Necromass Carbon Stock in a Secondary Atlantic Forest Fragment in Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
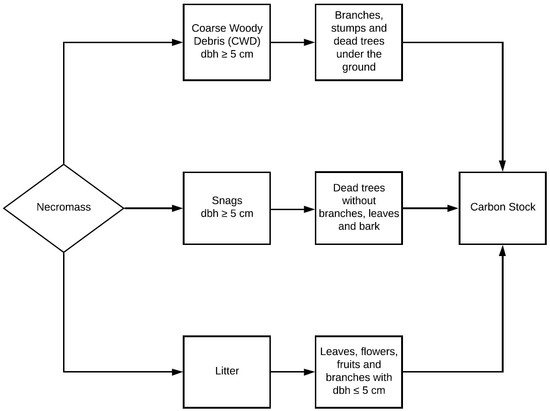
2.2. Quantification of Volume, Necromass and Carbon Stock
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Joly, C.A.; Metzger, J.P.; Tabarelli, M. Experiences from the Brazilian Atlantic Forest: Ecological findings and conservation initiatives. New Phytol. 2014, 204, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Fundação SOS Mata Atlântica and INPE. Atlas dos Remanescentes Florestais da Mata Atlântica Período 2016–2017; Relatório Técnico: São Paulo, Brazil, 2018; p. 63. [Google Scholar]
- Marcilio-Silva, V.; Marques, M.C. New paradigms for Atlantic Forest agriculture and conservation. Biodiversity 2017, 18, 201–205. [Google Scholar] [CrossRef]
- Sobral-Souza, T.; Vancine, M.H.; Ribeiro, M.C.; Lima-Ribeiro, M.S. Efficiency of protected areas in Amazon and Atlantic Forest conservation: A spatio-temporal view. Acta Oecol. 2018, 87, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Taubert, F.; Fischer, R.; Groeneveld, J.; Lehmann, S.; Müller, M.S.; Rödig, E.; Wiegand, T.; Huth, A. Global patterns of tropical forest fragmentation. Nature 2018, 554, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Scarano, F.R.; Ceotto, P. Brazilian Atlantic forest: Impact, vulnerability, and adaptation to climate change. Biodivers. Conserv. 2015, 24, 2319–2331. [Google Scholar] [CrossRef]
- Brinck, K.; Fischer, R.; Groeneveld, J.; Lehmann, S.; De Paula, M.D.; Pütz, S.; Sexton, J.O.; Song, D.; Huth, A. High resolution analysis of tropical forest fragmentation and its impact on the global carbon cycle. Nat. Commun. 2017, 8, 14855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, S.J.S.S.; Torres, C.M.M.E.; Jacovine, L.A.G.; Leite, H.G.; Gelcer, E.M.; Neves, K.M.; Schettini, B.L.S.; Villanova, P.H.; Silva, L.F.; Reis, L.P.; et al. Artificial neural networks: Modeling tree survival and mortality in the Atlantic Forest biome in Brazil. Sci. Total Environ. 2018, 645, 655–661. [Google Scholar] [CrossRef]
- Barbosa, R.I.; Castilho, C.V.; Perdiz, R.O.; Damasco, G.; Rodrigues, R.; Fearnside, P.M. Decomposition rates of coarse woody debris in undisturbed Amazonian seasonally flooded and unflooded forests in the Rio Negro-Rio Branco Basin in Roraima, Brazil. For. Ecol. Manag. 2017, 397, 1–9. [Google Scholar] [CrossRef]
- Araujo, L.S.; Komonen, A.; Lopes-Andrade, C. Influences of landscape structure on diversity of beetles associated with bracket fungi in Brazilian Atlantic Forest. Biol. Conserv. 2015, 191, 659–666. [Google Scholar] [CrossRef]
- Seibold, S.; Bässler, C.; Brandl, R.; Gossner, M.M.; Thorn, S.; Ulyshen, M.D.; Müller, J. Experimental studies of deadwood biodiversity—A review identifying global gaps in knowledge. Biol. Conserv. 2015, 191, 139–149. [Google Scholar] [CrossRef]
- Thibault, M.; Moreau, G. Enhancing bark-and wood-boring beetle colonization and survival in vertical deadwood during thinning entries. J. Insect Conserv. 2016, 20, 789–796. [Google Scholar] [CrossRef]
- Russell, M.B.; Fraver, S.; Aakala, T.; Gove, J.H.; Woodall, C.W.; D’Amato, A.W.; Ducey, M.J. Quantifying carbon stores and decomposition in dead wood: A review. For. Ecol. Manag. 2015, 350, 107–128. [Google Scholar] [CrossRef]
- Stutz, K.P.; Dann, D.; Wambsganss, J.; Scherer-Lorenzen, M.; Lang, F. Phenolic matter from deadwood can impact forest soil properties. Geoderma 2017, 288, 204–212. [Google Scholar] [CrossRef]
- Magalhães, T.M. Carbon stocks in necromass and soil pools of a Mozambican tropical dry forest under different disturbance regimes. Biomass Bioenergy 2017, 105, 373–380. [Google Scholar] [CrossRef]
- Krueger, I.; Schulz, C.; Borken, W. Stocks and dynamics of soil organic carbon and coarse woody debris in three managed and unmanaged temperate forests. Eur. J. For. Res. 2017, 136, 123–137. [Google Scholar] [CrossRef]
- Suzuki, S.N.; Tsunoda, T.; Nishimura, N.; Morimoto, J.; Suzuki, J.I. Dead wood offsets the reduced live wood carbon stock in forests over 50 years after a stand-replacing wind disturbance. For. Ecol. Manag. 2019, 432, 94–101. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Food and Agriculture Organization—FAO. Global Forest Resources Assessment; FRA2015 Brazil, Country Report. Rome, Italy, 2015. Available online: http://www.fao.org/3/a-az172e.pdf (accessed on 16 August 2019).
- Deus, K.H.P.D.; Figueiredo Filho, A.; Dias, A.N.; Bonete, I.P. Woody necromass stock in mixed ombrophilous forest using different sampling methods. Rev. Caatinga 2018, 31, 674–680. [Google Scholar] [CrossRef]
- Fonsêca, N.C.; Meunier, I.M.J.; Lins e Silva, A.C.B. Evaluation of the Plant Necromass Component: Methodological Approaches and Estimates in Atlantic Forest, Northeast Brazil. Floram 2019, 26, e20180383. [Google Scholar] [CrossRef]
- Universidade Federal de Viçosa—UFV. Departamento de Engenharia Agrícola. Estação Climatológica Principal de Viçosa. Boletim Meteorológico; Universidade Federal de Viçosa: Viçosa, Brazil, 2016. [Google Scholar]
- Ferreira Junior, W.G.; Schaefer, C.E.G.R.; Silva, A.F. Uma visão pedogeomorfológica sobre as formações florestais da Mata Atlântica. In Ecologia de Florestas Tropicais do Brasil, 2nd ed.; Martins, S.V., Ed.; Editora UFV: Viçosa, Brazil, 2012; pp. 141–174. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística—IBGE. Manual Técnico da Vegetação Brasileira. Manuais Técnicos em Geociências 2012, 2, 275. [Google Scholar]
- Brasil. Resolução n 392, de 25 Junho de 2007. Definição de Vegetação Primária e Secundária de Regeneração de Mata Atlântica no Estado de Minas Gerais; Brasília, DF: Ministério do Meio Ambiente/Conselho Nacional de Meio Ambiente: Brasília, Brazil, 2007. [Google Scholar]
- Torres, C.M.M.E.; Jacovine, L.A.G.; Soares, C.P.B.; Oliveira Neto, S.N.; Santos, R.D.; Castro Neto, F. Quantificação de biomassa e estocagem de carbono em uma floresta estacional semidecidual, no Parque Tecnológico de Viçosa, MG. Rev. Árvore 2013, 37, 647–655. [Google Scholar] [CrossRef]
- Harmon, M.E.; Whigham, D.F.; Sexton, J.; Olmsted, I. Decomposition and mass of woody detritus in the dry tropical forests of the northeastern Yucatan Peninsula, Mexico. Biotropica 1995, 27, 305–316. [Google Scholar] [CrossRef]
- Keller, M.; Palace, M.; Asner, G.P.; Pereira, R.; Silva, J.N.M. Coarse woody debris in undisturbed and logged forests in the eastern Brazilian Amazon. Glob. Chang. Biol. 2004, 10, 784–795. [Google Scholar] [CrossRef]
- Yuan, J.; Wei, X.; Shang, Z.; Cheng, F.; Hu, Z.; Zheng, X.; Zhang, S. Impacts of CWD on understory biodiversity in forest ecosystems in the Qinling Mountains, China. Pak. J. Bot. 2015, 47, 1855–1864. [Google Scholar]
- Chao, K.J.; Chen, Y.S.; Song, G.Z.M.; Chang, Y.M.; Sheue, C.R.; Phillips, O.L.; Hsieh, C.F. Carbon concentration declines with decay class in tropical forest woody debris. Forest Ecol. Manag. 2017, 391, 75–85. [Google Scholar] [CrossRef]
- Vital, B.R. Boletim Técnico: Métodos de Determinação de Densidade da Madeira, 1st ed.; Sociedade de Investigações Florestais: Viçosa, Brazil, 1984; p. 21. [Google Scholar]
- Associação Brasileira De Normas Técnicas—ABNT. Normas Técnicas NBR 11941; Associação Brasileira De Normas Técnicas—ABNT: São Paulo, Brazil, 2003. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 14 June 2019).
- Allen, S.E.; Grimshaw, H.M.; Rowland, A.P. Chemical analysis. In Methods in Plant Ecology, 2nd ed.; Moore, P.D., Chapman, S.B., Eds.; Blackwell Scientific Publications: Boston, MA, USA, 1986; pp. 285–344. [Google Scholar]
- Gautam, T.P.; Mandal, T.N. Effect of disturbance on biomass, production and carbon dynamics in moist tropical forest of eastern Nepal. For. Ecosyst. 2016, 3, 1–11. [Google Scholar] [CrossRef]
- Das, C.; Mondal, N.K. Litterfall, decomposition and nutrient release of Shorea robusta and Tectona grandis in a sub-tropical forest of West Bengal, Eastern India. J. For. Res. 2016, 27, 1055–1065. [Google Scholar] [CrossRef]
- Amaro, M.A. Quantificação do estoque volumétrico, de biomassa e de carbono em uma Floresta Estacional Semidecidual no Município de Viçosa-MG. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2010. [Google Scholar]
- Soares, C.P.B.; Oliveira, M.D. Equações para estimar a quantidade de carbono na parte aérea de árvores de eucalipto em Viçosa, Minas Gerais. Rev. Árvore. 2002, 26, 533–539. [Google Scholar] [CrossRef]
- Chambers, J.Q.; Higuchi, N.; Schimel, J.P.; Ferreira, L.V.; Melack, J.M. Decomposition and carbon cycling of dead trees in tropical forests of the central Amazon. Oecologia 2000, 122, 380–388. [Google Scholar] [CrossRef]
- Palace, M.; Keller, M.; Silva, H. Necromass production: Studies in undisturbed and logged Amazon forests. Ecol. Appl. 2008, 18, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, W.K.; Cornelissen, J.H.C.; Allison, S.D.; Bauhus, J.; Eggleton, P.; Preston, C.M.; Scarff, F.; Weeden, J.T.; Wirth, C.; Zanne, A.E. Plant traits and wood fates across the globe: Rotted, burned, or consumed? Glob. Chang. Biol. 2009, 15, 2431–2449. [Google Scholar] [CrossRef]
- Navarrete, D.; Sitch, S.; Aragão, L.E.; Pedroni, L.; Duque, A. Conversion from forests to pastures in the Colombian Amazon leads to differences in dead wood dynamics depending on land management practices. J. Environ. Manag. 2016, 171, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, S.P.; Cline, N.G.; Aumen, J.R.; Sedell, G.W.; et al. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 2004, 34, 59–234. [Google Scholar]
- Nascimento, R.G.M.; Machado, S.A.; Figueiredo Filho, A.; Higuchi, N. Modelo de projeção por classe diamétrica para florestas nativas: Enfoque na função probabilística de Weibull. Pesqui. Florest. Bras. 2012, 32, 209–219. [Google Scholar] [CrossRef]
- Naves, R.P.; Gandolfi, S.; Rother, D.C. Comparing patterns of density, diameter, and species abundance in areas in restoration process. Hoehnea 2015, 42, 737–748. [Google Scholar] [CrossRef]
- Kira, T.; Shidei, T. Primary production and turnover of organic matter in different forest ecosystems of the western Pacific. Jpn. J. Ecol. 1967, 17, 70–87. [Google Scholar]
- Odum, E.P. Strategy of ecosystem development. Science 1969, 164, 262. [Google Scholar] [CrossRef]
- Gough, C.M.; Curtis, P.S.; Hardiman, B.S.; Scheuermann, C.M.; Bond-Lamberty, B. Disturbance, complexity, and succession of net ecosystem production in North America’s temperate deciduous forests. Ecosphere 2016, 7, e01375. [Google Scholar] [CrossRef]
- Meyer, P.B.; Oliveira-Filho, A.T.; Botezelli, L.; Aurélio, M.; Fontes, L.; Garcia, P.O.; Santos, R.M. Dinâmica estrutural em um fragmento de Floresta Estacional Semideciduifólia em Lavras, MG, Brasil. CERNE 2015, 21, 259–265. [Google Scholar] [CrossRef]
- Rubino, D.L.; McCarthy, B.C. Evaluation of coarse woody debris and forest vegetation across topographic gradients in a southern Ohio forest. For. Ecol. Manag. 2003, 183, 221–238. [Google Scholar] [CrossRef]
- Barba, J.; Yuste, J.C.; Poyatos, R.; Janssens, I.A.; Lloret, F. Strong resilience of soil respiration components to drought-induced die-off resulting in forest secondary succession. Oecologia 2016, 182, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.B.; Castro, C.S.; Alvarado, L.D.A.; Read, J.M. Quantifying mortality of tropical rain forest trees using high-spatial-resolution satellite data. Ecol. Lett. 2004, 7, 52–59. [Google Scholar] [CrossRef]
- Fontes, C.G.; Chambers, J.Q.; Higuchi, N. Revealing the causes and temporal distribution of tree mortality in Central Amazonia. For. Ecol. Manag. 2018, 424, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.B.; Gregoire, T.G.; Couto, H.T.Z. Estimation of the volume, biomass and carbon content of coarse woody debris within two forest types in the State of São Paulo, Brazil. Forestry 2019, 92, 278–286. [Google Scholar] [CrossRef]
- Zaninovich, S.C.; Fontana, J.L.; Gatti, M.G. Atlantic Forest replacement by non-native tree plantations: Comparing aboveground necromass between native forest and pine plantation ecosystems. For. Ecol. Manag. 2016, 363, 39–46. [Google Scholar] [CrossRef]
- Moreira, A.B.; Gregoire, T.G.; Couto, H.T.Z. Wood density and carbon concentration of coarse woody debris in native forests, Brazil. For. Ecosyst. 2019, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Maraseni, T.N.; Mathers, N.J.; Harms, B.; Cockfield, G.; Apan, A.; Maroulis, J. Comparing and predicting soil carbon quantities under different land-use systems on the Red Ferrosol soils of southeast Queensland. J. Soil Water Conserv. 2008, 63, 250–256. [Google Scholar] [CrossRef]
- Chao, K.J.; Phillips, O.L.; Gloor, E.; Monteagudo, A.; Torres-Lezama, A.; Martínez, R.V. Growth and wood density predict tree mortality in Amazon forests. J. Ecol. 2008, 96, 281–292. [Google Scholar] [CrossRef]
- Chao, K.J.; Phillips, O.L.; Baker, T.R. Wood density and stocks of coarse woody debris in a northwestern Amazonian landscape. Can. J. For. Res. 2008, 38, 795–805. [Google Scholar] [CrossRef]
- Vieira, S.A.; Alves, L.F.; Duarte-Neto, P.J.; Martins, S.C.; Veiga, L.G.; Scaranello, M.A.; Picollo, M.C.; Camargo, P.B.; Carmo, J.B.; Sousa Neto, E.; et al. Stocks of carbon and nitrogen and partitioning between above and belowground pools in the Brazilian coastal Atlantic Forest elevation range. Ecol. Evol. 2011, 1, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, D.K.; Litton, C.M.; Giardina, C.P. Coarse woody debris carbon storage across a mean annual temperature gradient in tropical montane wet forest. For. Ecol. Manag. 2013, 291, 336–343. [Google Scholar] [CrossRef]
- Rice, A.H.; Pyle, E.H.; Saleska, S.R.; Hutyra, L.; Palace, M.; Keller, M.; Camargo, P.B.; Portilho, K.; Marques, D.F.; Wofsy, S.C. Carbon balance and vegetation dynamics in an old-growth Amazonian forest. Ecol. Appl. 2004, 14, 55–71. [Google Scholar] [CrossRef]
- Chao, K.J.; Phillips, O.L.; Baker, T.R.; Peacock, J.; Lopez-Gonzalez, G.; Vásquez Martínez, R.; Monteagudo, A.; Torres-Lezama, A. After trees die: Quantities and determinants of necromass across Amazonia. Biogeosciences 2009, 6, 1615–1626. [Google Scholar] [CrossRef]



| Variable | Year of Measurement | |
|---|---|---|
| 2010 | 2015 | |
| Botanical Families | 43 | 47 |
| Botanical Genera | 98 | 102 |
| Identified Species | 127 | 134 |
| Non - Identified Species | 3 | 5 |
| Shannon-Weaver Index (H’) | 3.97 | 4.01 |
| Density (stems ha−1) | 1,526 | 1,692 |
| Quadratic mean diameter (cm) | 12.27 | 12.31 |
| Basal Area (m2 ha−1) | 18.05 | 20.15 |
| Minimum DBH (cm) | 5.03 | 5.01 |
| Arithmetic mean diameter (cm) | 10.47 | 10.52 |
| Maximum DBH (cm) | 65.57 | 50.29 |
| Minimum Height (m) | 3.00 | 3.00 |
| Average Height (m) | 10.08 | 11.17 |
| Maximum Height (m) | 30.00 | 34.50 |
| Volume (m3 ha−1) | 128.12 | 152.04 |
| Aboveground biomass (Mg ha−1) * | 86.23 | 102.71 |
| Carbon stock in aboveground biomass (MgC ha−1) | 40.01 | 47.64 |
| Forest Dynamic | 2010–2015 | |
| Recruited Stems (stems ha−1) | 335 | |
| Mortality Stems (stems ha−1) | 169 | |
| Carbon stock in Recruitment (MgC ha−1) | 1.66 | |
| Carbon stock in Mortality (MgC ha−1) | 3.72 | |
| Gross increment in Carbon (MgC ha−1) ** | 9.69 | |
| Dead Wood | Class. of Decomp. | Dens. (g cm−3) | Var. Ran. (g cm−3) | Carbon Cont. (%) | Var. Ran. (%) |
|---|---|---|---|---|---|
| CWD | 1 | 0.577 ± 0.128 a | 0.358 − 0.913 | 45.49 ± 0.76 | 42.80 − 47.54 |
| 2 | 0.527 ± 0.167 a | 0.234 − 0.865 | |||
| 3 | 0.395 ± 0.108 b | 0.172 − 0.650 | 45.44 ± 1.31 | 43.25 − 46.63 | |
| 4 | 0.321 ± 0.165 c | 0.128 − 0.796 | |||
| Snags | - | 0.455 ± 0.174 | 0.128 − 0.913 | 45.49 ± 0.76 | 42.80 − 47.54 |
| Litter | - | - | - | 44.46 ± 2.83 | 33.00 – 47.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanova, P.H.; Torres, C.M.M.E.; Jacovine, L.A.G.; Soares, C.P.B.; da Silva, L.F.; Schettini, B.L.S.; da Rocha, S.J.S.S.; Zanuncio, J.C. Necromass Carbon Stock in a Secondary Atlantic Forest Fragment in Brazil. Forests 2019, 10, 833. https://doi.org/10.3390/f10100833
Villanova PH, Torres CMME, Jacovine LAG, Soares CPB, da Silva LF, Schettini BLS, da Rocha SJSS, Zanuncio JC. Necromass Carbon Stock in a Secondary Atlantic Forest Fragment in Brazil. Forests. 2019; 10(10):833. https://doi.org/10.3390/f10100833
Chicago/Turabian StyleVillanova, Paulo Henrique, Carlos Moreira Miquelino Eleto Torres, Laércio Antônio Gonçalves Jacovine, Carlos Pedro Boechat Soares, Liniker Fernandes da Silva, Bruno Leão Said Schettini, Samuel José Silva Soares da Rocha, and José Cola Zanuncio. 2019. "Necromass Carbon Stock in a Secondary Atlantic Forest Fragment in Brazil" Forests 10, no. 10: 833. https://doi.org/10.3390/f10100833