Cytotoxicity Evaluation of Anatase and Rutile TiO2 Thin Films on CHO-K1 Cells in Vitro
Abstract
:1. Introduction
2. Results
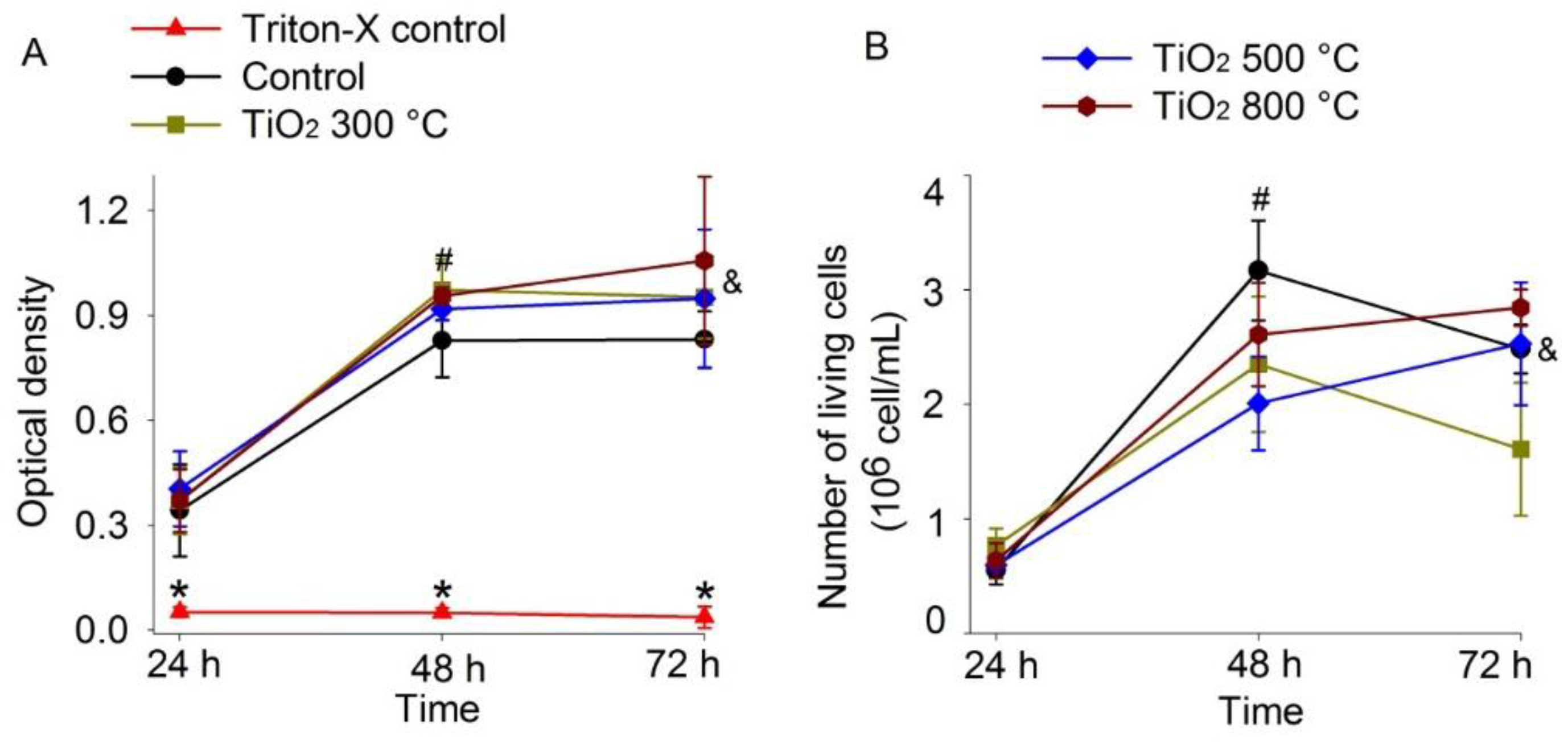
2.1. Cytotoxicity Analysis (MTT and Trypan Blue Assays)
2.2. Surface Roughness of TiO2 Thin Films
3. Discussion
4. Materials and Methods
4.1. TiO2 Thin Films
4.2. Cell Culture
4.3. MTT Cytotoxicity Assay
4.4. Trypan Blue Exclusion Assay
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| CMOS-MEMS | Complementary Metal Oxide Semiconductor and Micro-Electromechanical Systems |
| CHO-K1 | Chinese hamster ovary |
| DC | direct current |
| FESEM | Field Emission Scanning Electron Microscopy |
| MMT | 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide |
| RPM | revolution per minute |
| RMS | root-mean square surface roughness |
| SCCM | standard cubic centimeters per minute |
| TiO2 | Titanium Dioxide |
References
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, J.; Zhai, W. Effect of crystallographic phases of TiO2 on Hepatocyte Attachment Proliferation and Morphology. J. Biomater. Appl. 2005, 19, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Senain, I.; Nayan, N.; Saim, H. Structural and Electrical Properties of TiO2 Thin Film Derived from Sol-gel Method using Titanium (IV) Butoxide. Int. J. Integr. Eng. 2010, 4, 29–35. [Google Scholar]
- Constantin, D.G.; Apreutesei, M.; Arvinte, R.; Marin, A.; Andrei, O.C.; Munteanu, D. Magnetron sputtering technique used for coatings deposition; technologies and applications. Int. Conf. Mater. Sci. Eng. Brasov. Romania 2011, 2011, 29–33. [Google Scholar]
- Ghrairi, N.; Bouaicha, M. Structural, morphological, and properties of TiO2 thin films synthesized by the electrophoretic deposition technique. Nanoscale Res. Lett. 2012, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, S.; Kathyayini, S.R.; Dhivya, P.; Sridharan, M. Biocompatibility studies on TiO2 coated Ti surface. Int. Conf. Adv. Nanomat. Emerg. Eng. Technol. 2013. [Google Scholar] [CrossRef]
- Yin, Z.F.; Wu, L.; Yang, H.G.; Su, Y.H. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844–4858. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Wu, Z.W.; Wu, Y.Y.; Liu, Y.M. Electrochemical immunoassay of carcinoembryonic antigen based on TiO2-graphene/thionine/gold nanoparticles composite. Can. J. Chem. 2012, 90, 608–615. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, R.; Chai, Y.; Zhuo, Y.; He, Y.S.X.; Miao, X. A reagentless amperometric immunosensor for alpha-fetoprotein based on gold nanoparticles/TiO2 colloids/Prussian blue modified platinum electrode. Electroanalysis 2007, 19, 1402–1410. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Okon, S.L. Nanomaterial-based electrochemical immunosensors for clinically significant biomarkers. Materials 2014, 7, 4669–4709. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, Y.; Wu, C.; Guo, D.; Jiang, H.; Lu, X.; Wang, X. Photodynamic effect of mesoporous material: Titanium dioxide whiskers on SMMC-7721 cells. J. Nanosci. Nanotechnol. 2012, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Huerta, F.L.; Cervantes, B.; González, O.; Torres, J.H.; González, L.G.; Vega, R.; May, A.L.H.; Soto, E. Biocompatibility and surface properties of TiO2 thin films deposited by DC magnetron sputtering. Materials 2014, 7, 4088–4100. [Google Scholar] [CrossRef]
- Huerta, F.L.; Woo, R.M.G.; Lara, M.C.; Estrada, J.J.L.; Herrera, A.L.M. An integrated ISFET pH microsensor on a CMOS standard process. J. Sens. Technol. 2013, 3, 57–62. [Google Scholar] [CrossRef]
- Northup, S.J. Chapter 4: Nonclinical Medical Device Testing. In Biomaterials in the Design and Reliability of Medical Devices; Michael, M.N., Ed.; Landes Bioscience: Georgetown, TX, USA, 2003; pp. 144–171. [Google Scholar]
- Rickert, D.; Lendlein, A.; Peters, I.; Moses, M.A.; Franke, R.P. Biocompatibility testing of novel multifunctional polymeric biomaterials for tissue engineering applications in head and neck surgery: An overview. Eur. Arch. Otorhinolaryngol. 2006, 263, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity; ISO 10993–5:2006; International Organization for Standardization: Geneva, Switzerland, 2010.
- Helmus, M.N.; Gibbons, D.F.; Cebon, D. Biocompatibility: Meeting a key functional requirement of next-generation medical devices. Toxicol. Pathol. 2008, 36, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Grosskinsky, U. Biomaterial regulations for tissue engineering. Desalination 2006, 199, 265–267. [Google Scholar] [CrossRef]
- Lu, H.; Frazón, M.F.; Font, G.; Ruiz, M.J. Toxicity evaluation of individual and mixed enniatins using an in vitro method with CHO-K1-cells. Toxicol. in Vitro 2013, 27, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.J.; Festila, L.E.; Fernández, M. Comparison of basal cytotoxixity of seven carbamates in CHO-K1 cells. Toxicol. Environ. Chem. 2006, 88, 345–354. [Google Scholar] [CrossRef]
- Juan-García, E.F.A.; Font, G.; Ruiz, M.J. Reactive oxygen species induced by beauvericin, patulin and zearalenone in CHO-K1 cells. Toxicol. in Vitro 2009, 23, 1504–1509. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sorbello, G.S.A.L.; Saydam, G.; Banerjee, D.; Bertino, J.R. Chapter 38: Cytotoxicity and cell growth assays. In Cell Biology, Four-Volume Set: A Laboratory Handbook; Carter, N., Simons, K., Small, J., Hunter, T., Shotton, D., Eds.; Academic Press: New York, NY, USA, 2005; pp. 315–324. [Google Scholar]
- Putnam, K.P.; Bombick, D.W.; Doolittle, D.J. Evaluation of eight in vitro assays for assessing the cytotoxicity of cigarette smoke condensate. Toxicol. in Vitro 2002, 16, 599–607. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.A.; Duarte, M.A.H.; Matsumoto, M.A.; Marques, M.E.A.; Salvadori, D.M.F. Biocompatibility in vitro tests of mineral trioxide aggregate and regular and white Portland cements. J. Endod. 2005, 31, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar]
- Beausoleil, H.E.; Labrie, V.; Dubreuil, J.D. Trypan blue uptake by Chinese hamster ovary cultured epithelial cells: A cellular model to study Escherichia coli STb enterotoxin. Toxicon 2002, 40, 185–191. [Google Scholar] [CrossRef]
- Tolnai, S. A method for viable cell count. TCA Man./Tissue Cult. Assoc. 1975, 1, 37–38. [Google Scholar] [CrossRef]
- Yamane, H.; Konishi, K.; Iguchi, H.; Nakagawa, T.; Shibata, S.; Takayama, M.; Nishimura, K.; Sunami, K.; Nakai, Y. Assessment of hair cell death using the dye extrusion method. Acta Otolaryngol Suppl. 1998, 538, 7–11. [Google Scholar] [PubMed]
- Kaczmarek, D.; Domaradzki, J.; Wojcieszak, D.; Gornicka, B. XRD and AFM Studies of Nanocrystalline TiO2 Thin Films Prepared by Modified Magnetron Sputtering. In Proceedings International Spring Seminar on Electronics Technology, Budapest, Hungary, 7–11 May 2008; pp. 159–162.
- Jung, Y.S.; Lee, D.W.; Jeon, D.Y. Influence of the magnetron sputtering parameters on surface morphology of indium tin oxide thin films. Appl. Surf. Sci. 2004, 221, 136–142. [Google Scholar] [CrossRef]
- Brors, D.; Aletsee, C.; Schwager, K.; Mlynski, R.; Hansen, S.; Schäfers, M.; Ryan, A.F.; Dazert, S. Interaction of spiral ganglion neuron processes with alloplastic materials in vitro. Hear. Res. 2002, 167, 110–121. [Google Scholar] [CrossRef]
- Vila, M.C.; Buriel, B.M.; Chinarro, E.; Jurado, J.R.; Pastor, N.C.; Castro, J.E.C. Titanium oxide as substrate for neural cell growth. J. Biomed. Mater. Res. A 2009, 90, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Wang, Y.D.; Zheng, Y.F. In vitro biocompatibility study of nano TiO2 materials. Adv. Mater. Res. 2008, 47–50, 1438–1441. [Google Scholar] [CrossRef]
- Buchloh, S.; Stieger, B.; Meier, P.J.; Gauckler, L. Hepatocyte performance on different crystallographic faces of rutile. Biomaterials 2003, 24, 2605–2610. [Google Scholar] [CrossRef]
- Nakazawa, K.; Lee, S.W.; Fukuda, J.; Yang, D.H.; Kunitake, T. Hepatocyte spheroid formation on a titanium dioxide gel Surface and hepatocyte long-term culture. J. Mater. Sci. Mater. Med. 2006, 17, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Freshney, R.I. Chapter 1: Basic Principles of cell culture. In Culture of cells for Tissue Engineering; Vunjak-Novakovic, G., Freshney, R.I., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 3–22. [Google Scholar]
- Bai, Y.; Park, I.S.; Park, H.H.; Lee, M.H.; Bae, T.S.; Duncan, W.; Swain, M. The effect of annealing temperatures on surface properties, hydroxyapatite growth and cell behavior of TiO2 nanotubes. Surf. Interface Anal. 2011, 43, 998–1005. [Google Scholar] [CrossRef]
- McKeehan, M.; Warren, B.E. X-Ray Study of Cold Work in Thoriated Tungsten. J. Appl. Phys. 1953, 24, 52–56. [Google Scholar] [CrossRef]
- Gamper, N.; Stockand, J.D.; Shapiro, M.S. The use of Chinese hamster ovary (CHO) cells in the study of ion channels. J. Pharmacol. Toxicol. Methods 2005, 51, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, A.L.; Reigosa, M.; Arnal, P.M.; de Mele, M.F.L. Comparative study of the cytotoxic and genotoxic effects of titanium oxide and aluminum oxide nanoparticles in Chinese hamster ovary (CHO-K1) cells. J. Hazard. Mater. 2010, 177, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, F.; Tao, X.; Cheng, H. Ammonia-containing dimethyl sulfoxide: An im8roved solvent for the dissolution of formazan crystals in the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Anal. Biochem. 2012, 421, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Ngamwongsatit, P.; Banada, P.P.; Panbangred, W.; Bhunia, A.K. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. J. Microbiol. Methods 2008, 73, 211–215. [Google Scholar] [CrossRef] [PubMed]






| Percentage of Cell Viability | Control | TiO2 Films 300 °C | TiO2 Films 500 °C | TiO2 Films 800 °C |
|---|---|---|---|---|
| 24 h (n = 3) | 99.0 ± 0.6 | 99.2 ± 0.1 | 99.0 ± 0.5 | 98.5 ± 0.8 |
| 48 h (n = 3) | 99.6 ± 0.06 | 99.5 ± 0.08 | 99.3 ± 0.06 | 99.4 ± 0.02 |
| 72 h (n = 3) | 99.1 ± 0.09 | 99.0 ± 0.13 | 99.2 ± 0.12 | 99.0 ± 0.19 |
| TiO2 Films (Temperature) | RMS (nm) | Ra (nm) | Area (μm2) |
|---|---|---|---|
| 800 | 8.08 | 6.67 | 5 × 5 |
| 500 | 3.66 | 2.31 | 5 × 5 |
| 300 | 2.80 | 2.30 | 5 × 5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes, B.; López-Huerta, F.; Vega, R.; Hernández-Torres, J.; García-González, L.; Salceda, E.; Herrera-May, A.L.; Soto, E. Cytotoxicity Evaluation of Anatase and Rutile TiO2 Thin Films on CHO-K1 Cells in Vitro. Materials 2016, 9, 619. https://doi.org/10.3390/ma9080619
Cervantes B, López-Huerta F, Vega R, Hernández-Torres J, García-González L, Salceda E, Herrera-May AL, Soto E. Cytotoxicity Evaluation of Anatase and Rutile TiO2 Thin Films on CHO-K1 Cells in Vitro. Materials. 2016; 9(8):619. https://doi.org/10.3390/ma9080619
Chicago/Turabian StyleCervantes, Blanca, Francisco López-Huerta, Rosario Vega, Julián Hernández-Torres, Leandro García-González, Emilio Salceda, Agustín L. Herrera-May, and Enrique Soto. 2016. "Cytotoxicity Evaluation of Anatase and Rutile TiO2 Thin Films on CHO-K1 Cells in Vitro" Materials 9, no. 8: 619. https://doi.org/10.3390/ma9080619







