Electricity Recovery from Municipal Sewage Wastewater Using a Hydrogel Complex Composed of Microbially Reduced Graphene Oxide and Sludge
Abstract
:1. Introduction
2. Results and Discussion
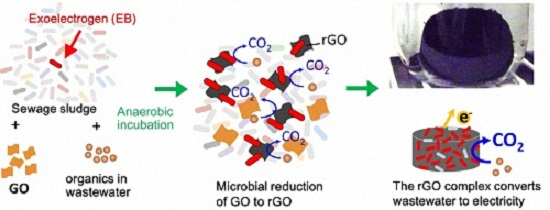
2.1. GO Reduction in Anaerobic Sludge
2.2. Electrochemical Cultivation of the rGO-Sludge Complex
2.3. Biomass Analysis of the rGO and GF Complexes
2.4. Electrochemical Comparison of the rGO and GF Complexes
2.5. Comparison of Microbial Communities in the rGO and GF Complexes
3. Discussion
4. Materials and Methods
4.1. Preparation of rGO and GF Complexes with Anaerobic Sludge
4.2. X-ray Photoelectron Spectroscopy (XPS) and Scanning Electron Microscopy (SEM) Analyses
4.3. Direct Cell Counting and Chemical Oxygen Demand (COD) Analyses
4.4. Electrochemical Cultivation
4.5. Electrochemical Analysis
4.6. Microbial Composition Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer—Can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.; Zhang, F.; Logan, B.E. Performance of two different types of anodes in membrane electrode assembly microbial fuel cells for power generation from domestic wastewater. J. Power Sources 2011, 196, 8293–8300. [Google Scholar] [CrossRef]
- Heidrich, E.; Curtis, T.; Dolfing, J. Determination of the internal chemical energy of wastewater. Environ. Sci. Technol. 2010, 45, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.K.; Lovley, D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 2003, 21, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, G.; Liu, Q.; Harnisch, F.; Zhou, Y.; Chen, Y.; Hanif, M.; Wang, S.; Peng, X.; Hou, H.; et al. Layered corrugated electrode macrostructures boost microbial bioelectrocatalysis. Energy Environ. Sci. 2012, 5, 9769–9772. [Google Scholar] [CrossRef]
- Yong, Y.-C.; Dong, X.-C.; Chan-Park, M.B.; Song, H.; Chen, P. Macroporous and monolithic anode based on polyaniline hybridized three-dimensional graphene for high-performance microbial fuel cells. ACS Nano 2012, 6, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, Z.; Yang, S.; Zhou, Y. Three-dimensional macroporous anodes based on stainless steel fiber felt for high-performance microbial fuel cells. J. Power Sources 2014, 258, 204–209. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, S.; Zhao, B.; Zhuang, L.; Wang, Y. Microbially-reduced graphene scaffolds to facilitate extracellular electron transfer in microbial fuel cells. Bioresour. Technol. 2012, 116, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Salas, E.C.; Sun, Z.; Luttge, A.; Tour, J.M. Reduction of graphen oxide via bacterial respiration. ACS Nano 2012, 4, 4852–4856. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qian, F.; Saltikov, C.W.; Jiao, Y.; Li, Y. Microbial reduction of graphene oxide by Shewanella. Nano Res. 2011, 4, 563–570. [Google Scholar] [CrossRef]
- Chae, H.K.; Siberio-Pérez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; YaghiA, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Duch, M.C.; Mansukhani, N.D.; Hersam, M.C.; Bouchard, D. Colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment. Environ. Sci. Technol. 2013, 47, 6288–6296. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Miyata, Y.; Doi, K.; Goto, Y.; Nagao, Y.; Tero, R.; Hiraishi, A. Graphene oxide-dependent growth and self-aggregation into a hydrogel complex of exoelectrogenic bacteria. Sci. Rep. 2016, 6, 21867. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Goto, Y.; Miyata, Y. Selective growth of and electricity production by marine exoelectrogenic bacteria in self-aggregated hydrogel of microbially reduced graphene oxide. C 2016, 2, 15. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon 2012, 50, 1853–1860. [Google Scholar] [CrossRef]
- Tanizawa, Y.; Okamoto, Y.; Tsuzuki, K.; Nagao, Y.; Yoshida, N.; Tero, R.; Iwasa, S.; Hiraishi, A.; Suda, Y.; Takikawa, H.; et al. Microorganism mediated synthesis of reduced graphene oxide films. J. Phys. Conf. Ser. 2012, 352, 012011. [Google Scholar] [CrossRef]
- Goto, Y.; Yoshida, N.; Umeyama, Y.; Yamada, T.; Tero, R.; Hiraishi, A. Enhancement of electricity production by graphene oxide in soil microbial fuel cells and plant microbial fuel cells. Front. Bioeng. Biotechnol. 2015, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Yoshida, N. Preliminary evaluation of a microbial fuelcell treating artificial dialysis wastewater using graphene oxide. AIP Conf. Proc. 2016, 1709, 020007. [Google Scholar]
- Zhang, T.; Shao, M.-F.; Ye, L. 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J. 2012, 6, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Kaniyoor, A.; Baby, T.T.; Ramaprabhu, S. Graphene synthesis via hydrogen induced low temperature exfoliation of graphite oxide. J. Mater. Chem. 2010, 20, 8467–8469. [Google Scholar] [CrossRef]
- Wu, T.; Cai, X.; Tan, S.; Li, H.; Liu, J.; Yang, W. Adsorption characteristics of acrylonitrile, p-toluenesulfonic acid, 1-naphthalenesulfonic acid and methyl blue on graphene in aqueous solutions. Chem. Eng. J. 2011, 173, 144–149. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Kim, J.-H. Microbial reduction of graphene oxide by Escherichia coli: A green chemistry approach. Colloids Surf. B Biointerfaces 2013, 102, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Khanra, P.; Kuila, T.; Kim, N.H.; Bae, S.H.; Yu, D.-S.; Lee, J.H. Simultaneous bio-functionalization and reduction of graphene oxide by baker’s yeast. Chem. Eng. J. 2012, 183, 526–533. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Boon, N.; Höfte, M.; Verstraete, W. Microbial phenazine production enhances electron transfer in biofuel cells. Environ. Sci. Technol. 2005, 39, 3401–3408. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sharbrough, E.; Liu, H. Quantification of the internal resistance distribution of microbial fuel cells. Environ. Sci. Technol. 2008, 42, 8101–8107. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, J.; Zhou, X.; Wang, X.; Li, B.; Santoro, C.; Grattieri, M.; Babanova, S.; Artyushkova, K.; Atanassov, P.; et al. Surface modification of microbial fuel cells anodes: Approaches to practical design. Electrochim. Acta 2014, 134, 116–126. [Google Scholar] [CrossRef]
- Santoro, C.; Guilizzoni, M.; Baena, J.C.; Pasaogullari, U.; Casalegno, A.; Li, B.; Babanova, S.; Artyushkova, K.; Atanassov, P. The effects of carbon electrode surface properties on bacteria attachment and start up time of microbial fuel cells. Carbon 2014, 67, 128–139. [Google Scholar] [CrossRef]
- Bond, D.R.; Lovley, D.R. Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl. Environ. Microbiol. 2005, 71, 2186–2189. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, M.; Hashimoto, K.; Watanabe, K. Use of cassette-electrode microbial fuel cell for wastewater treatment. J. Biosci. Bioeng. 2013, 115, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, M.; Yoshizawa, T.; Kouzuma, A.; Watanabe, K. Floating boards improve electricity generation from wastewater in cassette-electrode microbial fuel cells. J. Water Environ. Technol. 2015, 13, 221–230. [Google Scholar] [CrossRef]
- Kuever, J. The Family Desulfarculaceae. In The Prokaryotes; Springer: Berlin, Germany, 2014; pp. 41–44. [Google Scholar]
- Lovley, D.R.; Roden, E.E.; Phillips, E.; Woodward, J. Enzymatic iron and uranium reduction by sulfate-reducing bacteria. Mar. Geol. 1993, 113, 41–53. [Google Scholar] [CrossRef]
- Sun, H.; Spring, S.; Lapidus, A.; Davenport, K.; Del Rio, T.G.; Tice, H.; Nolan, M.; Copeland, A.; Cheng, J.-F.; Lucas, S.; et al. Complete genome sequence of Desulfarculus baarsii type strain (2st14T). Stand. Genom. Sci. 2010, 3, 276. [Google Scholar] [CrossRef] [PubMed]
- Sizova, M.V.; Panikov, N.S.; Spiridonova, E.M.; Slobodova, N.V.; Tourova, T.P. Novel facultative anaerobic acidotolerant Telmatospirillum siberiense gen. nov. sp. nov. isolated from mesotrophic fen. Syst. Appl. Microbiol. 2007, 30, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tsyganova, O.; Lee, D.-J.; Su, A.; Chang, J.-S.; Wang, A.; Ren, N. Anodic biofilm in single-chamber microbial fuel cells cultivated under different temperatures. Int. J. Gydrog. Energy 2012, 37, 15792–15800. [Google Scholar] [CrossRef]
- Liu, L.; Tsyganova, O.; Lee, D.-J.; Chang, J.-S.; Wang, A.; Ren, N. Double-chamber microbial fuel cells started up under room and low temperatures. Int. J. Hydrog. Energy 2013, 38, 15574–15579. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, B.; Cheng, M.; Feng, C. Effects of various organic carbon sources on simultaneous V reduction and bioelectricity generation in single chamber microbial fuel cells. Bioresours. Technol. 2016, 201, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, B.; Knorr, K.-H.; Schreck, K.; Tringe, S.G.; del Rio, T.G.; Loy, A.; Pester, M. Consortia of low-abundance bacteria drive sulfate reduction-dependent degradation of fermentation products in peat soil microcosms. ISME J. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Commault, A.S.; Lear, G.; Packe, M.A.R.; Weld, R.J. Influence of anode potentials on selection of Geobacter strains in microbial electrolysis cells. Bioresour. Technol. 2013, 139, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Ye, L.; Liu, F.; Li, Z.; Katayama, A. Evaluation of biodegradable plastics as solid hydrogen donors for the reductive dechlorination of fthalide by Dehalobacter species. Bioresours. Technol. 2013, 130, 478–485. [Google Scholar] [CrossRef] [PubMed]






| Cell Densities | Before Polarization | After 23 Days of Polarization |
|---|---|---|
| rGO | ||
| complex (108 cells cm−3) | 2.4 ± 0.17 | 8.4 ± 1.4 |
| complex (108 cells complex−1) | 46 ± 3.2 | 160 ± 27 |
| liquid culture (108 cells mL−1) | 0.40 ± 0.86 | 0.33 ± 0.10 |
| liquid culture (108 cells culture−1) | 360 ± 81 | 290 ± 81 |
| total (108 cells bottle−1) | 410 ± 75 | 450 ± 57 |
| complex (%) | 13 ± 2.8 | 38 ± 12 |
| liquid culture (%) | 87 ± 2.8 | 62 ± 12 |
| GF | ||
| complex (108 cells cm−3) | 4.0 ± 1.0 | 4.6 ± 0.63 |
| complex (108 cells complex−1) | 56 ± 14 | 87 ± 12 |
| liquid culture (108 cells mL−1) | 0.25 ± 0.63 | 0.61 ± 0.10 |
| liquid culture (108 cells culture−1) | 220 ± 55 | 550 ± 75 |
| total (108 cells bottle−1) | 280 ± 69 | 640 ± 87 |
| complex (%) | 20 ± 4.9 | 14 ± 1.7 |
| liquid culture (%) | 80 ± 4.9 | 86 ± 0.58 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, N.; Miyata, Y.; Mugita, A.; Iida, K. Electricity Recovery from Municipal Sewage Wastewater Using a Hydrogel Complex Composed of Microbially Reduced Graphene Oxide and Sludge. Materials 2016, 9, 742. https://doi.org/10.3390/ma9090742
Yoshida N, Miyata Y, Mugita A, Iida K. Electricity Recovery from Municipal Sewage Wastewater Using a Hydrogel Complex Composed of Microbially Reduced Graphene Oxide and Sludge. Materials. 2016; 9(9):742. https://doi.org/10.3390/ma9090742
Chicago/Turabian StyleYoshida, Naoko, Yasushi Miyata, Ai Mugita, and Kazuki Iida. 2016. "Electricity Recovery from Municipal Sewage Wastewater Using a Hydrogel Complex Composed of Microbially Reduced Graphene Oxide and Sludge" Materials 9, no. 9: 742. https://doi.org/10.3390/ma9090742