect of electric field (2.45 GHz) on sintering behavior of fully stabilized zirconia. J. Eur. Ceram. Soc. 2015, 35. [Google Scholar] [CrossRef]
- Takayama, S.; Link, G.; Sato, M.; Jelonnek, J. Possibility for Iron Production Using High-Power Millimeter Waves. IEEE Trans. Plasma Sci. 2015, 43, 3517–3521. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Olevsky, E.A.; Krikun, E.V. Microwave Sintering: Fundamentals and Modeling. J. Am. Ceram. Soc. 2013, 96, 1003–1020. [Google Scholar] [CrossRef]
- Wang, J.; Binner, J.; Pang, Y.; Vaidhyanathan, B. Microwave-enhanced densification of sol-gel alumina films. Thin Solid Films 2008, 516, 5996–6001. [Google Scholar] [CrossRef]
- Leonelli, C.; Veronesi, P.; Denti, L.; Gatto, A.; Iuliano, L. Microwave assisted sintering of green metal parts. J. Mater. Process. Technol. 2008, 205, 489–496. [Google Scholar] [CrossRef]
- Chang, H.Y.; Sheu, C.I.; Cheng, S.Y. Microwave enhanced sintering of tape-cast ferroelectric films. J. Eur. Ceram. Soc. 2007, 27, 3793–3797. [Google Scholar] [CrossRef]
- Rodriguez, H.; Suarez, M.; Perez, R.; Petit, A.; Loupy, A. Solvent-free synthesis of 4-aryl substituted 5-alkoxycarbonyl-6-methyl-3,4-dihydropyridones under microwave irradiation. Tetrahedron Lett. 2003, 44, 3709–3712. [Google Scholar] [CrossRef]
- Clark, D.E.; Folz, D.C.; West, J.K. Processing materials with microwave energy. Mater. Sci. Eng. A 2000, 287, 153–158. [Google Scholar] [CrossRef]
- Willert-Porada, M. Novel routes to microwave processing of ceramic materials. Mater. Res. Soc. Symp. Proc. 1994, 347. [Google Scholar] [CrossRef]
- Meek, T.T.; Blake, R.D. Ceramic—Ceramic seals by microwave heating. J. Mater. Sci. Lett. 1986, 5, 270–274. [Google Scholar] [CrossRef]
- Wroe, R.; Rowley, A.T. Evidence for a non-thermal microwave effect in the sintering of partially stabilized zirconia. J. Mater. Sci. 1996, 31, 2019–2026. [Google Scholar] [CrossRef]
- Thridandapani, R.R.; Folz, D.C.; Clark, D.E. Estimation of activation energies for sintering 8 mol % Yttria-Zirconia using conventional and microwave heating. Int. J. Appl. Ceram. Technol. 2014, 11, 938–945. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Semenov, V.E.; Link, G.; Thumm, M. Preferred orientation of pores in ceramics under heating by a linearly polarized microwave field. J. Appl. Phys. 2007, 101, 084915. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Link, G.; Thumm, M. The role of the native oxide shell on the microwave sintering of copper metal powder compacts. J. Alloys Compd. 2015, 627, 231–237. [Google Scholar] [CrossRef]
- Hassan, M.N.; Mahmoud, M.M.; El-Fattah, A.A.; Kandil, S. Microwave-assisted preparation of Nano-hydroxyapatite for bone substitutes. Ceram. Int. 2016, 42, 3725–3744. [Google Scholar] [CrossRef]
- Hassan, M.N.; Mahmoud, M.M.; El-Fattah, A.A.; Kandil, S. Microwave rapid conversion of sol–gel-derived hydroxyapatite into β-tricalcium phosphate. J. Sol-Gel Sci. Technol. 2015, 76, 74–81. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; El-Latif, M.M.A.; Mahmoud, M.M. Synthesis and characterization of nano-sized cobalt ferrite prepared via polyol method using conventional and microwave heating techniques. J. Alloys Compd. 2010, 506, 201–204. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Thumm, M. Crystallization of lithium disilicate glass using high frequency microwave processing. J. Eur. Ceram. Soc. 2015, 35, 2915–2922. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Folz, D.C.; Suchicital, C.T.A.; Clark, D.E. Crystallization of lithium disilicate glass using microwave processing. J. Am. Ceram. Soc. 2012, 95, 579–585. [Google Scholar] [CrossRef]
- Mahmoud, M.M. Crystallization of lithium disilicate glass using variable frequency microwave processing. In Materials Science and Engineering; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2007. [Google Scholar]
- Mahmoud, M.; Folz, D.; Suchicital, C.; Clark, D.; Fathi, Z. Variable frequency microwave (VFM) processing: A new tool to crystallize lithium disilicate glass. In Advances in Bioceramics and Biocomposites II, Ceramic Engineering and Science Proceedings; Mizuno, M., Wereszczak, A., Lara-Curzio, E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Volume 27, pp. 143–153. [Google Scholar]
- Mahmoud, M.; Folz, D.; Suchicital, C.; Clark, D. Microwave Crystallization of Lithium Disilicate Glass. In Proceedings of the 2004 AIChE Annual Meeting, Austin, TX, USA, 7–12 November 2004.
- Thumm, M.; Feher, L.; Link, G. Micro- and millimeter-wave processing of advanced materials at Karlsruhe Research Center. In Novel Materials Processing by Advanced Electromagnetic Energy Sources; Elsevier Science Ltd.: Oxford, UK, 2005; pp. 93–98. [Google Scholar]
- Berkemeier, F.H. Ionenleitende Borat- und Silikatglasschichten strukturelle und elektrische Charakterisierung mit Hilfe von TEM und Impedanzspektroskopie in Physik; University Münster: Münster, Germany, 2007; p. 152. [Google Scholar]
- Kumar, B.; Thomas, D.; Kumar, J. Space-Charge-Mediated Superionic Transport. in Lithium Ion. Conducting Glass-Ceramics. J. Electrochem. Soc. 2009, 156, A506–A513. [Google Scholar] [CrossRef]
- Bouchet, R.; Knauth, P.; Laugier, J.-M. Theoretical Analysis of IS of Polycrystalline Materials with Blocking or Conducting Grain Boundaries: From Microcrystals to Nanocrystals. J. Electrochem. Soc. 2003, 150, E348–E354. [Google Scholar] [CrossRef]







| Route Type | Ionic Conductivity (S/cm) @ RT |
|---|---|
| Melt-quenching [11] | 4 × 10−4 |
| Conventional sintering [18] | 3.99 × 10−4 |
| Sol-gel method [20] | 1.03 × 10−4 |
| Flame spray [3] | 2 × 10−4 |
| Element | Li | Al | P | Ge | O |
|---|---|---|---|---|---|
| Weight % | 2.85% | 3.45% | 22.20% | 23.70% | 44.40% |
| Atom % | 9.43% | 2.93% | 16.45% | 7.49% | 63.70% |
| Chemical formula | Li1.71 Al0.53 Ge1.36 P2.99 O11.9 | ||||
| Sample | Total Ionic Conductivity (S/cm) @ RT |
|---|---|
| LAGP As annealed glass | NA * |
| LAGP Conv.(550 °C/1 h + 630 °C/1 h + 800 °C/1 h) | 7.52 × 10−5 |
| LAGP Conv. (800 °C/6 h) | 1.3 × 10−4 |
| LAGP 30 GHz MW (800 °C/1 h) | 1.06 × 10−4 |
| LAGP 30 GHz MW (800 °C/6 h) | 2.77 × 10−4 |
| Sample | Total Ionic Conductivity (S/cm) @ RT | Grains Ionic Conductivity (S/cm) @ RT | Grain Boundaries Ionic Conductivity (S/cm) @ RT |
|---|---|---|---|
| LAGP Conv. (800 °C/6 h) | 1.3 × 10−4 | 3.06 × 10−4 | 2.25 × 10−4 |
| LAGP 30 GHz MW (800 °C/6 h) | 2.77 × 10−4 | 6.49 × 10−4 | 4.83 × 10−4 |
| Sample | Below 70 °C | Above 70 °C | Overall Range |
|---|---|---|---|
| LAGP Conv. (800 °C/6 h) | 0.44 eV | 0.34 eV | 0.37 eV ± 0.010 |
| LAGP 30 GHz MW (800 °C/6 h) | 0.38 eV | 0.32 eV | 0.33 eV ± 0.008 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, M.M.; Cui, Y.; Rohde, M.; Ziebert, C.; Link, G.; Seifert, H.J. Microwave Crystallization of Lithium Aluminum Germanium Phosphate Solid-State Electrolyte. Materials 2016, 9, 506. https://doi.org/10.3390/ma9070506
Mahmoud MM, Cui Y, Rohde M, Ziebert C, Link G, Seifert HJ. Microwave Crystallization of Lithium Aluminum Germanium Phosphate Solid-State Electrolyte. Materials. 2016; 9(7):506. https://doi.org/10.3390/ma9070506
Chicago/Turabian StyleMahmoud, Morsi M., Yuantao Cui, Magnus Rohde, Carlos Ziebert, Guido Link, and Hans Juergen Seifert. 2016. "Microwave Crystallization of Lithium Aluminum Germanium Phosphate Solid-State Electrolyte" Materials 9, no. 7: 506. https://doi.org/10.3390/ma9070506
APA StyleMahmoud, M. M., Cui, Y., Rohde, M., Ziebert, C., Link, G., & Seifert, H. J. (2016). Microwave Crystallization of Lithium Aluminum Germanium Phosphate Solid-State Electrolyte. Materials, 9(7), 506. https://doi.org/10.3390/ma9070506