Gas Diffusion Electrodes Manufactured by Casting Evaluation as Air Cathodes for Microbial Fuel Cells (MFC)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Characterization of the Active Layer (AL)
2.1.1. Influence of Fabrication Method on the Properties of the Active Layer
2.1.2. Effect of Composition of the Coagulation Bath on the Properties of the Active Layer
2.1.3. Effect of the Type of Activated Carbon on the Properties of the Active Layer
2.1.4. Effect of Active Carbon Contents on the Properties of the AL
2.2. Physico-Chemical Characterization of the Hydrophobic Gas Diffusion Layer (HGDL)
2.3. Electrode Characterization in Cathodic Half-Cell Environment
2.3.1. Electrode Polarization
2.3.2. Electrode Performance as a Function of Time
2.4. Electrode Performance in MFC (Full Cell Configuration)
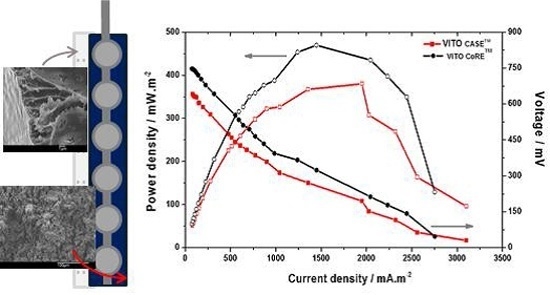
2.4.1. Power Output and Half-Cell Potentials
2.4.2. Fuel Cell Characterization
3. Materials and Methods
3.1. Materials
3.2. Manufacturing of the Gas Diffusion Cathodes
3.2.1. Active Layer (AL)
3.2.2. Hydrophobic Gas Diffusion Layer (HGDL)
3.3. Manufacturing of Freestanding Layers
3.4. Physicochemical Characterization Techniques
3.5. Electrode Characterization in Cathodic Half-Cell Configuration
3.6. Electrode Characterization in MFC (Full Cell Configuration)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AL | (electrochemically) active layer |
| Aw | Absorption coefficient |
| CDP | cell design point |
| CFP | capillary flow porometry |
| FEP | Fluorinated ethylene propylene |
| GDE | Gas diffusion electrode |
| HGDL | hydrophobic gas diffusion layer |
| LP | Liquid Permeability |
| LSV | Linear Sweep Voltammetry |
| MES | Microbial Electrochemical System |
| MFC | Microbial Fuel Cell |
| NEP | N-Ethylpyrrolidone |
| ORR | oxygen-reduction reaction |
| PSf | Polysulfone |
| PTFE | Polytetrafluoroethylene |
| SBET | BET Specific Surface Area |
| SEM | Scanning Electron Microscopy |
References
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schroder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.; Singh, A.; Van Bogaert, G.; Alvarez Gallego, Y.; Diels, L.; Vanbroekhoven, K. An introduction to the life cycle assessment (LCA) of bioelectrochemical systems (BES) for sustainable energy and product generation: Relevance and key aspects. Renew. Sustain. Energy Rev. 2011, 15, 1305–1313. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Richter, H.; Covalla, S.F.; Johnson, J.P.; Woodard, T.L.; Orloff, A.L.; Jia, H.; Zhang, M.; Lovley, D.R. Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells. Environ. Microbiol. 2008, 10, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Bullen, R.A.; Arnot, T.C.; Lakeman, J.B.; Walsh, F.C. Biofuel cells and their development. Biosens. Bioelectron. 2006, 21, 2015–2045. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Guerrini, E.; Grattieri, M.; Faggianelli, A.; Cristiani, P.; Trasatti, S. PTFE effect on the electrocatalysis of the oxygen reduction reaction in membraneless microbial fuel cells. Bioelectrochemistry 2015, 106, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.; Van Bogaert, G.; Porto-Carrero, C.; Diels, L.; Vanbroekhoven, K. Anode and cathode materials characterization for a microbial fuel cell in half cell configuration. Water Sci. Technol. 2011, 63, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.E.; Cheng, S.; Scott, K.; Logan, B.E. Microbial fuel cell performance with non-Pt catalysts. J. Power Sources 2007, 171, 275–281. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Liu, C.-S.; Zhuang, L.; Li, W.-S.; Zhou, S.-G.; Zhang, J.T. Manganese dioxide as an alternative cathode catalyst to platinum in microbial fuel cells. Biosens. Bioelectron. 2009, 24, 2825–2829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, S.; Pant, D.; Van Bogaert, G.; Logan, B.E. Power generation using an activated carbon and metal mesh cathode in a microbial fuel cell. Electrochem. Commun. 2009, 11, 2177–2179. [Google Scholar] [CrossRef]
- Forti, J.C.; Rocha, R.S.; Lanza, M.R.V.; Bertazzoli, R. Electrochemical synthesis of hydrogen peroxide on oxygen-fed graphite/PTFE electrodes modified by 2-ethylanthraquinone. J. Electroanal. Chem. 2007, 601, 63–67. [Google Scholar] [CrossRef]
- McLean, G.F.; Niet, T.; Prince-Richard, S.; Djilali, N. An assessment of alkaline fuel cell technolgy. Int. J. Hydrogen Energy 2002, 27, 507–526. [Google Scholar] [CrossRef]
- Bidault, F.; Brett, D.J.L.; Middleton, P.H.; Brandon, N.P. Review of gas diffusion cathodes for alkaline fuel cells. J. Power Sources 2009, 187, 39–48. [Google Scholar] [CrossRef]
- Yang, W.; He, W.; Zhang, F.; Hickner, M.A.; Logan, B.E. Single-Step Fabrication Using a Phase Inversion Method of Poly(vinylidene fluoride) (PVDF) Activated Carbon Air Cathodes for Microbial Fuel Cells. Environ. Sci. Technol. Lett. 2014, 1, 416–420. [Google Scholar] [CrossRef]
- Yang, W.; Kim, K.-Y.; Logan, B.E. Development of carbon free diffusion layer for activated carbon air cathode of microbial fuel cells. Bioresour. Technol. 2015, 197, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, P.; Moreels, J.P.; Claes, A.; Beckers, H. Electrode diaphragm electrode assembly for alkaline water electrolysers. Int. J. Hydrogen Energy 2009, 34, 9305–9315. [Google Scholar] [CrossRef]
- Alvarez, G.Y.; Vermeiren, Y.; Claes, A.V.; Adriansens, W. Gas Diffusion Electrode, Method of Producing Same, Membrane Electrode Assembly Comprising Same and Method of Producing Membrane Electrode Assembly Comprising Same. U.S. Patent 9105933 B2, 11 August 2015. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Yasuda, H.; Tsai, J.T. Pore size of microporous polymer membranes. J. Appl. Polym. Sci. 1974, 18, 805–819. [Google Scholar] [CrossRef]
- Doi, S.; Hamanaka, K. Pore size control technique in the spinning of polysulfone hollow fiber ultrafiltration membranes. Desalination 1991, 80, 167–180. [Google Scholar] [CrossRef]
- Radovanovic, P.; Thiel, S.; Hwang, S. Formation of asymmetric polysulfone membranes by immersion precipitation. J. Membr. Sci. 1992, 65, 231–246. [Google Scholar] [CrossRef]
- Vermeiren, P.; Leysen, R.; Beckers, H.; Moreels, J.P.; Claes, A. The influence of manufacturing parameters on the properties of macroporous Zirfon® separators. J. Porous Mater. 2008, 15, 259–264. [Google Scholar] [CrossRef]
- Mulder, G.; Coenen, P.; Martens, A.; Spaepen, J. The development of a 6 kW fuel cell generator based on alkaline fuel cell technology. Int. J. Hydrogen Energy 2008, 33, 3220–3224. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; De Smet, M.; Diels, L.; Vanbroekhoven, K. Use of novel permeable membrane and air cathodes in acetate microbial fuel cell. Electrochim. Acta 2010, 55, 7710–7716. [Google Scholar] [CrossRef]
- ElMekawy, A.; Srikanth, S.; Vanbroekhoven, K.; De Wever, H.; Pant, D. Bioelectro-catalytic valorization of dark fermentation effluents by acetate oxidizing bacteria in bioelectrochemical system. J. Power Sources 2014, 262, 183–191. [Google Scholar] [CrossRef]
- Alvarez-Gallego, Y.; Dominguez-Benetton, X.; Pant, D.; Diels, L.; Vanbroekhoven, K.; Genné, I.; Vermeiren, P. Development of gas diffusion electrodes for cogeneration of chemicals and electricity. Electrochim. Acta 2012, 82, 415–426. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J. Electrocatalytic oxygen reduction reaction in PEM Fuel Cell. In Electrocatalysts and Catalyst Layers; Zhang, J., Ed.; Springer Verlag: London, UK, 2008; pp. 89–134. [Google Scholar]
- Cifrain, M.; Kordesch, K.V. Advances, aging mechanism and lifetime in AFCs with circulating electrolytes. J. Power Sources 2004, 127, 234–242. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Ren, N.; Wang, H.; Lee, H.; Li, N.; Zhao, Q. Accelerated start-up of two-chambered microbial fuel cells: Effect of anodic positive poised potential. Electrochim. Acta 2009, 54, 1109–1114. [Google Scholar] [CrossRef]
- Srikanth, S.; Mohan, S.V. Influence of terminal electron acceptor availability to the anodic oxidation on the electrogenic activity of microbial fuel cell (MFC). Bioresour. Technol. 2012, 123, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, S.B.; Srikanth, S.; Venkata Mohan, S.; Pant, D. Development of exoelectrogenic bioanode and study on feasibility of hydrogen production using abiotic VITO-CoRE™ and VITO-CASE™ electrodes in a single chamber microbial electrolysis cell (MEC) at low current densities. Bioresour. Technol. 2015, 195, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Pant, D.; Logan, B.E. Long-term performance of activated carbon air cathodes with different diffusion layer porosities in microbial fuel cells. Biosens. Bioelectron. 2011, 30, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pant, D.; Zhang, F.; He, J.L.; Logan, B.E. Long-Term Performance of Chemically and Physically Modified Activated Carbons in Air Cathodes of Microbial Fuel Cells. ChemElectroChem 2014, 1, 1859–1866. [Google Scholar] [CrossRef]








| Polymer | Surface Energy (Polymer), mN·m−1 | Thickness, cm | Resistance, Ω·cm | εhydrophilic, % | εTOTAL, % | SBET, m2·g−1 | Pore Diameter, µm |
|---|---|---|---|---|---|---|---|
| PSf | 41 | 0.0357 | 194 | 10 | 65 | 230 | 0.5 |
| PTFE | 20 | 0.0510 | 14 | 29 | 66 | 454 | 0.1 |
| Non-Solvent | Type of Carbon Powder | Thickness, cm | Resistance, Ω·cm | Aw, mg·cm−2·s−1/2 | εhydrophilic, % | εTOTAL, % | SBET, m2·g−1 |
|---|---|---|---|---|---|---|---|
| H2O | Norit SX1G | 0.076 | 145 | 0.27 | 8 | 74 | 207 |
| H2O | Printex | 0.068 | 321 | – | 1 | 72 | 18 |
| NMP/H2O | Norit SX1G | 0.070 | 99 | 0.34 | 9 | 73 | 172 |
| NMP/H2O | Printex | 0.066 | nm * | – | 0.5 | 65 | 15 |
| C, wt % | Thickness, cm | Resistance, Ω·cm | Aw, mg cm−2 s−1/2 | εhydrophilic, % | εTOTAL, % | SBET, m²·g−1 |
|---|---|---|---|---|---|---|
| 65 | 0.071 | 655 | 0.42 | 8 | 73 | 177 |
| 70 | 0.076 | 145 | 0.27 | 8 | 74 | 207 |
| 75 | 0.067 | 86 | 0.33 | 10 | 70 | 201 |
| Polymer Additive | wt % | Thickness, cm | Mean Pore Size (CFP), μm | εhydrophilic, % | εTOTAL, % | LP, L·h−1·cm−2·bar−1·106 |
|---|---|---|---|---|---|---|
| FEP | 0 | 0.091 | 0.405 | 32 | 79 | 3.6 |
| FEP | 10 | 0.097 | 0.162 | 28 | 78 | 3.7 |
| FEP | 20 | 0.096 | 0.202 | 27 | 81 | 1.7 |
| FEP | 40 | 0.106 | 0.115 | 15 | 75 | 2.8 |
| FEP | 60 | 0.103 | 0.159 | 7 | 74 | 2.5 |
| FEP | 70 | 0.107 | 0.116 | 4 | 73 | 2.8 |
| FEP | 80 | 0.117 | 0.136 | 3 | 66 | 4.2 |
| PTFE Algoflon | 10 | 96 | 0.358 | 21 | 80 | 3.7 |
| PTFE 636N | 10 | 0.105 | 0.137 | 24 | 63 | 4.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srikanth, S.; Pant, D.; Dominguez-Benetton, X.; Genné, I.; Vanbroekhoven, K.; Vermeiren, P.; Alvarez-Gallego, Y. Gas Diffusion Electrodes Manufactured by Casting Evaluation as Air Cathodes for Microbial Fuel Cells (MFC). Materials 2016, 9, 601. https://doi.org/10.3390/ma9070601
Srikanth S, Pant D, Dominguez-Benetton X, Genné I, Vanbroekhoven K, Vermeiren P, Alvarez-Gallego Y. Gas Diffusion Electrodes Manufactured by Casting Evaluation as Air Cathodes for Microbial Fuel Cells (MFC). Materials. 2016; 9(7):601. https://doi.org/10.3390/ma9070601
Chicago/Turabian StyleSrikanth, Sandipam, Deepak Pant, Xochitl Dominguez-Benetton, Inge Genné, Karolien Vanbroekhoven, Philippe Vermeiren, and Yolanda Alvarez-Gallego. 2016. "Gas Diffusion Electrodes Manufactured by Casting Evaluation as Air Cathodes for Microbial Fuel Cells (MFC)" Materials 9, no. 7: 601. https://doi.org/10.3390/ma9070601
APA StyleSrikanth, S., Pant, D., Dominguez-Benetton, X., Genné, I., Vanbroekhoven, K., Vermeiren, P., & Alvarez-Gallego, Y. (2016). Gas Diffusion Electrodes Manufactured by Casting Evaluation as Air Cathodes for Microbial Fuel Cells (MFC). Materials, 9(7), 601. https://doi.org/10.3390/ma9070601