3.1. Structural Analysis
Figure 1 shows the FT-IR spectra of SMPU, OOBA, and SMPU-OOBAm composites (e.g., P1, P2, P3, P4, P5). In the spectrum of OOBA (
Figure 1a), the C=O stretching vibration was detected at approximately 1683 cm
−1, which suggest the formation of hydrogen bond associations. Thus, OOBA molecules may form dimers and create a long lath-like structure with a three-ring core, which is the basic structure of mesogen, to form the LC phase. In the spectrum of SMPU, the N–H stretching vibration is detected at approximately 3323 cm
−1 and 3286 cm
−1, whereas the C–N stretching vibration occurs at 1533 cm
−1 and 1531 cm
−1. In the SMPU-OOBAm composites (e.g., P4), the N–H stretching vibration shifts to a low wavenumber, which implies the formation of a hydrogen bond between the OOBA and the hard segment of SMPU. Additionally, P4 shows a new frequency at approximately 1685 cm
−1, which can be ascribed to the C=O stretching vibration of OOBA. The frequency at approximately 1166 cm
−1 also likely results from the C–O stretching vibration of OOBA. These results suggest that OOBA has successfully incorporated into SMPU and has formed an SMPU-OOBAm composite.
Figure 1b also shows that all SMPU-OOBAm composites have similar FT-IR results to P4. The peak density at 1685 cm
−1 obviously increases from P1 to P5, which confirms the increased OOBA content. In the previous literature, the LC phase was lost when LC mesogens containing pyridine moieties were attached to the SMPU containing carboxyl groups through hydrogen bonding between pyridine and COOH [
24]. However, in this work, the SMPU-OOBAm composites tend to keep the intrinsic LC properties of OOBA because of their dimer structure. The first advantage of the SMPU-OOBAm composites is that the liquid crystalline properties of OOBA can be used in the form of polymeric materials for many applications, being more conducive to the applications of liquid crystal materials.
3.2. Morphology Analysis
The surface morphology of the SMPU-OOBAm composites was investigated systematically via SEM and XRD. As shown in
Figure 2, the SEM images of the pure SMPU exhibit a smooth surface with no hole, but the surface becomes rough after OOBA is incorporated into SMPU (
Figure 2b–d). When the OOBA-content increases, more cracks are observed. These broken surfaces demonstrate that the SMPU-OOBAm forms a two-phase separated structure (
Figure 2c,d): the OOBA phase and the SMPU matrix. For example, for P5, some self-assembled crystals are observed, which the free OOBA molecules form, but the other OOBA molecules could not be precisely estimated because they are wrapped into a notably small size by the SMPU matrix. It has been confirmed that the incorporated OOBA enters the polyurethane matrix and self-assembles into the free OOBA phase in the composites. Unlike the previous SMPU-HOBA systems, OOBA does not form individual lamellar crystals in the SMPU-OOBAm composites, and the OOBA molecules have better dispersion in the SMPU matrix. This might affect the structure, morphology, and properties of the SMPU greatly.
Wide-angle X-ray diffraction (WAXD) experiments were used to further study the morphology of the SMPU-OOBAm composites. As shown in
Figure 3a, only two weak, broad peaks are observed in the pure SMPU at 19.5° and 23.8°, assigned to the (120) and (132) crystal faces, represents the crystal of polyethylene glycol, which indicates that the pure SMPU contains only a semi-crystalline soft phase and an amorphous hard phase. In addition, the pure OOBA shows many sharp peaks in the range of 10°~35°, which indicates a complete crystalline structure of OOBA [
28,
29]. When the OOBA is incorporated into the SMPU, nearly all crystalline peaks of OOBA remain detected, e.g., P2 and P5 (see
Figure 3a), and the intensity of these crystalline peaks increases when the OOBA content increases (see
Figure 3b). The crystalline peaks of the PEG soft phase are also intensified with the increase in OOBA content. This result indicates that OOBA promotes the crystallinity of the soft phase of SMPU. However, the intensity of the crystalline peaks in SMPU-OOBAm is not as strong as that of pure OOBA, which implies a reduced crystallinity of OOBA. Moreover, a new peak at 16.2° is observed; which also intensifies with an increase of OOBA-content. According to the literature [
30], we know that pure OOBA can form two different crystals (
α and
β crystalline phases) at low temperature. Therefore, this new peak may be ascribed to the second crystal type (
β phase) of OOBA. Thus, the crystallizability of the first crystal type (
α phase) of OOBA is restrained, and OOBA subsequently tends to form a second
β phase when the OOBA content increases. This phenomenon can also be reflected in the thermal properties and phase transitions.
3.3. Thermal Properties
The thermal properties of the SMPU-OOBAm composites were investigated by TGA and DSC. The TG curves show that all samples appear to decompose in two stages, which corresponds to the decomposition of the soft (at 200–300 °C) and hard segments (at 350–450 °C) of the SMPU matrix (see
Figure 4a). The TG results also show that the decomposition temperatures of the SMPU-OOBAm composites increase when the OOBA-content increases, which implies that the decomposition of SMPU-OOBAm is restrained when OOBA is incorporated. However, when the OOBA-content increases, the decomposition quantity in the first stage increases because of the increased decomposition of OOBA. Additionally, a broad weak peak is observed at about 210 °C on the DTG curves. This result demonstrates that the thermal stability of the composites at the first stage improves (see
Figure 4b). The improved thermal stability make the composites good polymeric materials.
The second DSC heating curves demonstrate that the pure SMPU shows an exothermic peak at −20 °C and an obvious endothermic peak at 50 °C (see
Figure 5a), which correspond to the cold crystallization and crystal-melting temperature of the soft phase, respectively. Pure OOBA shows four obvious endothermic peaks (
Figure 5a). Among them, the first two peaks at 78 °C and 101 °C can be attributed to the crystal-melting transitions of the
α and
β crystalline phases, respectively. The
β crystal-melting transition also indicates that OOBA enters the smectic phase. The third peak at 107 °C indicates a smectic-nematic phase transition, and the fourth peak at 148 °C suggests a phase transition from the nematic phase to the isotropic phase. When OOBA is incorporated into the SMPU matrix, the two crystal-melting transitions in the composites (e.g., P3) at 49 °C and 74 °C can be attributed to the crystal-melting of the soft segment of SMPU and the
α phase of OOBA, respectively. Meanwhile, a new weak exothermic peak which can be attributed to the
β crystal-melting transition appears at 89 °C. When the molar ratio of OOBA/MDI is lower than 0.4 (m ≤ 0.4), the
β crystal-melting transitions cannot be detected on the second DSC heating curve (see
Figure 5b), because the SMPU polymer chain affects the order structure of OOBA. In addition, another weak exothermic peak is detected at 183 °C and is attributed to the crystal melting of the hard segment of SMPU. This result indicates that the crystallizability of the hard segment of SMPU is also enhanced, because the OOBA crystals provide the nuclear seeds of crystallization for the hard phase. However, the cooling curves demonstrate that pure SMPU shows two weak peaks at 105 °C and −14 °C (see
Figure 5c), which are the crystallization temperatures of the hard and soft segments of SMPU, respectively; whereas SMPU-OOBAm shows a series of endothermic peaks upon cooling. For P5, the lowest and highest endothermic peaks at 15 °C and 133.6 °C are attributed to the crystallization temperatures of the soft phase and hard phase of SMPU, and these crystallization temperatures are also higher than those of pure SMPU. When the OOBA-content increases, the crystallization temperature of the soft phase of SMPU shifts to a higher temperature range. It is confirmed again that the soft phase of composites with higher OOBA-content has higher crystallinity. In addition, the endothermic peaks at 37 °C should be attributed to the crystallization temperature of the OOBA phase, whereas the endothermic peak at 80 °C shows the phase transition from the isotropic phase to the LC phase. These phase transitions are also verified by the following POM investigations.
The influence of heat treatment was also investigated in this study. In
Figure 5d, the effect of OOBA in the composites on the thermal properties during the heat treatment was investigated with DSC by heating sample P3 to 100, 120, 140, 160, and 180 °C. When P3 was thermally treated at 120 °C and 140 °C, the cold crystallization peak of the soft phase of SMPU was detected at −11 °C. However, the peak disappears after the thermal treatment at 100 °C, 160 °C, and 180 °C. A possible reason is that the movement of the polymer chains is frozen at low temperature (<100 °C), and the composites cannot form cold crystallization; the higher treatment temperature (≥160 °C) can promote the movement of the polymer chains and cause a complete crystallization of the soft segments when they cool to a low temperature. Thus, there is no cold crystallization in the second heating process. In addition,
Figure 5d demonstrates that the crystallization temperatures of both the soft and hard phases of SMPU shift to higher temperatures when the thermal treatment temperature increases. This result also demonstrates that the heat treatment can promote the crystallizability of the soft and hard segments of SMPU. However, the heat treatment results show that the crystal melting transition of OOBA is maintained at 74 °C in all composites. Thus, OOBA can aggregate into an isolated phase and retain its intrinsic LC properties in the SMPU-OOBAm composites.
For understanding the crystallization behaviors of the hard phase in the SMPU-OOBAm composites, the isothermal crystallization kinetics were investigated using DSC analysis. Detailed calculations are provided in
Section 2.5. The crystallization temperature was fixed at 137 °C in this experiment.
Figure 6a shows that the exothermic peak of SMPU-OOBAm widens when the OOBA-content increases during the isothermal crystallization process, which suggests the increased crystallization time. According to Equation (1), the relationships between relative crystallinity
(Xt) and time are shown in
Figure 6b. The curves exhibit an S pattern. The crystallization rate of the hard phase is slow during the initial stage and during the later period of crystallization. Moreover, the slope of the curves decreases when the OOBA-content increases. Thus, the crystallization rate of the hard phase decreases when the OOBA-content increases. The Avrami equation (Equation (2)) was used to denote the complete crystallization behaviours of the SMPU-OOBAm composites. Generally, and for convenient analysis, the Avrami equation is converted to Equation (3) via linearization.
Figure 6c presents the dependence of log{−ln(1 −
X(
t))} on log(
t). It was found that log{−ln(1 −
X(
t))} is linearly related to log(
t), but the curve slightly deviates during the later period of crystallization. This deviation implies the existence of a secondary crystallization, which should be attributed to the phase transformation from sphero-crystal to lamella-crystal. Finally, based on the original data and fitting a straight line, the kinetic parameters
n,
K, and
t1/2 of the isothermal crystallization were easily calculated. The results are summarized in
Table 2. The
n value of all samples is ~1.3, which further indicates that the crystallization mechanism of the hard segment of SMPU in all composites is the nucleated mechanism. The crystallization rate decreases because the
K value decreases when the OOBA-content increases. One possible reason for this is that the increased OOBA content might affect the aggregation of the hard segment of SMPU, though they provide more crystal seeds.
3.5. Liquid-Crystalline Properties
The liquid-crystalline properties and the phase transition behaviors of SMPU-OOBAm composites were investigated by POM. As we known, OOBA exhibits two crystalline forms, followed by smectic and nematic LC phases upon heating. Typical POM textures of OOBA upon heating are shown in
Figure 8. At low temperatures, OOBA forms a stable crystalline phase (see
Figure 8a). When the temperature increases to 112 °C, the colourful crystalline texture turns into a smectic LC phase (see
Figure 8b). When the temperature continuously increases, the smectic LC texture converts to the nematic LC phase at 135 °C (see
Figure 8c). As is known, an amorphous substance has no birefringence, and the field of vision is dark. The OOBA has good liquidity in the nematic phase. Thus, it is hard to get a whole continuous nematic texture at 135 °C. Therefore, the dark areas seen in
Figure 8c resulted from either the isotropic phase of part of the OOBA or the amorphous substrate. When the temperature reaches 139 °C, the LC texture begins to disappear and the field of vision becomes dark (
Figure 8d). This is the isotropic temperature of OOBA, which exactly corresponds to the exothermic peak on the DSC curve (see
Figure 5a).
For the SMPU-OOBAm composites, sample P5 has a bright abundant crystalline texture at 28 °C (
Figure 9a) because the crystals of the soft segment of SMPU and OOBA mixed together. When the temperature increases to over 50 °C, part of the bright crystalline texture disappears (see
Figure 9b), and the field of vision becomes darker, which suggests the crystal melting of the soft segment of SMPU. When the temperature reaches 76 °C, the composites enter the smectic phase (see
Figure 9c). This result is consistent with the DSC curve (see
Figure 5b). At 95 °C, the POM image shows the nematic phase, which suggests the phase transition from smectic phase to nematic phase because of the OOBA. When the temperature reaches 106 °C (see
Figure 9e), the nematic phase gradually disappears. Compared with pure OOBA, the LC phase transition temperatures shift to lower temperatures in all composites. These results confirm that the aggregation of OOBA mesogen was restrained by the SMPU matrix, which significantly affected the LC phase transitions. In addition, the POM images also demonstrate that some weak crystalline texture is maintained above 180 °C (see
Figure 9f), which implies the crystal formation of the hard segment of SMPU. These phenomena are also identified on the DSC curves.
Additionally, a comparison study of the POM textures of the composites is shown in
Figure 10. The results demonstrate that all composites showed similar LC phase transitions with relatively lower temperatures than pure OOBA. In the composites with lower OOBA contents (e.g., P2 and P4), the OOBA phase tended to disperse into the isolated OOBA phase in an SMPU matrix. As shown in
Figure 9, the black phase is the SMPU phase, and the bright phase is the OOBA LC phase. When the OOBA-content was higher, the OOBA phase could also form a continuous OOBA phase in the composites. This result confirms that the crystalline behaviours and LC properties of OOBA are maintained in all SMPU-OOBAm composites. It is thus confirmed again that SMPU-OOBAm composites can make the OOBA mesogens be used in many polymeric materials applications, including flexible displays, electronic papers, etc.
3.6. Shape Memory Properties
Triple-shape memory behaviours of SMPU-OOBAm composites were examined in a thermo-mechanical analysis using a TA Instruments DMA800 with tension clamps in controlled-force mode. In this experiment, the rectangular samples were first elongated at 100 °C, and then fixed at 80 °C, while the second elongation and fixation were carried out at 80 °C and 0 °C, respectively. Thereafter, the two step shape recovery is tested each for 40 min at 80 °C and 100 °C, respectively. The strain deformation, strain fixation, and strain recovery process were recorded for analysis.
Figure 11 presents the strain-time-temperature curves of P1, P2, P3, and P4, respectively. The PEG-based SMPU was reported to show only dual-shape memory effects in previous studies. However,
Figure 11 demonstrates that all composites show a two-step shape recovery with typical triple-shape memory effects. For example, in sample P1, after 62% strain is deformed at 100 °C, only 38% strain is fixed at 80 °C because of the higher fixation temperature, which is ascribed to the crystallization of the OOBA crystals. However, more than 98% strain fixation is obtained when the temperature decreases to 0 °C because of the further crystallization of the soft phase of the SMPU matrix. During the strain recovery process, P1 recovers 62.6% strain at 80 °C, and the remaining 13.8% strain is quickly recovered when the temperature increases to 100 °C. Finally, more than 90% of the total strain is recovered after the temperature is maintained at 100 °C for approximately 20 min. The strain recovery temperature in the second step corresponds exactly to the crystal-melting temperature of OOBA (76 °C) as mentioned in the DSC and POM results. However, when the OOBA-content increases, the strain recovery ratio in the second step tends to reduce from 90% (P1), 79% (P2), and 78% (P3) to 77% (P4). One possible reason for this is that OOBA has lubricated the SMPU polymer chains as a plasticizer. Although the crystallizability of the hard segment of SMPU is enhanced, the OOBA molecules in the composites may destroy the entire network structure of SMPU, which results in permanent deformation. The shape recovery thus decreases as the OOBA-content increases. However, all composites show good triple-SME. Both the strain fixation and strain recovery are programmable. Therefore, the SMPU-OOBAm can potentially be used in smart optical devices, electronic devices, and sensors.
3.7. Self-Healing Properties
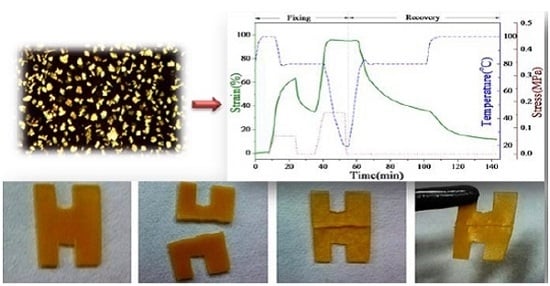
The SMPU-OOBAm composites were also found to show self-healing properties in this work. As shown in
Figure 12, a specimen with a H pattern was prepared using sample P2. First, the sample was cut into two parts before testing (
Figure 12), which were subsequently placed together. When the temperature was increased to 80 °C for 10 min, the sample became soft because of the crystal-melting of the soft segment of SMPU. When heated to 100 °C, the gap between the two cut parts gradually decreased. When the temperature reached 130 °C and the samples had been treated for 10 min, the two cut parts joined together completely (see
Figure 12c). After cooling to room temperature, the healed sample was obtained (see
Figure 12d). Thus, SMPU-OOBAm exhibits thermal-induced self-healing properties. According to the described structure and morphological analysis, the self-healing process is a thermal-induced self-healing mechanism, which can be explained as follows: the OOBA forms an isolated phase, and the SMPU matrix provides the backbone. When the temperature increases to 100 °C, the crystalline OOBA enters the LC state, and a good fluidity is obtained in OOBA molecules at 130 °C. Thus, the OOBA molecules bleed from the SMPU matrix and spontaneously enter the gap. After cooling to room temperature, the OOBA molecules in the gap form crystals via the crystallization process. Therefore, the gap between two cut parts can be sealed. Therefore, the SMPU-OOBAm composites might also have many promise self-healing applications in the fields of flexible printed circuit, self-protecting textiles, and self-healing concrete.