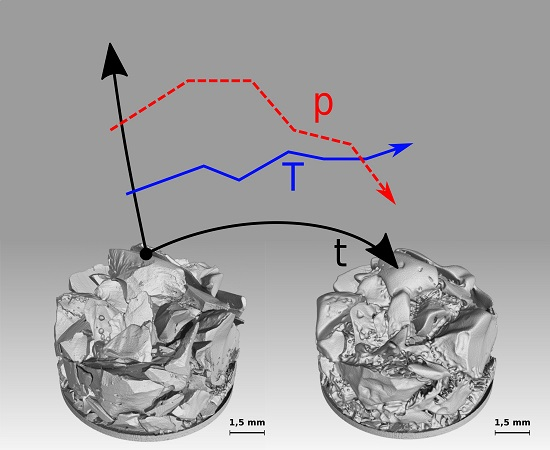
Micro-Tomographic Investigation of Ice and Clathrate Formation and Decomposition under Thermodynamic Monitoring
Abstract
:1. Introduction
2. Results
2.1. Formation and Decay of Ice, THF and 1,3-Dioxolane Clathrate
2.1.1. Thermal Expansion of Pure Nitrogen
2.1.2. Melting of Ice/Clathrate
2.1.3. Thermal Expansion after Melting
2.1.4. Thermal Contraction before Crystallization
2.1.5. Crystallization
2.1.6. Thermal Contraction after Crystallization
2.2. Pressure Monitored µCT Imaging of Ice and THF Clathrate
2.2.1. Ice Sample
2.2.2. THF Clathrate Sample
2.3. Nitrogen Uptake in THF Clathrate
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Experimental Setup
4.2.1. µCT Setup
4.2.2. Pressure-Monitored Cooling Stage
4.3. Temperature Management
4.4. Pressure Management
4.4.1. Thermal Expansion and Contraction in Non-Uniform Temperature Fields
4.4.2. Pressure Loss due to Leakage
4.5. Image Reconstruction and Post Processing
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bartels-Rausch, T.; Bergeron, V.; Cartwright, J.H.E.; Escribano, R.; Finney, J.L.; Grothe, H.; Gutiérrez, P.J.; Haapala, J.; Kuhs, W.F.; Pettersson, J.B.C.; et al. Ice structures, patterns, and processes: A view across the icefields. Rev. Mod. Phys. 2012, 84, 885–944. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, P.V. Ice Physics (Oxford Classic Texts in the Physical Sciences); Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Klinger, J. Extraterrestrial Ice: A review. J. Phys. Chem. 1983, 87, 4209–4214. [Google Scholar] [CrossRef]
- Consolmagno, G.J. Ice-rich moons and the physical properties of ice. J. Phys. Chem. 1983, 87, 4204–4208. [Google Scholar] [CrossRef]
- Hubbard, W.B. Planetary Interiors; Van Nostrand Reinhold: New York, NY, USA, 1984. [Google Scholar]
- Cavazzoni, C.; Chiarotti, G.L.; Scandolo, S.; Tosatti, E.; Bernasconi, M.; Parrinello, M. Superionic and metallic states of water and ammonia at Giant planet conditions. Science 1999, 283, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Roush, T.L. Physical state of ices in the outer solar system. J. Geophys. Res. Planets 2001, 106, 33315–33323. [Google Scholar] [CrossRef]
- Lin, J.-F.; Gregoryanz, E.; Struzhkin, V.V.; Somayazulu, M.; Mao, H.; Hemley, R.J. Melting behavior of H2O at high pressures and temperatures. Geophys. Res. Lett. 2005, 32, L11306/1–L11306/4. [Google Scholar] [CrossRef]
- Blake, D.; Allamandola, L.; Sandford, S.; Hudgins, D.; Freund, F. Clathrate hydrate formation in amorphous cometary ice analogs invacuo. Science 1991, 254, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.A.; Pohorille, A.; Jenniskens, P.; Blake, D.F. Probing the structure of cometary ice. Orig. Life Evol. Biosph. 1995, 25, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Chou, I.M.; Sharma, A.; Burruss, R.C.; Hemley, R.J.; Goncharov, A.F.; Stern, L.A.; Kirby, S.H. Diamond-anvil cell observations of a new methane hydrate phase in the 100-MPa pressure range. J. Phys. Chem. A 2001, 105, 4664–4668. [Google Scholar] [CrossRef]
- Prieto-Ballesteros, O.; Kargel, J.S.; Fernandez-Sampedro, M.; Selsis, F.; Martinez, E.S.; Hogenboom, D.L. Evaluation of the possible presence of clathrate hydrates in Europa’s icy shell or seafloor. Icarus 2005, 177, 491–505. [Google Scholar] [CrossRef]
- Fortes, A.D.; Choukroun, M. Phase behaviour of ices and hydrates. Space Sci. Rev. 2010, 153, 185–218. [Google Scholar] [CrossRef]
- Miller, S.L.; Smythe, W.D. Carbon dioxide clathrate in the Martian ice cap. Science 1970, 170, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Longhi, J. Phase equilibrium in the system CO2-H2O: Application to Mars. J. Geophys. Res. Planets 2006, 111, E06011/1–E06011/16. [Google Scholar] [CrossRef]
- Trainer, M.G.; McKay, C.P.; Tolbert, M.A.; Toon, O.B. CO2 clathrates on Mars and the CH4 question: A laboratory investigation. Astron. Soc. Pac. Conf. Ser. 2009, 420, 123–129. [Google Scholar]
- Von Stackelberg, M.; Müller, H.R. Feste Gashydrate II. Struktur und Raumchemie. Z. Electrochem. 1954, 58, 25–39. [Google Scholar]
- Sloan, E.D.; Koh, C. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Buffett, B.A. Clathrate hydrates. Annu. Rev. Earth Planet. Sci. 2000, 28, 477–507. [Google Scholar] [CrossRef]
- Mahajan, D.; Taylor, C.E.; Mansoori, G.A. An introduction to natural gas hydrate/clathrate: The major organic carbon reserve of the Earth. J. Pet. Sci. Eng. 2007, 56, 1–8. [Google Scholar] [CrossRef]
- Milkov, A.V. Global estimates of hydrate-bound gas in marine sediments: How much is really out there? Earth Sci. Rev. 2004, 66, 183–197. [Google Scholar] [CrossRef]
- Demirbas, A. Methane hydrates as potential energy resource: Part 1–Importance, resource and recovery facilities. Energy Convers. Manag. 2010, 51, 1547–1561. [Google Scholar] [CrossRef]
- Demirbas, A. Methane hydrates as potential energy resource: Part 2–Methane production processes from gas hydrates. Energy Convers. Manag. 2010, 51, 1562–1571. [Google Scholar] [CrossRef]
- Makogon, Y.F. Natural gas hydrates–A promising source of energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Mimachi, H.; Takahashi, M.; Takeya, S.; Gotoh, Y.; Yoneyama, A.; Hyodo, K.; Takeda, T.; Murayama, T. Effect of long-term storage and thermal history on the gas content of natural gas hydrate pellets under ambient pressure. Energy Fuels 2015, 29, 4827–4834. [Google Scholar] [CrossRef]
- Struzhkin, V.V.; Militzer, B.; Mao, W.L.; Mao, H.; Hemley, R.J. Hydrogen storage in molecular clathrates. Chem. Rev. 2007, 107, 4133–4151. [Google Scholar] [CrossRef] [PubMed]
- Strobel, T.A.; Hester, K.C.; Koh, C.A.; Sum, A.K.; Sloan, E.D. Properties of the clathrates of hydrogen and developments in their applicability for hydrogen storage. Chem. Phys. Lett. 2009, 478, 97–109. [Google Scholar] [CrossRef]
- Stern, L.A.; Circone, S.; Kirby, S.H.; Durham, W.B. Anomalous preservation of pure methane hydrate at 1 atm. J. Phys. Chem. B 2001, 105, 1756–1762. [Google Scholar] [CrossRef]
- Stern, L.A.; Circone, S.; Kirby, S.H.; Durham, W.B. Temperature, pressure, and compositional effects on anomalous or “self” preservation of gas hydrates. Can. J. Phys. 2003, 81, 271–283. [Google Scholar] [CrossRef]
- Takeya, S.; Ripmeester, J.A. Dissociation behavior of clathrate hydrates to ice and dependence on guest molecules. Angew. Chem. 2008, 120, 1296–1299. [Google Scholar] [CrossRef]
- Istomin, V.A.; Yakushev, V.S.; Makhonina, N.A.; Kwon, V.G.; Chuvilin, E.M. Self-preservation phenomenon of gas hydrate. Gas Ind. Russ. 2006, 4, 16–27. [Google Scholar]
- Chuvilin, E.M.; Guryeva, O.M. Experimental study of self-preservation effect of gas hydrates in frozen sediments. In Proceedings of the 9th International Conference on Permafrost, Fairbanks, AK, USA, 28 June–3 July 2008.
- Falenty, A.; Kuhs, W.F.; Glockzin, M.; Rehder, G. “Self-preservation” of CH4 hydrates for gas transport technology: Pressure–temperature dependence and ice microstructures. Energy Fuels 2014, 28, 6275–6283. [Google Scholar] [CrossRef]
- Falenty, A.; Kuhs, W.F. “Self-preservation” of CO2 gas hydrates—Surface microstructure and ice perfection. J. Phys. Chem. B 2009, 113, 15975–15988. [Google Scholar] [CrossRef] [PubMed]
- Kuhs, W.F.; Genov, G.; Staykova, D.K.; Hansen, T. Ice perfection and onset of anomalous preservation of gas hydrates. Phys. Chem. Chem. Phys. 2004, 6, 4917–4920. [Google Scholar] [CrossRef]
- Takeya, S.; Ripmeester, J.A. Anomalous preservation of CH4 hydrate and its dependence on the morphology of hexagonal ice. Chem. Phys. Chem. 2010, 11, 70–73. [Google Scholar] [PubMed]
- Veluswamy, H.P.; Wong, A.J.H.; Babu, P.; Kumar, R.; Kulprathipanja, S.; Rangsunvigit, P.; Linga, P. Rapid methane hydrate formation to develop a cost effective large scale energy storage system. Chem. Eng. J. 2016, 290, 161–173. [Google Scholar] [CrossRef]
- Buch, V.; Devlin, J.P.; Monreal, I.A.; Jagoda-Cwiklik, B.; Uras-Aytemiz, N.; Cwiklik, L. Clathrate hydrates with hydrogen-bonding guests. Phys. Chem. Chem. Phys. 2009, 11, 10245–10265. [Google Scholar] [CrossRef] [PubMed]
- Pinzer, B.R.; Schneebeli, M. Snow metamorphism under alternating temperature gradients: Morphology and recrystallization in surface snow. Geophys. Res. Lett. 2009, 36. [Google Scholar] [CrossRef]
- Pinzer, B.R.; Schneebeli, M.; Kaempfer, T.U. Vapor flux and recrystallization during dry snow metamorphism under a steady temperature gradient as observed by time-lapse micro-tomography. Cryosphere 2012, 6, 1141–1155. [Google Scholar] [CrossRef]
- Hammonds, K.; Lieb-Lappen, R.; Baker, I.; Wang, X. Investigating the thermophysical properties of the ice–snow interface under a controlled temperature gradient. Cold Reg. Sci. Technol. 2015, 120, 157–167. [Google Scholar] [CrossRef]
- Hammonds, K.; Baker, I. Investigating the thermophysical properties of the ice–snow interface under a controlled temperature gradient. Part 2. Analysis. Cold Reg. Sci. Technol. 2016, 125, 12–20. [Google Scholar] [CrossRef]
- Murshed, M.M.; Klapp, S.A.; Enzmann, F.; Szeder, T.; Huthwelker, T.; Stampanoni, M.; Marone, F.; Hintermüller, C.; Bohrmann, G.; Kuhs, W.F.; et al. Natural gas hydrate investigations by synchrotron radiation X-ray cryo-tomographic microscopy (SRXCTM). Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Jin, Y.; Hayashi, J.; Nagao, J.; Suzuki, K.; Minagawa, H.; Ebinuma, T.; Narita, H. New method of assessing absolute permeability of natural methane hydrate sediments by microfocus X-ray computed tomography. Jpn. J. Appl. Phys. 2007, 46, 3159–3162. [Google Scholar] [CrossRef]
- Jin, S.; Takeya, S.; Hayashi, J.; Nagao, J.; Kamata, Y.; Ebinuma, T.; Narita, H. Structure analyses of artificial methane hydrate sediments by microfocus X-ray computed tomography. Jpn. J. Appl. Phys. 2004, 43, 5673–5675. [Google Scholar] [CrossRef]
- Jin, S.; Nagao, J.; Takeya, S.; Jin, Y.; Hayashi, J.; Kamata, Y.; Ebinuma, T.; Narita, H. Structural investigation of methane hydrate sediments by microfocus X-ray computed tomography technique under high-pressure conditions. Jpn. J. Appl. Phys. 2006, 45, L714–L716. [Google Scholar] [CrossRef]
- Jin, Y.; Nagao, J.; Hayashi, J.; Shimada, W.; Ebinuma, T.; Narita, H. Observation of Xe hydrate growth at gas-ice interface by microfocus X-ray computed tomography. J. Phys. Chem. C 2008, 112, 17253–17256. [Google Scholar] [CrossRef]
- Ohno, H.; Narita, H.; Nagao, J. Different modes of gas hydrate dissociation to ice observed by microfocus X-ray computed tomography. J. Phys. Chem. Lett. 2011, 2, 201–205. [Google Scholar] [CrossRef]
- Chaouachi, M.; Falenty, A.; Sell, K.; Enzmann, F.; Kersten, M.; Haberthür, D.; Kuhs, W.F. Microstructural evolution of gas hydrates in sedimentary matrices observed with synchrotron X-ray computed tomographic microscopy. Geochem. Geophys. Geosystems 2015, 16, 1711–1722. [Google Scholar] [CrossRef]
- Jin, Y.; Konno, Y.; Nagao, J. Pressurized subsampling system for pressured gas-hydrate-bearing sediment: Microscale imaging using X-ray computed tomography. Rev. Sci. Instrum. 2014, 85. [Google Scholar] [CrossRef] [PubMed]
- Kerkar, P.; Jones, K.W.; Kleinberg, R.; Lindquist, W.B.; Tomov, S.; Feng, H.; Mahajan, D. Direct observations of three dimensional growth of hydrates hosted in porous media. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Ta, X.H.; Yun, T.S.; Muhunthan, B.; Kwon, T.-H. Observations of pore-scale growth patterns of carbon dioxide hydrate using X-ray computed microtomography. Geochem. Geophys. Geosystems 2015, 16, 912–924. [Google Scholar] [CrossRef]
- Kerkar, P.B.; Horvat, K.; Jones, K.W.; Mahajan, D. Imaging methane hydrates growth dynamics in porous media using synchrotron X-ray computed microtomography. Geochem. Geophys. Geosystems 2014, 15, 4759–4768. [Google Scholar] [CrossRef]
- Takeya, S.; Honda, K.; Yoneyama, A.; Hirai, Y.; Okuyama, J.; Hondoh, T.; Hyodo, K.; Takeda, T. Observation of low-temperature object by phase-contrast X-ray imaging: Nondestructive imaging of air clathrate hydrates at 233 K. Rev. Sci. Instrum. 2006, 77. [Google Scholar] [CrossRef] [Green Version]
- Takeya, S.; Honda, K.; Kawamura, T.; Yamamoto, Y.; Yoneyama, A.; Hirai, Y.; Hyodo, K.; Takeda, T. Imaging and density mapping of tetrahydrofuran clathrate hydrates by phase-contrast X-ray computed tomography. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Takeya, S.; Yoneyama, A.; Ueda, K.; Hyodo, K.; Takeda, T.; Mimachi, H.; Takahashi, M.; Iwasaki, T.; Sano, K.; Yamawaki, H.; et al. Nondestructive imaging of anomalously preserved methane clathrate hydrate by phase contrast X-ray imaging. J. Phys. Chem. C 2011, 115, 16193–16199. [Google Scholar] [CrossRef]
- Takeya, S.; Honda, K.; Gotoh, Y.; Yoneyama, A.; Ueda, K.; Miyamoto, A.; Hondoh, T.; Hori, A.; Sun, D.; Ohmura, R.; et al. Diffraction-enhanced X-ray imaging under low-temperature conditions: Non-destructive observations of clathrate gas hydrates. J. Synchrotron Radiat. 2012, 19, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Takeya, S.; Yoneyama, A.; Ueda, K.; Mimachi, H.; Takahashi, M.; Sano, K.; Hyodo, K.; Takeda, T.; Gotoh, Y. Anomalously preserved clathrate hydrate of natural gas in pellet form at 253 K. J. Phys. Chem. C 2012, 116, 13842–13848. [Google Scholar] [CrossRef]
- Takeya, S.; Gotoh, Y.; Yoneyama, A.; Hyodo, K.; Takeda, T. Observation of the growth process of icy materials in interparticle spaces: Phase-contrast X-ray imaging of clathrate hydrate. Can. J. Chem. 2015, 93, 983–987. [Google Scholar] [CrossRef]
- Grady, L. Random walks for image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2006, 28, 1768–1783. [Google Scholar] [CrossRef] [PubMed]
- Butkovich, T.R. Thermal expansion of ice. J. Appl. Phys. 1959, 30, 350–353. [Google Scholar] [CrossRef]
- Tse, J.S.; McKinnon, W.R.; Marchi, M. Thermal expansion of structure I ethylene oxide hydrate. J. Phys. Chem. 1987, 91, 4188–4193. [Google Scholar] [CrossRef]
- Tse, J.S. Thermal expansion of the clathrate hydrates of ethylene oxide and tetrahydrofuran. J. Phys. Colloq. 1987, 48, C1-543–C1-549. [Google Scholar] [CrossRef]
- Handa, Y.P. Heat capacities in the range 95 to 260 K and enthalpies of fusion for structure-II clathrate hydrates of some cyclic ethers. J. Chem. Thermodyn. 1985, 17, 201–208. [Google Scholar] [CrossRef]
- Petrenko, V.F.; Whitworth, R.W. Physics of Ice; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Devarakonda, S.; Groysman, A.; Myerson, A.S. THF–water hydrate crystallization: An experimental investigation. J. Cryst. Growth 1999, 204, 525–538. [Google Scholar] [CrossRef]
- VLE-Calc-Vapor-Liquid Equilibrium Database and Distillation Calculator. Available online: http://vle-calc.com/ (accessed on 11 June 2016).
- Handa, Y.P. Enthalpies of fusion and heat capacities for H218O ice and H218O tetrahydrofuran clathrate hydrate in the range 100–270 K. Can. J. Chem. 1984, 62, 1659–1661. [Google Scholar] [CrossRef]
- Demurov, A.; Radhakrishnan, R.; Trout, B.L. Computations of diffusivities in ice and CO2 clathrate hydrates via molecular dynamics and Monte Carlo simulations. J. Chem. Phys. 2002, 116, 702–709. [Google Scholar] [CrossRef]
- Blackford, J.R. Sintering and microstructure of ice: A review. J. Phys. Appl. Phys. 2007, 40, R355–R385. [Google Scholar] [CrossRef]
- Dash, J.G.; Rempel, A.W.; Wettlaufer, J.S. The physics of premelted ice and its geophysical consequences. Rev. Mod. Phys. 2006, 78, 695–741. [Google Scholar] [CrossRef]
- Mellor, M.; Smith, J.H. Creep of snow and ice. Phys. Snow Ice Proc. 1967, 1, 843–855. [Google Scholar]
- Theile, T.; Löwe, H.; Theile, T.C.; Schneebeli, M. Simulating creep of snow based on microstructure and the anisotropic deformation of ice. Acta Mater. 2011, 59, 7104–7113. [Google Scholar] [CrossRef]
- Iizuka, A.; Hayashi, S.; Tajima, H.; Kiyono, F.; Yanagisawa, Y.; Yamasaki, A. Gas separation using tetrahydrofuran clathrate hydrate crystals based on the molecular sieving effect. Sep. Purif. Technol. 2015, 139, 70–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Debenedetti, P.G.; Prud’homm, R.K.; Pethica, B.A. Differential scanning calorimetry studies of clathrate hydrate formation. J. Phys. Chem. B 2004, 108, 16717–16722. [Google Scholar] [CrossRef]
- Feistel, R.; Wagner, W. Sublimation pressure and sublimation enthalpy of H2O ice Ih between 0 and 273.16 K. Geochim. Cosmochim. Acta 2007, 71, 36–45. [Google Scholar] [CrossRef]
- Brunke, O.; Neuser, E.; Suppes, Al.; Chandgdar, S. High resolution industrial CT systems: Advances and comparison with synchrotron-based CT. E-J. Nondestruct. Test. 2014, 20, 1–9. [Google Scholar]
- Finke, H.L.; Gross, M.E.; Waddington, G.; Huffman, H.M. Low-temperature thermal data for the nine normal paraffin hydrocarbons from octane to hexadecane. J. Am. Chem. Soc. 1954, 76, 333–341. [Google Scholar] [CrossRef]
- Gucker, F.T.; Marsh, G.A. Refrigerating capacity of two-component systems. Ind. Eng. Chem. 1948, 40, 908–915. [Google Scholar] [CrossRef]
- CSC-Elmer. Available online: https://www.csc.fi/web/elmer (accessed on 15 June 2016).
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes in C: The Art of Scientific Computing, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Harmston, D. Materials challenge, diversification and the future. In Proceeding of the 40th International SAMPE Symposium and Exhibition, Anaheim, CA, USA, 8–11 May 1995; Society for the Advancement of Material and Process Engineering, Ed.; SAMPE: Covina, CA, USA, 1995. [Google Scholar]
- Weise, H.-P.; Kowalewsky, H.; Wenz, R. Behaviour of elastomeric seals at low temperature. Vacuum 1992, 43, 555–557. [Google Scholar] [CrossRef]
- Torquato, S. Random Heterogeneous Materials: Microstructure and Macroscopic Properties; Springer: Berlin, Germany, 2002. [Google Scholar]
- Berryman, J.G. Relationship between specific surface area and spatial correlation functions for anisotropic porous media. J. Math. Phys. 1987, 28, 244–245. [Google Scholar] [CrossRef]












| Pressure (mbar) | Ice | THF CH | DXL CH |
|---|---|---|---|
| at ramp bottom | 969 | 969 | 977 |
| at ramp top | 1064 | 1137 | 1126 |
| at melting point (solid samples) | 1041 | 1049 | 1045 |
| extrapolated from melting point (liquid samples) to ramp top | 1052 | 1063 | 1072 |
| left for guest gas | 12 | 74 | 54 |
| vapor pressure over solution at ramp top | 17 | 92 | 44 |
| Parameter | Value |
|---|---|
| tube voltage (kV) | 70 |
| focal spot size (µm) | 2.5 |
| magnification factor (-) | 20 |
| voxel edge length (µm) | 5.0 |
| no. of images per scan (-) | 1500 |
| exposure time per image (ms) | 1000 |
| no. of averaged images per increment (-) | 3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arzbacher, S.; Petrasch, J.; Ostermann, A.; Loerting, T. Micro-Tomographic Investigation of Ice and Clathrate Formation and Decomposition under Thermodynamic Monitoring. Materials 2016, 9, 668. https://doi.org/10.3390/ma9080668
Arzbacher S, Petrasch J, Ostermann A, Loerting T. Micro-Tomographic Investigation of Ice and Clathrate Formation and Decomposition under Thermodynamic Monitoring. Materials. 2016; 9(8):668. https://doi.org/10.3390/ma9080668
Chicago/Turabian StyleArzbacher, Stefan, Jörg Petrasch, Alexander Ostermann, and Thomas Loerting. 2016. "Micro-Tomographic Investigation of Ice and Clathrate Formation and Decomposition under Thermodynamic Monitoring" Materials 9, no. 8: 668. https://doi.org/10.3390/ma9080668